Published online May 26, 2015. doi: 10.4331/wjbc.v6.i2.28
Peer-review started: January 31, 2015
First decision: March 6, 2015
Revised: March 23, 2015
Accepted: April 8, 2015
Article in press: April 9, 2015
Published online: May 26, 2015
Processing time: 112 Days and 7.4 Hours
Poisonous organisms are represented in many taxa, including kingdom Animalia. During evolution, animals have developed special organs for production and injection of venoms. Animal venoms are complex mixtures, compositions of which depend on species producing venom. The most known and studied poisonous terrestrial animals are snakes, scorpions and spiders. Among marine animals, these are jellyfishes, anemones and cone snails. The toxic substances in the venom of these animals are mainly of protein and peptide origin. Recent studies have indicated that the single venom may contain up to several hundred different components producing diverse physiological effects. Bites or stings by certain poisonous species result in severe envenomations leading in some cases to death. This raises the problem of bite treatment. The most effective treatment so far is the application of antivenoms. To enhance the effectiveness of such treatments, the knowledge of venom composition is needed. On the other hand, venoms contain substances with unique biological properties, which can be used both in basic science and in clinical applications. The best example of toxin application in basic science is α-bungarotoxin the discovery of which made a big impact on the studies of nicotinic acetylcholine receptor. Today compositions of venom from many species have already been examined. Based on these data, one can conclude that venoms contain a large number of individual components belonging to a limited number of structural types. Often minor changes in the amino acid sequence give rise to new biological properties. Change in the living conditions of poisonous animals lead to alterations in the composition of venoms resulting in appearance of new toxins. At the same time introduction of new methods of proteomics and genomics lead to discoveries of new compounds, which may serve as research tools or as templates for the development of novel drugs. The application of these sensitive and comprehensive methods allows studying either of venoms available in tiny amounts or of low abundant components in already known venoms.
Core tip: Animal venoms are complex mixtures mostly of peptides and proteins. Recent studies have indicated that the single venom may contain up to several hundred different components producing diverse physiological effects. The knowledge of venom composition as well as structure and properties of its components on the one hand may give the clue for better treatment of bites and stings on the other hand it may lead to the discovery of new medicines. Recent developments in research methods gave a great impulse to animal venom studies, which may result in the entry of new drugs to the market.
- Citation: Utkin YN. Animal venom studies: Current benefits and future developments. World J Biol Chem 2015; 6(2): 28-33
- URL: https://www.wjgnet.com/1949-8454/full/v6/i2/28.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i2.28
Poisonous organisms are wide spread throughout the globe and represented in many biological taxa. A large number of poisonous species belongs to the kingdom Animalia. Both vertebrates (fishes, amphibians, reptiles, birds, and mammals) and invertebrates (coelenterates, worms, arthropods, molluscs, echinoderms) may be poisonous. Venomous animals permanently or periodically contain in their organisms substances, which are toxic to other species. Even small doses of such compounds in the body of another animal cause painful disorders and sometimes death. Some venomous animals have special venom glands producing venom; others contain toxic substances in the various tissues of the body. A number of animals have a specialized wounding apparatus (organ) for the introduction of venom into the body of a predator or a prey. In the coelenterates (hydras, sea anemones, jellyfishes) these are stinging cells, in arthropods (scorpions, bees, wasps) - multicellular glands connected with the sting, and in fishes - similar glands connected with spines on the fins (e.g., scorpionfish) and on gill covers (sea dragon). In many animals (millipedes, spiders, and snakes), venom glands are associated with mouthparts and venom is injected into the prey’s body at the bite or puncture. In venomous animals, which have venom glands, but do not have a special apparatus for the venom injection, such as amphibians (salamanders, newts, frogs and others), glands are located in various skin sites. Of venomous terrestrial animals the most studied are snakes, scorpions and spiders. Among the marine animals, these are jellyfishes, anemones and cone snails. Animal venoms are complex mixtures, compositions of which depend on species producing venom.
The history of the venom study traces back more than two millennia. The earliest survived detailed synopsis of venomous animals and their bites belongs to Aristotle (384-322 BC. Aristotle “Historia Animalium”). Modern scientific understanding of venomous snakes and venoms began to take shape thanks to the Italian scientists Francesco Redi and Felice Fontana, who worked in the city of Pisa in the 17 and 18 centuries, respectively. Physician, biologist, linguist and poet Francesco Redi[1] (1626-1697) published in 1664 a treatise on poisonous snakes “Osservazioni intorno alle vipere”. He found that snake bile is not toxic as it was accepted at that time, and toxicity is in the venom released from the teeth at the bite. Redi is considered as one of the founders of toxinology, a specialist area of science dealing with animal, microbial, and plant venoms, poisons and toxins. A hundred years later, in the 18 century, another Italian, Felice Fontana, discovered snake venom glands and obtained snake venom, which he used for a variety of experiments with animals. Fontana corrected Redi: venom acts on the animals not by getting in stomach as Redi believed, but in the blood.
Intensive modern biochemical studies of venoms began in the middle of the last century, although some proteins in the venoms of snakes have been discovered much earlier. For example, one of the earliest reports on snake venom phosphodiesterase activity refers to 1932[2]. However, only since the late 50’s early 60’s of the last century, a broader study of snake venom proteins began. For example, in 1959 snake venom protease was isolated by the Japanese group[3], and in 1963 Chang and Lee[4] published a paper, which described isolation of α-bungarotoxin. The latter work has become a classic. To date, it is the most cited in the field of venom studies, and α-bungarotoxin is still one of the most selective markers of certain subtypes of nicotinic acetylcholine receptor. According to the PubMed database, more than 1000 articles devoted to the study of venoms are published annually, and the total number of publications is more than 40000. About half of these publications deal with snake venoms.
The exact historical moment, when people have learned to benefit from venoms and to use them for medical purposes, is very difficult to trace. However, in ancient Rome animal venoms were used to produce drug for the treatment of smallpox, leprosy, and fever, as well as for wound healing. Many doctors and philosophers of ancient Greece wrote about the action of snake venom. The Greek physician Nicander of Colophon described the action of snake venom and the composition of antidotes. Particularly, snake venom was widespread as a remedy in the Middle Ages and until the 19 century it was part of many antidotes. Back in the 1st century AD, there was a mixture containing snake venom - the so-called theriac. It was manufactured in pharmacies in Europe until the 18 century. But the real use of snake venom began in the late 19 century, when French scientist Albert Calmette found that if animals were injected with venom in small doses, their blood serum became a strong antidote[5]. First snake venoms were used only for antivenom production, but then their use has expanded. It was reported that in patients with epilepsy seizures were ceased after they being bitten by a rattlesnake, and in 1934 it was found that cobra venom in small doses has a potent analgesic activity - many times greater than morphine, with the difference that the venom does not cause drug addiction. Since that time venom was part of the drug against asthma, hypertension and even leprosy. So far, ointments and cremes (Cobroxin, Viprosal, Cobratoxan, etc.) based on venoms are produced in many countries. However, such use of venoms is hardly effective as their content in the preparations is extremely low, and the effect is achieved due to nonspecific action. Nevertheless, there is a certain demand for these products.
Animal venoms are extremely rich and complex natural sources of biologically active molecules that have a variety of molecular targets and functions. Venoms are classified by origin - venoms of snakes, scorpion venoms, spider venoms, etc., or by their effects on the body - neurotoxic, hemotoxic venoms, etc. Animal venoms usually are aqueous solutions containing a significant number of components, most of the peptide and protein nature[6]. Several hundred different substances - toxins, may be present in single venom. Since one of the main objectives of the venom is killing or immobilization of the prey, its components are highly toxic. Animal toxins cause significant dysfunction of the nervous, cardiovascular and muscular systems. Poisoning by animal venoms cause serious illness in humans and can lead to death. For the treatment of such poisoning, antisera obtained against crude venom are generally used[7].
The venoms usually consist of enzymes (phospholipases, proteases, oxidases, etc.), proteins without enzymatic activity (neurotoxins, disintegrin, etc.), and peptides. Such a complex composition of the venom results in the combined effects on vital systems of the body and severe poisoning. Peptide toxins acting on ion channels (e.g., ion channel blockers) are widely represented in venoms. Generally, they affect nervous system and are the principal active components of spider, scorpion, Cone snail and Elapidae snake venoms[8]. Spider and scorpion neurotoxins act mostly on voltage-gated ion channels, while snake neurotoxins - on ligand-gated channels, in particular on nicotinic acetylcholine receptors. Diverse conotoxins from Cone snails block both voltage gated channels and acetylcholine receptors.
In venoms, two classes - oxidases and hydrolases, represent enzymes. The highest enzyme content is in the snake venoms. Only single oxidase, L-amino acid oxidase, is found in venoms so far, while hydrolases are very abundant and include phospholipases А2, proteinases/peptidases, acetylcholinesterases, and hyaluronidases. Non-enzymatic proteins are more diversified and, for example, in snakes include so called three finger toxins, disintegrins, proteinase inhibitors, nerve growth factor, etc[9]. Peptides and polypeptides are the principal constituents of spider, scorpion and cone snail venoms and comprise various neurotoxins, antimicrobial peptides, immuno-modulating peptides, etc.
Such a wide range of peptides and proteins with different biological functions makes animal venoms valuable source of new compounds both for use in basic research and for the development of new medicines.
Despite the long history of venom handling, only few toxins found real application. Several toxins are widely used in basic research as biochemical tools. α-Bungarotoxin mentioned above is the best example. Discovered more than fifty years ago[4] it is still unsurpassed as selective and efficient pharmacological tool for the study of α7 and muscle type nicotinic acetylcholine receptors. α-Conotoxins, peptide neurotoxins, are more versatile tools for this receptor studies as they distinguish various subtypes and even different binding sites within one receptor molecule[10]. Some scorpion and spider toxins show very high selectivity toward particular types of voltage-gated ion-channels and are used in investigations on these channels[8]. Venom proteins affecting hemostasis also represent useful tools for exploring different processes within this sophisticated system.
Several venom components especially those from snake venoms have found extensive applications in the diagnosis of haemostatic disorders. Some diagnostics marketed by Pentapharm Ltd. (Switzerland) can be mentioned as examples. Thus, prothrombin activator Ecarin from Echis carinatus venom is used for the detection of abnormal type of prothrombin, serine protease Protac® from Agkistrodon contortrix-for determination of protein C and protein S, and metalloprotease RVV-X from Daboia russelli-for determination of FX and screening assay for lupus anti-coagulants[11].
A new fibrin adhesive (sealant) was developed by a group of researchers from the Center for the Study of Venoms and Venomous Animals, in Sao Paulo State, Brazil. This sealant is a biological and biodegradable product produced without using human blood. The components of this novel sealant were extracted from large animals and a serine proteinase isolated from Crotalus durissus terrificus snake venom. A new fibrin adhesive was tested in periodontal surgery and found to be a good alternative to traditional sutures[12].
The application of snake venom proteinase in biotechnology was proposed by Australian company Venom Supplies Pty Ltd. The serine proteinase notanarin, which is a structural homologue of blood coagulation factor Xa, was positioned as an alternative to this factor in molecular biology for recombinant protein cleavage (http://www.venomsupplies.com/cleavage-compound/).
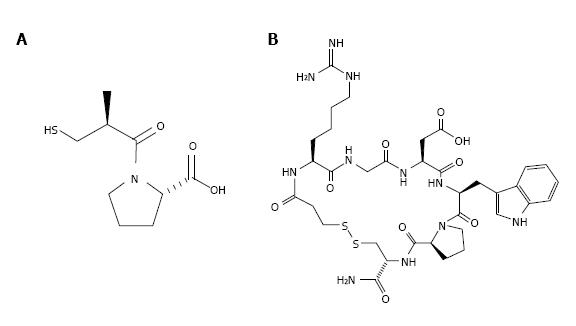
It should be noted that despite the great promises, only few medications are developed on basis of animal venom ingredients. The barriers on this difficult road are perfectly considered in a recent review[13]. Yet, several drugs based on the venom components have been successfully elaborated. In this row, Captopril® or Enalpril® (Figure 1A), a drug that treats hypertension, should be mentioned first. It was developed in the late 1970s and early 1980s based on bradykinin potentiating peptide, a peptide from the pit viper Bothrops jararaca venom. Captopril is an angiotensin-converting enzyme inhibitor and its development became a paradigm for “rational drug design”. The year production of captopril is about 1000 tons.
Two more drugs based on animal toxins, anticoagulants Integrilin® (Eptifibatide, Figure 1B) and Aggrastat® (Triofiban), are used now to prevent heart attacks or the formation of blood clots. Eptifibatide is a cyclic heptapeptide derived from a protein found in the venom of the south eastern pygmy rattlesnake Sistrurus miliarius barbouri. It belongs to the class of the so-called arginine-glycine-aspartate (RGD) - mimetics, reversibly binds to platelets and selectively blocks the platelet glycoprotein IIb/IIIa receptor. Eptifibatide is marketed by Millennium Pharmaceuticals, Inc. and Schering Corporation and distributed by Schering Corporation. Tirofiban is a peptidomimetic based on disintegrin echistatin from the saw-scaled viper Echis carinatus. It also mimics the RGD sequence and is an antiplatelet drug[14]. Tirofiban is administered to reduce the rate of thrombotic cardiovascular events.
Recently a real neurotoxic peptide was turned into a medicine. This is Prialt® (Ziconotide), an analgesic from cone snail venom[15]. Ziconotide is a synthetic form of ω-conotoxin M-VIIA, peptide from the venom of Conus magus marine snail; it acts as a specific blocker of neuronal N-type calcium channels and is used to treat a variety of chronic pain syndromes.
The above examples show that the new drugs can be developed based on proteins and peptides from animal venoms. The number of new compounds found in venoms is growing steadily and some of them can be used for this purpose as well.
In relation to subjects discussed above it is reasonable to assume that one of the areas in the future venom research will be the search for new compounds, which may serve as a basis for the development of new medicines or used as new molecular tools. Since all available venoms have already been well studied, rare or very small animals will be used as research objects. Also previously unexplored compounds present in the venoms in extremely small quantities may be included in studies. In this direction, progress in high-throughput “omics” methodologies (proteomics, transcriptomics and genomics) make a great contribution to the identification and characterization of new scaffolds[16,17]. In particular, in the last decade, the utilization of these protocols for deciphering the toxin composition of venoms in great details becomes a common practice. Even the new term “venomics”, which is the proteomic characterization of venom proteomes, has emerged. For example, the venom profiling of the rear-fanged snake Thamnodynastes strigatus using venomics has revealed new types of biologically important molecules including a new kind of matrix metalloproteinase that is unrelated to the classical snake venom metalloproteinases[18].
The search for prodrugs of medications acting on central nervous system among animal venom components is especially promising. The data available show that some of venom peptides appear to be capable of go through the blood brain barrier (BBB). Thus, bee venom apamin, a peptide interacting with certain types of potassium channels, and its nontoxic analogue can penetrate BBB[19] that makes these peptides promising candidates for BBB shuttles. Other peptide chlorotoxin from scorpion venom was shown also penetrate BBB and its fluorescently labelled analogue was used for detecting cancer foci and metastases noninvasively[20]. Chlorotoxin-linked dye Tumor Paint BLZ-100 that “light ups” cancer cells so that surgeons can precisely target brain tumors is entering phase 1b study in human patients. Some venom proteins also possess capability to cross BBB (for review see[21]). These findings open up the new possibilities for emergence of medicines for the central nervous system treatment.
It should be noted that the genome of king cobra Ophiophagus hannah has been sequenced recently[22]. However, no new types of venom proteins or peptides revealed by this study have been reported so far.
Another way for the progress in the area of animal venoms is the development of new drugs based on known toxins. However even well known toxins may have unexpected or unwanted effects in vivo, as their targets may be expressed differently in the body. This problem requires a careful study in each particular case before a compound can be considered as a drug lead. Currently, several companies are moving in this direction. Thus, Celtic Biotech Iowa Inc. develops novel therapeutic products for the treatment of solid cancers and pain, which are based on crotoxin from pit-viper Crotalus durissus terrificus and cardiotoxin from cobra Naja atra. ReceptoPharm, United States-based biotechnology company, has completed phase I trials with modified versions of toxins from cobra venoms. The company plans to conduct further clinical trials in multiple sclerosis, motor neuron disease, adrenomyeloneuropathy and viral infections. The United States biotechnology company Kineta begins randomized placebo controlled trials in psoriatic arthritis and psoriasis of drug candidate ShK-186, which is a synthetic peptide that blocks Kv1.3 potassium ion channels. ShK-186 was derived from ShK neurotoxin of Caribbean anemone Stichodactyla helianthus. It should be noted that the work in this sector is very risky and competitive. A number of companies, which have begun work on the development of new toxin-based drugs, were forced to stop operation or ceased to exist.
A few words about antivenom therapy should be said here as well. It is currently the only available effective treatment for envenomation. “Omics” technologies have also a strong impact in this field. Transcriptomic analyses can identify regional variations in venom composition, which should be taken into account at development of specific and efficacious antivenoms. Utilizing transcriptomic analyses cDNA sequences encoding main toxic proteins can be easily determined. Large amount of particular cDNA can be prepared and directly used for immunization. For example, cDNA encoding a novel P-II type metalloproteinase from Bothrops asper venom glands was cloned, sequenced and used for DNA immunization of animals[23]. The equine antibodies induced by immunization with plasmid encoding the metalloproteinase completely neutralized the hemorrhagic activity of the whole Bothrops asper venom. This technique opens a new way to the treatment of envenomation.
Despite the great progress in animal venom studies, some challenges remain yet in this area. Thus, the development of effective, cheap and safe antivenoms is in great demand. To address this problem, the composition of animal venoms specific to the area and its relation to the known one should be established. Until now, even the venoms of some very dangerous poisonous animals are not fully characterized. This concerns, for example, the Australian jellyfishes. The box jellyfish Chironex fleckeri, causing so-called Irukandji syndrome, produces extremely potent and rapid-acting venom. Envenomed victims can show symptoms ranging from headaches, severe pain, nausea and vomiting to pulmonary oedema, cardiac failure and severe hypertension resulting in death. However, the exact venom composition is not known and no well established treatment exists. The same is true for some other venomous animals (e.g., scorpions and spiders) inhabiting other part of globe. The problem is complicated in rural areas where even existing antivenoms are not always available.
In conclusion, it is still largely expected that introduction of new “omics” methodologies in venom studies will greatly enhance the discovery of new toxins which may be used as templates for drug design. These methodologies provide a number of advantages for the development of new tools for basic research, clinical diagnostics, and novel medicines.
| 1. | Redi F; Osservazioni Intorno alle Vipere. Florence: All'Insegna della Stella. Available from: http://www.books.google.ru/books/reader?id=h3FVAAAAcAAJ&hl=ru&printsec=frontcover&output=reader&pg=GBS.PP1. |
| 2. | Uzawa S. Über die phosphomonoesterase und die phosphodiesterase. J Biochem Toyko. 1932;15:19-28. |
| 3. | Maeno H, Morimura M, Mitsuhashi S, Sawai Y, Okonogi T. Studies on Habu snake venom. 2b. Further purification and enzymic and biological activities of H alpha-proteinase. Jpn J Microbiol. 1959;3:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Chang CC, Lee CY. Isolation of neurotoxins from the venom of bungarus multicinctus and their modes of neuromuscular blocking action. Arch Int Pharmacodyn Ther. 1963;144:241-257. [PubMed] |
| 5. | Calmette A. The Treatment of Animals Poisoned with Snake Venom by the Injection of Antivenomous Serum. Br Med J. 1896;2:399-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Casewell NR, Wüster W, Vonk FJ, Harrison RA, Fry BG. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol. 2013;28:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 689] [Article Influence: 49.2] [Reference Citation Analysis (1)] |
| 7. | Gutiérrez JM. Improving antivenom availability and accessibility: science, technology, and beyond. Toxicon. 2012;60:676-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Kalia J, Milescu M, Salvatierra J, Wagner J, Klint JK, King GF, Olivera BM, Bosmans F. From foe to friend: using animal toxins to investigate ion channel function. J Mol Biol. 2015;427:158-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | McCleary RJ, Kini RM. Non-enzymatic proteins from snake venoms: a gold mine of pharmacological tools and drug leads. Toxicon. 2013;62:56-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Lebbe EK, Peigneur S, Wijesekara I, Tytgat J. Conotoxins targeting nicotinic acetylcholine receptors: an overview. Mar Drugs. 2014;12:2970-3004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Schoni R. The use of snake venom-derived compounds for new functional diagnostic test kits in the field of haemostasis. Pathophysiol Haemost Thromb. 2005;34:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Barbosa MD, Gregh SL, Passanezi E. Fibrin adhesive derived from snake venom in periodontal surgery. J Periodontol. 2007;78:2026-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Harvey AL. Toxins and drug discovery. Toxicon. 2014;92:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Hashemzadeh M, Furukawa M, Goldsberry S, Movahed MR. Chemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: A review. Exp Clin Cardiol. 2008;13:192-197. [PubMed] |
| 15. | Pope JE, Deer TR. Ziconotide: a clinical update and pharmacologic review. Expert Opin Pharmacother. 2013;14:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Fox JW, Serrano SM. Exploring snake venom proteomes: multifaceted analyses for complex toxin mixtures. Proteomics. 2008;8:909-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Brahma RK, McCleary RJ, Kini RM, Doley R. Venom gland transcriptomics for identifying, cataloging, and characterizing venom proteins in snakes. Toxicon. 2015;93:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Ching AT, Paes Leme AF, Zelanis A, Rocha MM, Furtado Mde F, Silva DA, Trugilho MR, da Rocha SL, Perales J, Ho PL. Venomics profiling of Thamnodynastes strigatus unveils matrix metalloproteinases and other novel proteins recruited to the toxin arsenal of rear-fanged snakes. J Proteome Res. 2012;11:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Oller-Salvia B, Teixidó M, Giralt E. From venoms to BBB shuttles: Synthesis and blood-brain barrier transport assessment of apamin and a nontoxic analog. Biopolymers. 2013;100:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ. Tumor paint: a chlorotoxin: Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882-6888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 318] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 21. | Osipov A, Utkin Y. Effects of snake venom polypeptides on central nervous system. Cent Nerv Syst Agents Med Chem. 2012;12:315-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Vonk FJ, Casewell NR, Henkel CV, Heimberg AM, Jansen HJ, McCleary RJ, Kerkkamp HM, Vos RA, Guerreiro I, Calvete JJ. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc Natl Acad Sci USA. 2013;110:20651-20656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 356] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 23. | Arce-Estrada V, Azofeifa-Cordero G, Estrada R, Alape-Girón A, Flores-Díaz M. Neutralization of venom-induced hemorrhage by equine antibodies raised by immunization with a plasmid encoding a novel P-II metalloproteinase from the lancehead pitviper Bothrops asper. Vaccine. 2009;27:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
P- Reviewer: Audi S, Febbraio F, Kitagawa S, Malli R S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/