Published online Nov 26, 2014. doi: 10.4331/wjbc.v5.i4.409
Revised: June 30, 2014
Accepted: August 27, 2014
Published online: November 26, 2014
Processing time: 281 Days and 19.8 Hours
Brain-derived neurotrophic factor (BDNF) attracts increasing attention from both research and clinical fields because of its important functions in the central nervous system. An adequate amount of BDNF is critical to develop and maintain normal neuronal circuits in the brain. Given that loss of BDNF function has been reported in the brains of patients with neurodegenerative or psychiatric diseases, understanding basic properties of BDNF and associated intracellular processes is imperative. In this review, we revisit the gene structure, transcription, translation, transport and secretion mechanisms of BDNF. We also introduce implications of BDNF in several brain-related diseases including Alzheimer’s disease, Huntington’s disease, depression and schizophrenia.
Core tip: Brain-derived neurotrophic factor (BDNF) plays essential roles in the central nervous system (CNS) through a specific tropomyosin-related kinase receptor B (TrkB), contributing to neuronal survival, neurite outgrowth, and synaptic function. It is critically required for normal development and functions of the brain. A number of reports have shown an importance of BDNF in the pathophysiology of brain-associated diseases. This review describes molecular mechanisms underlying gene expression, transport, and secretion of BDNF and its function in the CNS. We further introduce recent findings on the possible involvement of BDNF in the pathophysiology of neurodegenerative and psychiatric diseases, and the potential effectiveness of enhancing BDNF/TrkB system for therapeutic applications.
- Citation: Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J Biol Chem 2014; 5(4): 409-428
- URL: https://www.wjgnet.com/1949-8454/full/v5/i4/409.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i4.409
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin (NT) family, was purified in 1982 from pig brain as a cell survival-promoting factor for sensory neurons[1] about 30 years after the discovery of nerve-growth factor (NGF)[2]. In the past three decades, two other neurotrophins, neurotrophin 3 (NT3) and neurotrophin 4 (NT4), have been identified in the mammalian brain and their function in neuronal survival, neurite outgrowth, and synaptic plasticity in the nervous system extensively investigated[3]. Each neurotrophin has a common receptor p75NTR and a specific tropomyosin-related kinase receptor (Trk); TrkA for NGF, TrkB for BDNF and NT4, TrkC for NT3. All neurotrophins are synthesized as 32 kD precursor proteins called pro-neurotrophins that are intra- and extracellularly cleaved to produce the mature neurotrophin form[4-6]. Mature neurotrophins bind to Trk receptors with high affinity while pro-neurotrophins preferentially bind to p75NTR[4-6]. Neurotrophins exert their biological function through several signaling pathways activated by Trk receptors or p75NTR after binding of neurotrophins. Binding of the BDNF dimer to TrkB induces dimerization and autophosphorylation of TrkB, resulting in sequential phosphorylation in three intracellular signaling pathways; Mitogen-activated protein kinase/extracellular signal-regulated protein kinase, phospholipase Cγ (PLCγ), and phosphatidylinositol 3-kinase (PI3K) pathways[7-10].
BDNF is the most studied and characterized neurotrophin in the central nervous system (CNS) and has received remarkable attention from clinicians because of its importance in the development and maintenance of normal brain functions. This is especially important, as growing evidence suggests a role of BDNF in the pathophysiology of brain-associated illnesses including both neurodegenerative and psychiatric diseases[11,12]. Appropriate intracellular processes including transcription from the BDNF gene, translation to protein, BDNF protein sorting to secretory vesicles, BDNF-containing vesicle transport, and BDNF secretion are essential to achieve normal BDNF functions as well as to activate the signaling pathways after TrkB phosphorylation. As decreased expression levels of BDNF in several brain regions are evident in postmortem studies of patients with the brain-related diseases[11,12], precise knowledge about BDNF gene expression is critical. Specifically, the responsible gene of Huntington’s disease, huntingtin, has been shown to directly regulate intracellular transport of BDNF[13,14]. Furthermore, we recently reported impaired BDNF secretion and TrkB signaling in a cellular model of schizophrenia[15].
In this article, we review both converting (from gene to mature protein) and spatial regulation (transport and secretion) processes of BDNF. We will also highlight recent findings suggesting implications of BDNF in the pathophysiology of the brain-related diseases.
BDNF mRNA and protein are abundantly expressed in the hippocampus, cerebral cortex, amygdala, and cerebellum in the mammalian brain[16-19]. Although BDNF is widely expressed in other tissues such as the heart, kidney, lung and testis, BDNF expression is higher in the brain than any other tissue during development[20]. It is also known that BDNF expression is dramatically increased in the rodent visual cortex with a peak at postnatal days 20-40 when visual plasticity is high[21,22].
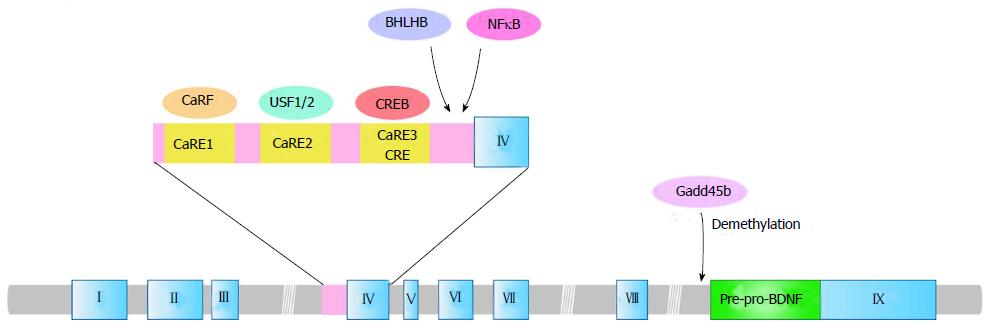
The BDNF gene consists of at least eight 5’ exons (exon I-VIII) with each respective promoter and one 3’ exon (exon IX) encoding BDNF protein in both human and rodent[23-25] (Figure 1). Pruunsild et al[25] recently identified two human-specific exons named exons Vh and VIIIh. Exon Vh has a specific promoter while exon VIIIh is not linked to an independent promoter. Transcription of the BDNF gene is initiated at each of 5’ noncoding exons that are spliced onto the pre-proBDNF protein-coding 3’exon IX[24,25]. Rodent exon I and human exons I, VII and VIII of the BDNF gene contain the ATG sequence from which translation could be started, which could produce the distinct pre-proBDNF proteins with longer amino acid sequences at the N-terminal end of the protein[24,25]. Other exons are untranslated exons, and translation of the transcripts (mRNAs) containing these exons only starts from the ATG sequence located at the exon IX[25]. Moreover, transcription of the BDNF gene terminates at two alternative polyadenylation sites in exon IX, giving rise to two distinct populations of mRNA with either short (approximately 0.35 kb) or long (approximately 2.85 kb) 3’ untranslated regions (3’ UTRs)[26,27]. To produce an identical pre-proBDNF protein, the evolutionary change in BDNF gene structure has remained a complex transcription mechanism that results in the expression of multiple BDNF mRNA variants. The diversity of BDNF mRNA leads to different neuronal distribution. The short 3’ UTR BDNF mRNA variant is restricted to the cell body in hippocampal neurons while the long 3’ UTR mRNAs are also observed in dendrites[27], indicating a specific dendritic transport system for the long 3’ UTR BDNF mRNA and local dendritic translation.
Using multiple promoters in BDNF transcription allows for appropriate responses to intracellular processes and varying extracellular environments. Membrane depolarization is a well-known neuronal gene expression regulator, and more than 300 gene transcriptions have been identified as activity-dependent in neurons[28]. The transcription of BDNF in neurons is positively regulated by membrane depolarization induced by seizures[29], sensory stimuli[30-32] and activation of glutamate receptors such as N-methyl-D-aspartate (NMDA) receptors[33-35], suggesting its important function in experience-dependent modifications of neural circuits and brain development. Neuronal activity stimulates transcription initiation of the BDNF gene, which is regulated by elevation of intracellular Ca2+ concentration via NMDA receptors (NMDAR) or L-type voltage-gated calcium channels (L-VGCC)[35]. The activity- and Ca2+-dependent transcription of the BDNF gene occurs predominantly through exons I and IV. The promoter region of BDNF exon IV contains three Ca2+-response elements CaRE1, CaRE2 (also known as USF-binding element, UBE) and CaRE3 (also known as calcium response element, CRE)[36-38] (Figure 1). CaRE1 is located at 64-73 bp upstream of the transcription initiation site in exon IV. CaRE2 and CaRE3 are 43-52 bp and 29-36 bp upstream, respectively[38]. Regulation of exon IV transcription via CaRE3 has been extensively investigated. Cyclic AMP-responsive element binding protein (CREB), which is phosphorylated at multiple sites by calcium/calmodulin (CaM)-dependent protein kinases, cAMP-dependent protein kinase A (PKA) and MAPK cascades binds to CaRE3/CRE to activate the promoter[37-39]. Knock-in mice with a subtle mutation at the CREB binding site in endogenous CaRE3/CRE showed a disrupted sensory experience-dependent increase of the BDNF exon IV transcript in the cortex[40]. Greenberg and colleagues identified novel transcription regulators for BDNF exon IV such as CaRF (calcium responsive factor) and USFs (upstream stimulatory factors) for CaRE1 and CaRE2, respectively[36,41] (Figure 1). CaRF knockout mice showed reduced exon IV mRNA levels in the cerebral cortex with normal levels in the hippocampus and striatum, suggesting brain region-specific regulation of CaRF-CaRE1 at the BDNF promoter IV[42]. Recently, Zheng et al[37] revealed intriguing Ca2+-dependent activation mechanisms of each CaRE, using cultured cortical neurons at DIV 11. Ca2+ entry through NMDAR or L-VGCC stimulated only CaRE1 and CaRE3 but not CaRE2, and CaRE1 and CaRE3 were activated via different sets of protein kinases depending on the route of calcium entry[37]. For example, CaRF is essential only for the L-VGCC-induced, not the NMDAR-induced, transcription of BDNF exon IV[37,38], indicating that other transcriptional regulators stimulate the NMDAR-dependent activation of CaRE1. Furthermore, the L-VGCC-dependent CaRE1 activation depends on protein kinase A (PKA), calcium/calmodulin-dependent protein kinase I (CaMKI), and CaMKIV, while CaRF activation requires MEK, PI3K and CaMKII[37,38]. On the other hand, CaRE3 activity is regulated by Ca2+ influx through both L-VGCC and NMDAR. MEK, PI3K, and PKA activity are required in both the L-VGCC - and NMDA-induced CaRE3 activation[37,38]. CaM and CaMKIV are needed in the L-VGCC-induced CaRE3 activation while CaMKI and CaMKIV are required in the NMDAR-mediated CaRE3 activation[37,38]. Although CaREs play a central role in the regulation of BDNF promoter IV, there are also other possible regulators for the promoter including basic helix-loop-helix B2 (BHLHB2) and nuclear factor-κB (NF-κB)[43,44] (Figure 1). Furthermore, neuronal activity stimulates BDNF promoter I as well as promoter IV. Several transcription factor binding sites such as CREB, USFs, myocyte enhancer factor 2D, and NF-κB have been suggested to stimulate BDNF promoter I[45-47], though the regulatory mechanisms have not been elucidated. Futhermore, Timmusk and colleagues have identified a cis-element PasRE (bHLH-PAS transcription factor response element) in both promoter I and IV. They also demonstrated that transcription factors ARNT2 (aryl hydrocarbon receptor nuclear translocator 2, a basic helix-loop-helix (bHLH)-PAS transcription factor) and NPAS4 (neuronal PAS domain protein 4) bind to PasRE (bHLH-PAS transcription factor response element) in both promoters, which is required for full induction of neuronal activity-dependent transcription from each exon[48,49].
Epigenetic regulations in transcription of the BDNF gene have also been reported. Decreased methylation of cytosine residues in CpG dinucleotides (CpG) of BDNF promoter IV, at least in part, mediates neuronal activity-dependent induction of BDNF transcription. Methyl-CpG-binding protein 2 (MeCP2), a member of the methyl-CpG-binding protein family, binds to methylated DNA in the BDNF promoter IV region and functions as a transcriptional regulator[50]. It is still controversial whether MeCP2 functions as an activator or a repressor for BDNF transcription. Because MeCP2 protein is released from the BDNF promoter IV region in response to neuronal activity-induced reduction of CpG methylation, it had been recognized as a transcriptional repressor for the BDNF gene[50]. However, both BDNF mRNA and protein levels were decreased in MeCP2-null mice[51], suggesting a possible repressive function of MeCP2 on BDNF expression. Furthermore, a combination mechanism of MeCP2 and CREB in BDNF transcription[52], and an additional function of MeCP2 other than transcriptional regulation, has also been indicated[3]. Recent findings demonstrated a novel epigenetic regulation in BDNF exon IX. Gadd45b, a neural immediate early gene, promotes activity-dependent demethylation in the BDNF exon IX promoter region[53] (Figure 1). Considering that electric stimulation-induced BDNF transcription was dramatically reduced in Gadd45b knock-out mice and that Gadd45b interacts and demethylates the promoter region of BDNF exon IX encoding pre-pro-BDNF[53], a gating function of Gadd45b in neuronal activity-dependent BDNF gene transcription was indicated.
The secretion process of neurotrophins involves either the constitutive or regulated pathway, depending on whether secretion occurs spontaneously or in response to neuronal activity, respectively. Unlike other neurotrophins including NGF and NT-3 that are mainly secreted by the constitutive pathway, BDNF seems to be preferentially sorted into the regulated pathway[54-56]. As described above, the 32 kDa precursor form of BDNF (pre-pro-BDNF) is translated at the rough endoplasmic reticulum (ER) and then proteolytically cleaved to generate the 13 kDa mature form of BDNF. The pre-pro-BDNF is conveyed to the Golgi apparatus and sorted into the membrane stacks of the trans-Golgi network (TGN) where BDNF-containing dense core vesicles (DCVs) bud off. The pro-region of BDNF is implicated in the sorting step of BDNF into secretory vesicles[57,58]. A single nucleotide polymorphism (SNP) at nucleotide 196 in the pro-region of the human BDNF gene that produces an amino acid substitution from valine to methionine (val66met) has a negative effect on the sorting of BDNF into vesicles[57]. The BDNF pro-region also binds to the lipid-raft-associated sorting receptor carboxypeptidase E (CPE) in the TGN, which is an important step for sorting into secretory vesicles[58]. Furthermore, a trans-membrane protein sortilin which mainly resides in the membrane of the Golgi apparatus interacts with the pro-region of BDNF and regulates its sorting. BDNF vesicular sorting was impaired in pheochromocytoma PC12 cells that express the luminal domain sortilin lacking the transmembrane and cytoplasmic domains[59].
BDNF-containing vesicles are transported to secretion sites by motor protein complexes in polarized neurons, although the subcellular localization of BDNF secretion sites in neurons of the adult CNS is still controversial. Transport and secretion of BDNF-containing vesicles into axons and dendrites have been observed in cultured cortical and hippocampal neurons by visualization using exogenously transfected fluorescent protein-tagged BDNF[56,60,61], while knock-in mice expressing Myc-tagged BDNF showed specific localization of BDNF-containing vesicles at presynaptic terminals in the adult (8-wk-old) hippocampus[62]. Intriguingly, the Golgi apparatus present in dendrites was revealed in cultured hippocampal neurons and adult rat hippocampus, with fluorescent protein-tagged BDNF localized to the dendritic Golgi, indicating a local BDNF secretory pathway as well as local translation in dendrites[63,64]. Although BDNF transport properties are still not fully elucidated, bidirectional (anterograde and retrograde) trafficking of BDNF-containing vesicles in axons and dendrites have been reported in cultured neurons[56,65,66]. Furthermore, the anterograde BDNF vesicle transport is microtubule-based with a motor protein kinesin and a coordinator dynactin[13,66]. Several important studies revealed that huntingtin, a polyglutamine-containing protein associated with Huntington disease (HD), and CPE are also involved in the motor protein complex for BDNF-containing vesicle transport and affect properties such as velocity and direction[13,14,65,66].
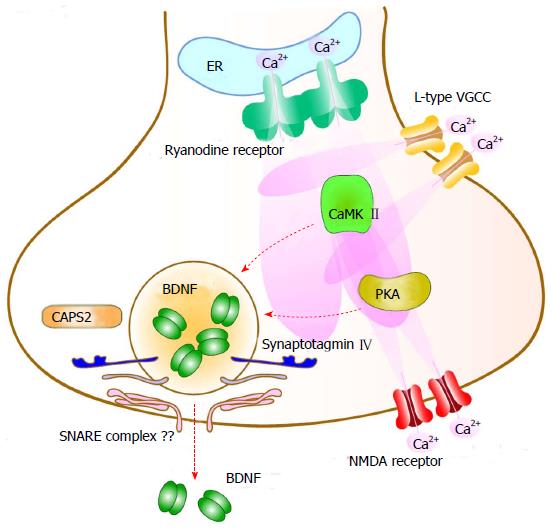
Evidence suggests that BDNF can be secreted from the cell soma, axon and dendrites of cultured cortical and hippocampal neurons in a neuronal activity (depolarization)-dependent manner[67,68] (Figure 2). Lessmann et al[68] clearly showed that activity-dependent BDNF secretion requires Ca2+ influx via ionotropic glutamate receptors or L-type VGCC and subsequent amplification of the initial Ca2+ elevation through Ca2+-induced Ca2+ release from internal stores (the endoplasmic reticulum) via ryanodine receptors. Activation of Trk receptors or Na+ channels, on the other hand, is not directly associated with BDNF secretion[68-70]. They further revealed that activation of both CaMKII and PKA were also required for secretion[67,70] (Figure 2). Considering the shared signaling pathways between the activity-dependent secretion and transcription of BDNF, decreased BDNF levels after secretion may be restored immediately with simultaneous induction of BDNF transcription. Although molecular mechanisms underlying the vesicle fusion step in BDNF secretion remain unclear, two proteins are suggested to regulate this step: Synaptotagmin IV and Ca2+-dependent activator protein for secretion 2 (CAPS2). Synaptotagmin IV, a soluble NSF attachment protein receptor (SNARE) complex binding protein localized to BDNF containing vesicles, negatively regulates BDNF secretion at both axons and dendrites in hippocampal neurons[71]. It was also reported that CAPS2, a DCV-associated protein, enhanced activity-dependent BDNF secretion efficiency[72,73]. However, it is still unclear whether BDNF-containing vesicles use SNARE-dependent membrane fusion similar to synaptic vesicle exocytosis.
It is a matter of continuous debate where and how pre-pro-BDNF is converted to the mature form of BDNF in the CNS. It was previously concluded that pro-neurotrophins are cleaved by proteases such as furin and pro-protein convertases (PCs) in TGN or in DCVs prior to secretion[74]. Several recent studies, however, showed that hippocampal neurons secreted a considerable amount of pro-BDNF that was subsequently processed extracellularly to mature-BDNF by plasmin or matrix metalloproteinases[6,75]. More recently, immediate intracellular conversion of pro-BDNF to mature-BDNF along with co-localization of the cleaved pro-peptide region and mature-BDNF in secretory vesicles in hippocampal neurons were confirmed[62,76]. Resolving the discrepancy of whether neurons secrete pro-BDNF is a very important issue because pro-BDNF preferentially binds to p75NTR rather than TrkB, which in turn causes cell death or synaptic depression in neurons[77,78]. Where pro-neurotrophin cleavage takes place and which proteinases participate in the process might be dependent upon the developmental stage of neurons or the location of BDNF translation (the cell soma or dendrites)[79].
Neurons exhibit several distinct features such as a polarized structure, inter-connected circuits, and electrical activity. Many studies have revealed a variety of BDNF functions associated with these features in the developing and mature brain. BDNF acts as a neurite outgrowth and elongation factor, pro-survival factor, and synaptic regulator in the CNS. BDNF promotes axon initiation through two distinct signaling pathways. BDNF induces TrkB-dependent local elevation and stabilization of cAMP/PKA activity that are essential for axon initiation in undifferentiated neurites of hippocampal neurons[80,81]. Furthermore, BDNF/TrkB-induced Akt phosphorylation reduces GSK-3 activation which in turn decreases production of the active form of collapsin response mediator protein-2 that plays a critical role in microtubule assembly during axon elongation and branching in rat hippocampal neurons[82,83]. BDNF also promotes axon elongation and branching of sensory neurons both in vitro and in vivo[83,84]. A BDNF concentration gradient produced by a BDNF-containing micropipette demonstrated an attractive turning effect on the axonal growth cone in cultured Xenopus spinal neurons[83,84]. Interestingly, application of specific inhibitors for cAMP or PKA resulted in repulsive turning in the same BDNF gradient, indicating the critical role of cAMP levels to determine the growth cone’s response between attraction and repulsion to the same BDNF gradient[83]. A comprehensive study on BDNF function in dendritic growth done by Wirth et al[85] revealed that overexpressed BDNF affected pyramidal cells by increasing length and number of apical dendritic segments in layer VI, and basal dendrites in layer V in rat cortical slice cultures. It is important that such BDNF-promoted dendritogenesis and dendrite growth were observed only in BDNF-overexpressed neurons themselves, suggesting the autocrine action of BDNF[85]. Studies using primary cultured neurons have revealed the importance of MAP kinase and PI3 kinase activation via TrkB phosphorylation to promote BDNF-dependent dendritic growth[86,87]. It is also important to note that acute elevation in BDNF concentration promotes total growth of dendrites and the number of primary dendrites while gradual BDNF elevation increases branching number[88].
BDNF also plays a role in synapse formation and stabilization[89]. TrkB knockout mice showed a significant decline in the number of hippocampal synaptic structures[90]. TrkB conditional-mutant mice in which TrkB is deleted in the cerebellum resulted in a reduction of inhibitory synapses[90]. BDNF as a regulator of synaptogenesis in vivo was also confirmed in Xenopus optic axons[91]. Using the time-lapse imaging technique with GFP-tagged synaptobrevin II as a marker for functional synapses, BDNF-induced retinal ganglion cell axon arborization and synaptogenesis were shown[91]. Furthermore, neutralization of endogenous BDNF by function-blocking antibodies for BDNF rapidly (within 2 h) dismantled pre-existing GFP-synaptobrevin clusters, suggesting its importance in synaptic stabilization[92]. Exogenously applied BDNF induced functional excitatory and inhibitory synapse formation in cultured rat hippocampal neurons[93] and increased excitatory synapse numbers in rat hippocampal slices[94]. On the other hand, there are lines of evidence showing synaptogenic function of BDNF and TrkB in inhibitory GABAergic connections in the visual cortex[95-97], cerebellum[98], organotypic slice cultures of the rat hippocampus[99] and organotypic cerebellar cultures of mice[100]. BDNF serves as an important modulator for synaptic connections in mature neuronal circuits as well as in developing neuronal circuits. BDNF modulates synaptic efficacy and synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) in the developed CNS (for reviews, see[3,101,102]). For example, acute and chronic application of exogenous BDNF potentiates synaptic efficacy by increasing glutamate transmission in rat brain slices of the hippocampus[103], visual cortex[104], and hippocampal dissociated cultures[105,106]. Modulation of NMDA receptor function[107,108] and ion channels including Nav[109], Kv1.3[110], and TRPC3 channels[111] by BDNF contributes to enhancement of excitatory synaptic efficacy. BDNF also promotes spine growth[112,113] and membrane insertion of NMDA[114] and AMPA[115] receptors at the postsynaptic cites of excitatory synapses. Contrary to its enhancing action on excitatory synapses, BDNF has been reported to modulate the efficacy of GABAergic synapse transmission both presynaptically and postsynaptically in a bi-directional manner. Presynaptic GABA transmission was suppressed by acute BDNF application in hippocampal slices[116] as well as chronic application in hippocampal cultures[117], while chronic BDNF in cultured hippocampal neurons potentiates presynaptic GABA transmission[118]. BDNF shows a bi-directional modulation also on postsynaptic GABAA receptor function. BDNF potentiated[119] and depressed[120,121] GABAergic postsynaptic currents through modulating GABAA receptor functions and regulating membrane insertion of GABAA receptors. Interestingly, BDNF decreased and increased inhibitory postsynaptic currents (IPSCs) when recorded from excitatory and inhibitory neurons, respectively[122]. Furthermore, IPSCs amplitude was increased by acute BDNF treatment in hippocampal slices obtained from postnatal day 6 rats whereas the opposite effect was observed in slices from postnatal day 14 rats[123]. These data indicate the complicated function of BDNF, especially in the modulation of basic properties of developed GABAergic synapses, depending on the region in the brain, stage of maturity, and duration of exposure to BDNF.
It has been frequently reported that synaptic BDNF is involved in LTP and LTD. LTP was first identified in the rat hippocampus as a long-lasting activity-dependent synaptic modification[124,125], and it is the leading hypothesis of the mechanism underlying experience-dependent learning and memory. Important roles of BDNF/TrkB signaling in specific high-frequency electrical stimuli (tetanus or Theta burst stimulation)-induced LTP in brain slices prepared from the hippocampus and cortex have been shown (for reviews, see[102,126]). A severe impairment in hippocampal LTP was confirmed in independent lines of BDNF knockout and heterozygous mice[127]. Interestingly, in heterozygous mice in which BDNF expression in the hippocampus is suppressed to approximately 60% of wild type levels, the same degree of impairment was found in LTP as that of knockout mice. Furthermore, supplementation of exogenous BDNF rescued this impairment, suggesting that BDNF plays a critical role in hippocampal LTP and a threshold level of BDNF is essential to assure LTP induction[128]. Of note, acute hippocampus-specific deletion of the BDNF gene in adult mice showed impaired novel object recognition and spatial learning in the Morris water maze[129]. These mice also demonstrated reduced extinction of conditioned fear in spite of normal acquisition and expression fear, suggesting a critical role for BDNF in hippocampal-dependent cognitive function and memory extinction[129]. TrkB knockout mice also exhibited impaired hippocampal LTP, illustrating the importance of TrkB in hippocampal-dependent learning involved in spatial memory tasks[130]. Mutant TrkB knock-in mice carrying a point mutation in the kinase domain of TrkB demonstrated that PLCγ-dependent activation of CREB and CaMKIV phosphorylation are critical factors for BDNF/TrkB-dependent hippocampal LTP[131]. Recently, mammalian target of rapamycin (mTOR) was also identified as a contributor to BDNF/TrkB-dependent hippocampal LTP[132]. Considering that PI3K and Akt are the upstream regulators of mTOR, the PI3K-AKT-mTOR pathway is thought to play a significant role in BDNF-dependent LTP. The PI3K-AKT pathway was also shown to enhance dendritic transport of PSD-95 to postsynaptic sites depending on BDNF/TrkB signaling[113], suggesting its involvement in persistent structural changes of spines after LTP induction. In contrast to mature BDNF, pro-BDNF mainly plays a role in LTD which is induced by low frequency stimulation (LFS). It has been reported that pro-BDNF enhanced NMDA receptor (NR2B)-dependent LTD in the hippocampus through p75NTR activation[78]. Application of pro-BDNF facilitated NR2B-mediated hippocampal LTD only in the wild type, but not in p75NTR knockout mice[78]. Interestingly, other forms of synaptic plasticity such as NMDA receptor-dependent LTP and NMDA receptor-independent LTD were intact in p75NTR knockout mice[78], suggesting a specific role of pro-BDNF in hippocampal LTD. Considering that p75NTR activation negatively affects dendrite complexity and spine density in hippocampal neurons[133], secreted pro-BDNF, which escapes processing by proteinases, modulates neurite morphology and synaptic plasticity in the opposite way as mature BDNF. Taken together, BDNF is deeply associated with neuronal connectivity through modulating the development of neural circuitry and regulating synaptic efficacy throughout the CNS. Therefore, impairment of BDNF function in the developing and mature brain is implicated in many psychiatric and neurodegenerative diseases.
A critical non-synonymous SNP in the human BDNF gene was first reported in 2003[57], involving an amino acid substitution at valine 66 to methionine (Val66Met)[57]. The BDNF Val66Met polymorphism predominantly affects the sorting process of synthesized BDNF into secretory vesicles[57], resulting in reduction in activity-dependent BDNF secretion[12,57]. Inconsistent with critical BDNF roles in hippocampal LTP, met allele carriers (BDNFmet carriers) showed impaired performance in episodic memory[57,134], verbal memory[135], and cognitive performance[136]. However, it is still controversial whether carrying the met allele causes significant reduction in human hippocampal volume despite the essential roles of BDNF in neurite development[12,137]. A mouse model of the polymorphism BDNFmet/met knock-in mice showed an approximately 15% reduction in hippocampal volume and dendritic arbor complexity[138]. Cultured neurons from BDNFmet/met knock-in mice exhibited normal levels of total BDNF protein but significantly reduced amounts of activity-dependent secretion of BDNF[138]. Interestingly, the BDNFmet/met knock-in mice showed anxiety-related behaviors that did not improve with chronic antidepressant fluoxetine treatment[138]. Detailed electrophysiological analysis of hippocampal slices of BDNFmet/met knock-in mice revealed normal basal glutamatergic transmission but reduced NMDA receptor-mediated current and NMDA receptor-dependent LTP[139]. It is clear that further investigation of BDNF transport and secretion processes are important for more detailed understanding of the neurotrophin and for the development of BDNF-based therapy for patients with brain-related diseases.
BDNF has been implicated in multiple brain-related diseases, as it plays a critical role in neuronal development, survival, and synaptic function. Although studies show involvement of BDNF in the pathophysiology of multiple brain-related illnesses such as bipolar disorder[140], Parkinson’s disease[140], stroke, epilepsy[141], eating disorders[142], and substance use[142], we review four neuropsychiatric illnesses associated with BDNF dysfunction.
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disorder with noticeable impairment of cognitive function. Patients with AD show progressive loss of synapses and neurons especially in the entorhinal cortex and hippocampus, which causes a loss of their ability to acquire new memories[11,143-145]. A wide range of evidence exists due to brain cohort differences[146], though decreased expression levels of BDNF protein and mRNA have been consistently reported in the hippocampus and cortex of individuals with AD[147-151] as well as in serum[152] (Table 1). Furthermore, firm conclusions regarding involvement of the Val66Met polymorphism on disease onset or progression of AD have not been made (for review, see[140]). On the other hand, Nagahara et al[153] recently reported that BDNF gene delivery to the entorhinal cortex ameliorated entorhinal cortical and hippocampal neuronal degeneration in a mouse model of AD. Mice in this model expressed the human amyloid precursor protein (APP) with mutations and exhibited AD-like pathology such as cortical plaques, progressive cell loss in the entorhinal cortex and cognitive decline by 6-7 mo after birth[153,154]. Direct injection of lentiviral vector constitutively expressing BDNF into the entorhinal cortex of the mice after disease onset reversed synapse loss in both the entorhinal cortex and hippocampus, and restored learning and memory deficits[153]. Such BDNF gene delivery was similarly effective to the reduced memory function in normally aged (24-mo-old) rats and primates[153]. Because the entorhinal cortex is a primary input to the hippocampus, the rescued synaptic loss in the hippocampus of these model animals could be attributed to BDNF transport from the cortical region. Other AD mouse models with amyloid-β overproduction caused by APP mutations exhibited significant reduction in cortical BDNF mRNA[155]. Hippocampal BDNF protein is reduced in the 5XFAD transgenic mice expressing five familial AD mutant forms of human APP, with memory deficits rescued by a specific TrkB agonist, 7,8-dihydroxyflavone (7,8-DHF), without affecting endogenous BDNF levels[156]. Furthermore, neural stem cell (NSC) transplantation also improved cognitive function in AD model mice via BDNF secreted by NSC[157]. BDNF-based therapy is increasingly expected to ameliorate the symptoms of AD.
| BDNF expression levels in brain diseases andanimal models | Possible involvement of BDNF in therapies |
| Alzheimer’s disease (patients) | |
| Decreased expression levels of BDNF mRNA | |
| Hippocampus: ref.[147,148] | |
| Cortex: ref.[148,149] | |
| Decreased expression levels of BDNF protein | |
| Serum: ref.[152] | |
| Hippocampus: ref.[150] | |
| Cortex: ref.[150,151]; no change ref.[247] | |
| Alzheimer’s disease (animal models) | Alzheimer’s disease (animal models) |
| Decreased expression levels of BDNF mRNA | BDNF gene delivery ameliorated age-related cognitive impairment in aged primates and rats. ref.[153] |
| Cortex: Aβ transgenic strains | 7, 8-DHF improved memory deficits in 5XFAD transgenic mouse. ref.[156] |
| (APPNLh and TgCRND8) ref.[155] | |
| Decreased expression levels of BDNF protein | |
| Hippocumpus: 5XFAD transgenic mouse ref.[156] | |
| Huntington disease (patients) | |
| Decreased expression levels of BDNF mRNA | |
| Serum: ref.[165] | |
| Cortex: ref.[162] | |
| Caudate: decreased TrkB mRNA ref.[162] | |
| Decreased expression levels of BDNF protein | |
| Cortex: ref.[163,165] | |
| Striatum: ref.[163] | |
| Cerebellum: ref.[163] | |
| Substantia nigra: ref.[163] | |
| Caudate and Putamen: ref.[164] | |
| Huntington disease (animal models) | Huntington disease (animal models) |
| Decreased expression levels of BDNF mRNA | BDNF overexpression improved motor function in R6/2 mice. ref.[167] |
| Cortex: R6/2 mice, ref.[166,167] | BDNF overexpression ameliorated YAC128 mice phenotype. ref.[168] |
| Decreased expression levels of BDNF protein | Environmental enrichment-increased BDNF improved the phenotype in R6/1 mice. ref.[169] |
| Striatum: YAC128 mice, ref.[168]; | Sertraline-increased BDNF improved the phenotype in R6/2 mice. ref.[170] |
| R6/1 mice ref.[169] | 7, 8-DHF or 4’-DMA-7,8-DHF improved motor deficits in N171-82Q mice. ref.[171] |
| Depression (patients) | Depression (patients) |
| Decreased expression levels of BDNF mRNA | Antidepressants increased BDNF in serum. ref.[191-193,248] |
| Hippocampus: ref.[185] (suicidal) | Antidepressants increased BDNF and TrkB in the cerebellum. ref.[194] |
| Cortex: ref.[185] (suicidal), | |
| decreased TrkB mRNA ref.[188] | |
| Amygdala: ref.[187] | |
| Decreased expression levels of BDNF protein | |
| Serum: ref.[189-191] | |
| Hippocampus ref.[185] (suicidal)[186] (suicidal) | |
| Cortex: ref.[185] (suicidal)[186] (suicidal) | |
| Depression (animal models) | Depression (animal models) |
| Decreased expression levels of BDNF mRNA | Antidepressants increased BDNF in the rat brain |
| Hippocampus: social defeat stress, ref.[195]; | Tricyclic antidepressants (TCA; including imipramine, desipramine), ref.[202-204] |
| restrain stress, ref.[196,197] (also TrkB); | Selective serotonin reuptake inhibitors (SSRI; fluoxetine, paroxetine, sertraline), ref.[202,204-206,251] |
| maternal deprivation, ref.[198]; | Noradrenergic and specific serotonergic antidepressants (NaSSA; mirtazapine, mianserin), ref.[202,207] |
| dexamethasone, ref.[199]; | Monoamine oxidase inhibitors (MAOIs; tranylcypromine), ref.[202-204] |
| corticosterone, ref.[196,200,201]; | |
| foot shock stress, ref.[249] | |
| Cortex: social defeat stress, ref.[195]; | Electroconvulsive seizures induced BDNF expression. ref.[202,208-211] |
| maternal deprivation, ref.[198]; | 7,8-DHF showed an antidepressant-like effect. ref.[212] |
| dexamethasone, ref.[199]; | Direct BDNF infusion into the rat midbrain induced antidepressant-like effects. ref.[213] |
| cold swim stress, ref.[250] | |
| Amygdala: social defeat stress, ref.[193] | |
| Decreased expression levels of BDNF protein | |
| Hippocampus: maternal deprivation, ref.[198]; | |
| corticosterone, ref.[200,201]; | |
| cold swim stress, ref.[250] | |
| Schizophrenia (patients) | Schizophrenia (patients) |
| Decreased expression levels of BDNF mRNA | Clozapine treatment increased serum BDNF mRNA levels. ref.[235,236] |
| Serum: ref.[236,252]; | |
| decreased TrkB mRNA ref.[253] | |
| Cortex: ref.[122,233,254,255]; | |
| decreased TrkB mRNA ref.[255]; | |
| incrased trunkated TrkB mRNA ref.[256] | |
| Serum: ref.[235,257-260] | |
| Hippocampus: ref.[231]; | |
| abnormal expression of TrkB ref.[261]; | |
| increased BDNF mRNA ref.[230] | |
| Cortex: ref.[232-234]; | |
| increase BDNF mRNA ref.[230,231] | |
| Cerebrospinal fluid ref.[234] | |
| Schizophrenia (animal models) | Schizophrenia (animal models) |
| Decreased expression levels of BDNF mRNA | Mk-801-induced decline in hippocampal BDNF mRNA was normalized by olanzapine, but exacerbated by haloperidol. ref.[238] |
| Hippoaumpus: phencyclidine, ref.[238]; | Haloperidol or risperidone decreased BDNF protein in the rat frontal cortex and hippocampus. ref.[241] |
| MK-801 ref.[239]; | Haloperidol decreased BDNF and TrkB protein in the rat hippocampus. ref.[242] |
| ibotenic acid lesions, ref.[240] | Risperidon, olanzapine, haloperidol, and chlorpromazine decreased BDNF levels in the rat striatum and hippocampus. ref.[243] |
| Cortex: phencyclidine, ref.[238] | Haloperidol down-regulated BDNF mRNA expression in the rat hippocampus. ref.[262] |
| Amygdala: phencyclidine, ref.[238] |
Huntington’s disease (HD) is a neurodegenerative and autosomal dominant disease caused by repeats of the CAG trinucleotide in the huntingtin (htt) gene, which produces abnormal htt proteins with polyglutamine expansion (polyQ). Degeneration of striatal neurons is thought to induce progressive psychiatric, cognitive and motor dysfunction[158]. Possible link between HD and BDNF function has been reported. Given that substantial levels of BDNF protein are detectable in adult rodent striatum while TrkB mRNA, but not BDNF mRNA, is present along with the fact that cortical neurons projecting to the striatum contain high levels of BDNF mRNA, most striatal BDNF is anterogradely transported from the cortex[159]. It was revealed that wild-type htt but not mutant (polyQ) htt stimulated transcription from BDNF exon II in the cerebral cortex[160,161]. Postmortem studies have revealed reduced BDNF mRNA and protein levels in the cortex[162,163], caudate-putamen[163,164], striatum[163], cerebellum[163], and substantia nigra[163] in patients with HD. Decreased serum BDNF levels in patients was also reported[165]. The mouse models of HD expressing exon 1 of the human HD gene with expanded polyQ region recapitulate many of the features of human HD such as progressive behavioral deficit, impaired motor functions, cognitive decline, and premature death[166-169]. Reduced expression of BDNF was confirmed in the cortex[166,167] and striatum[168,169] of three HD mouse models (R6/1, R6/2, and YAC128). The antidepressant sertraline increased BDNF protein levels in the hippocampus and striatum, improving lifespan and motor performance, and ameliorated brain atrophy in R6/2 HD mice[170]. Genetically overexpressed BDNF in the cerebral cortex and striatum of YAC128 HD mice alleviated loss of striatal neurons and motor dysfunction, and improved procedural learning[168]. BDNF overexpression in the striatum improved cortico-striatal connectivity and motor function in R6/2 HD mice[167]. Furthermore, 7,8-DHF and its derivative 4’-dimethylamino-7,8- dihydroxyflavone (4’-DMA-7,8-DHF) extended survival, ameliorated brain atrophy, and improved motor deficits in N171-82Q HD mice[171]. Importantly, forebrain-specific BDNF-knock-out mice (Emx-BDNF KO mice) show a similar phenotype to that observed in the mouse models of HD[172,173]. In contrast to evidence suggesting involvement of BDNF in the pathophysiology of HD, no association between the Val66Met polymorphism of the BDNF gene and onset/progression of HD has been reported[174-178].
Gauthier et al[13] have unveiled an important htt function in BDNF transport. Mutant htt reduced post-Golgi microtubule-based axonal transport velocity of BDNF-containing vesicles while wild-type htt accelerated transport velocity in cultured cortical neurons[13]. These findings suggest that mutations in htt lead to decreased amounts of BDNF in the striatum by inhibiting both BDNF transcription and axonal transport of BDNF-containing vesicles in cortical neurons. Furthermore, the direction of transport was regulated by phosphorylation of htt at serine 421[14]. Phosphorylated htt recruited motor protein kinesin-1 to BDNF-containing vesicles to facilitate anterograde transport, though wild-type htt enhances both anterograde and retrograde transport efficiency in cortical neurons. In contrast, retrograde transport was increased in the absence of htt phosphorylation at the serine 421 site[14]. Interestingly, mutant htt impairs the post-Golgi transport only in val-BDNF-containing vesicles but not in met-BDNF, which in turn produces a reduction in the amount of activity-dependent secretion of val-BDNF in cell lines[179]. Severely reduced BDNF levels in R6/1 HD mice hippocampus and striatum were rescued by environmental enrichment[169]. The enrichment, consisting of small cardboard boxes, small open wooden boxes, cylindrical cardboard tunnels, and folded sheets of paper prevented body weight loss and ameliorated motor symptoms of the mice[169]. Considering that the striatum itself does not produce BDNF and receives BDNF supply from the cortex, such enrichment may compensate by attenuating the mutant htt-induced impairment of anterograde transport of BDNF to the striatum[169,170]. Given the functional interaction between BDNF and htt, exogenous supplementation of BDNF or TrkB agonists to the striatum would be the primary choice for the treatment of HD patients.
Several lines of evidence implicate BDNF in the pathophysiology of psychiatric disorders[10-12,180]. Specifically, reduced BDNF expression levels and impaired BDNF function have been reported in patients with depression and schizophrenia[181-184]. Reduced BDNF[185-187] or TrkB[188] levels in the prefrontal cortex[185,186,188], hippocampus[185,186], amygdala[187], and serum[189-191] have been demonstrated in patients with depression, especially in suicide victims[185,186,189]. Considering that BDNF protein levels were unchanged in hippocampal and cortical tissue of suicide subjects who had been treated with antidepressants[186], increased levels of BDNF in these brain regions may be facilitated by treatment with antidepressants. Decreased serum BDNF levels have been also reported in patients with depression[189-191], which was recovered by antidepressant drug treatment[191-193]. Increased expression of BDNF or TrkB mRNA in the cerebellum[194] was also shown after chronic antidepressant treatment in subjects with depression. Decreased BDNF and TrkB expression levels are also evident in rodent models of depression. A variety of stressors on rodents such as social defeat[195], restraint (immobilization)[196,197], maternal deprivation[198], and glucocorticoid administration[196,199-201] can reduce BDNF and TrkB expression levels in the hippocampus, cortex, and amygdala. In the normal rat brain, hippocampal and cortical BDNF/TrkB levels were increased after administration of tricyclic antidepressants (TCA; including imipramine, desipramine)[202-204], selective serotonin reuptake inhibitors (SSRI; fluoxetine, paroxetine, sertraline)[202,204-206], noradrenergic and specific serotonergic antidepressants (NaSSA; mirtazapine, mianserin)[202,207], and monoamine oxidase inhibitors (MAOIs; tranylcypromine)[202-204]. Electroconvulsive seizures also significantly induced BDNF expression in the cortex[202,208] and hippocampus[202,209-211]. 7,8-DHF, a specific TrkB agonist, and its O-methylated metabolites showed an antidepressant-like effect in the forced swimming and tail suspension tests[212]. Of note, direct BDNF infusion into the rat midbrain induced antidepressant-like effects[213]. Interestingly, TrkB phosphorylation was also increased by antidepressants in the rat brain[214]. An antidepressant imipramine, however, induced TrkB phosphorylation even in the conditional BDNF knock-out mice, suggesting the antidepressant-induced TrkB activation might be BDNF-independent[215]. Despite compelling evidence of BDNF contribution to depression onset and symptoms, meta-analysis with inconsistent results from genetic association studies of the BDNF val66met polymorphism revealed no significant association between the val66met polymorphism and depression[216]. It must be noted, however, that the polymorphism may affect the development of depression more in men than in women[216].
Chronic stress-induced hypothalamic pituitary-adrenal (HPA) axis abnormalities result in increased serum levels of glucocorticoids that are implicated in the pathogenesis of depression[10,217,218]. The HPA axis is an important endocrinological coping mechanism against stressful stimuli that includes release of hormones such as corticotropin-releasing hormone (CRH) from the paraventricular nucleus (PVN), CRH-trigged adrenocorticotropic hormone (ACTH) from the anterior pituitary, and ACTH-induced glucocorticoids from the adrenal glands. We recently reported that the glucocorticoid receptor (GR), which has both genomic and non-genomic properties in cortical neurons, requires TrkB interaction for full activation of the BDNF-induced PLCγ pathway[219]. Chronic treatment with glucocorticoids decreased GR protein levels, and inhibited TrkB-GR interaction and resultant BDNF-induced glutamate release via the PLCγ pathway[219]. On the other hand, Jeanneteau et al[220] showed TrkB activation by acute administration of glucocorticoids in cortical neurons. The activation of TrkB by glucocorticoids promoted neuronal survival through the transcriptional function of GR and Akt signaling pathway activation[220]. They also revealed that BDNF/TrkB signaling regulates GR transcriptional activity by stimulating GR phosphorylation at S155 and S287[221,222]. These results suggest a possible involvement of the impaired reciprocal interaction between BDNF and the HPA axis in the pathogenesis of depressive disorders. Taken together, BDNF-based therapy for depressed patients could target GR function or the direct activation of TrkB[12].
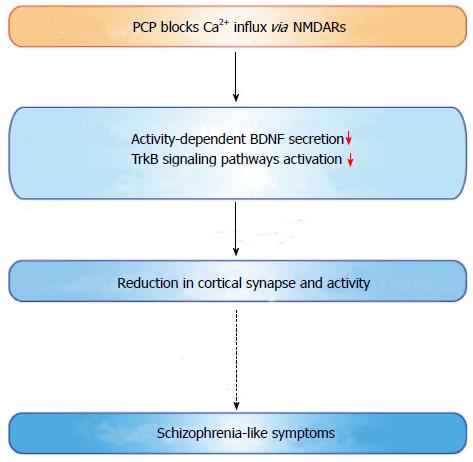
Approximately 1% of the world population is affected by schizophrenia. This psychiatric condition manifests in three major ways: Positive symptoms (hallucinations, delusions, thought and movement disorders), negative symptoms (flat affect, lack of pleasure and ability to begin and sustain planned activities), and cognitive symptoms (impaired ability to understand and use information, and problems with “working memory”)[223]. Several brain regions have been implicated in the pathophysiology of this illness. Studies on postmortem brain tissue of schizophrenia patients demonstrates that the overall number of neurons in the prefrontal cortex is not decreased[224]. However, reduced synaptophysin (presynaptic protein) immunoreactivity and dendritic spine density of pyramidal cells were observed in the cortex[225,226], suggesting synaptic dysfunction in the pathogenesis of the disease. Because symptoms usually start between ages 16 and 30 with only rare cases reported after age 45[227], deficits in synaptic maturation and overall brain development may be heavily involved in disease onset. Expression levels of BDNF or TrkB have been investigated in brains of patients with schizophrenia. Whether or not BDNF levels are altered in brain tissue or serum of patients with schizophrenia is a controversial topic[184,228,229]. Some postmortem studies demonstrated elevated BDNF protein levels in the hippocampal and cortical tissue in schizophrenic patients[230,231], while decreased BDNF levels have also been shown in these brain regions[231-234] (Table 1). Reduced BDNF mRNA and protein levels in serum of patients with schizophrenia have been consistently reported (Table 1). There are some reports showing that clozapine treatment increased serum BDNF mRNA levels in patients[235,236]. A meta-analysis examining blood BDNF levels in schizophrenia found a moderate effect of reduced BDNF in patents with schizophrenia compared with controls[237]. Furthermore, val/met and met/met BDNF genotypes were reported in a meta-analysis to increase the risk of schizophrenia[142]. Decreased BDNF mRNA and protein levels have also been confirmed in animal models of schizophrenia induced by phencyclidine (PCP)[238], MK-801[239] or ibotenic acid[240]. In contrast to human patients, antipsychotic administration in rodents significantly reduces BDNF expression levels (Table 1). Second-generation antipsychotics such as risperidone and olanzapine, in addition to first-generation antipsychotics such as haloperidol and chlorpromazine, all tend to reduce BDNF protein levels in the rat cortex, hippocampus, and striatum[241-243] (Table 1). Although it is challenging to approach the molecular mechanisms underlying the pathophysiology of schizophrenia, PCP, a psychomimetic drug, is known to produce beneficial animal and cellular models for schizophrenia. PCP acts as a non-competitive NMDA receptor blocker and generates schizophrenia-like behavioral changes in humans and rodents[244,245]. Interestingly, sub-chronic PCP (2 mg/kg, i.p. twice daily for 7 d followed by 6 wk drug-free) administration decreased BDNF mRNA levels in many brain regions including the cortical, hippocampal, and amygdaloid regions in adult rats[238], while higher doses of PCP (10 mg/kg, i.p. for 14 d) increased BDNF levels in the hippocampus and entorhinal cortex of rat pups[246]. We recently demonstrated that PCP suppressed the activity-dependent secretion of BDNF in cultured cortical neurons through blockade of Ca2+ influx via NMDA receptors[15] (Figure 3). Decreased BDNF secretion subsequently caused a reduction in the activation of TrkB-dependent signaling pathways and resultant synaptic loss in cortical neurons[15], which is consistent with the observation that patients demonstrate reduced cortical synaptic structure[225-227]. Because of the multifactorial etiology of schizophrenia, whether the BDNF-based approach would be effective or not is unclear in this disease. Ultimately, the multiple, essential roles of BDNF in synaptic function, however, could influence future therapy for these patients.
Many efforts are being dedicated to understanding the intracellular processes of BDNF to develop BDNF-based effective treatments for the brain-related diseases. Although brain diseases are associated with an overall decrease in BDNF function, varying BDNF levels in specific brain regions may affect symptom attenuation. As BDNF does not cross the blood-brain barrier, researchers are challenged to develop BDNF gene or protein delivery methods to the CNS[11]. Molecules that increase BDNF or TrkB expression levels, TrkB phosphorylation or membrane insertion, or conversion from pro-BDNF to mature BDNF are important and novel treatment options[12]. Even if BDNF itself is not the main gene responsible for the particular brain disease, BDNF-based treatments may provide valuable symptom relief. It is also critical to elucidate the basic molecular mechanisms of BDNF expression, transport, and secretion so that new therapeutic approaches may be developed to treat these debilitating neuropsychiatric conditions.
| 1. | Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549-553. [PubMed] |
| 2. | Cohen S, Levi-Montalcini R, Hamburger V. A nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proc Natl Acad Sci USA. 1954;40:1014-1018. [PubMed] |
| 3. | Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1547] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 4. | Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735-738. [PubMed] |
| 5. | Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299-309. [PubMed] |
| 6. | Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603-614. [PubMed] |
| 7. | Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1840] [Cited by in RCA: 1978] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 8. | Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850-860. [PubMed] |
| 9. | Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56 Suppl 1:73-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H. Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience. 2013;239:157-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 683] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 12. | Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 594] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 13. | Gauthier LR, Charrin BC, Borrell-Pagès M, Dompierre JP, Rangone H, Cordelières FP, De Mey J, MacDonald ME, Lessmann V, Humbert S. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127-138. [PubMed] |
| 14. | Colin E, Zala D, Liot G, Rangone H, Borrell-Pagès M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Adachi N, Numakawa T, Kumamaru E, Itami C, Chiba S, Iijima Y, Richards M, Katoh-Semba R, Kunugi H. Phencyclidine-induced decrease of synaptic connectivity via inhibition of BDNF secretion in cultured cortical neurons. Cereb Cortex. 2013;23:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511-526. [PubMed] |
| 17. | Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459-2464. [PubMed] |
| 18. | Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295-2313. [PubMed] |
| 19. | Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431-448. [PubMed] |
| 20. | Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 228] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Gorba T, Klostermann O, Wahle P. Development of neuronal activity and activity-dependent expression of brain-derived neurotrophic factor mRNA in organotypic cultures of rat visual cortex. Cereb Cortex. 1999;9:864-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Patz S, Wahle P. Developmental changes of neurotrophin mRNA expression in the layers of rat visual cortex. Eur J Neurosci. 2006;24:2453-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1-12. [PubMed] |
| 24. | Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 782] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 25. | Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 550] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 26. | Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475-489. [PubMed] |
| 27. | An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B. Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 536] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 28. | Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 513] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 29. | Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937-948. [PubMed] |
| 30. | Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444-9448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 415] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Bozzi Y, Pizzorusso T, Cremisi F, Rossi FM, Barsacchi G, Maffei L. Monocular deprivation decreases the expression of messenger RNA for brain-derived neurotrophic factor in the rat visual cortex. Neuroscience. 1995;69:1133-1144. [PubMed] |
| 32. | Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411-4419. [PubMed] |
| 33. | Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545-3550. [PubMed] |
| 34. | Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA. 1991;88:10037-10041. [PubMed] |
| 35. | Zafra F, Lindholm D, Castrén E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793-4799. [PubMed] |
| 36. | Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383-395. [PubMed] |
| 37. | Zheng F, Zhou X, Luo Y, Xiao H, Wayman G, Wang H. Regulation of brain-derived neurotrophic factor exon IV transcription through calcium responsive elements in cortical neurons. PLoS One. 2011;6:e28441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Zheng F, Zhou X, Moon C, Wang H. Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol. 2012;4:188-200. [PubMed] |
| 39. | Koppel I, Timmusk T. Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology. 2013;75:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 41. | Chen WG, West AE, Tao X, Corfas G, Szentirmay MN, Sawadogo M, Vinson C, Greenberg ME. Upstream stimulatory factors are mediators of Ca2+-responsive transcription in neurons. J Neurosci. 2003;23:2572-2581. [PubMed] |
| 42. | McDowell KA, Hutchinson AN, Wong-Goodrich SJ, Presby MM, Su D, Rodriguiz RM, Law KC, Williams CL, Wetsel WC, West AE. Reduced cortical BDNF expression and aberrant memory in Carf knock-out mice. J Neurosci. 2010;30:7453-7465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Jiang X, Tian F, Du Y, Copeland NG, Jenkins NA, Tessarollo L, Wu X, Pan H, Hu XZ, Xu K. BHLHB2 controls Bdnf promoter 4 activity and neuronal excitability. J Neurosci. 2008;28:1118-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Kairisalo M, Korhonen L, Sepp M, Pruunsild P, Kukkonen JP, Kivinen J, Timmusk T, Blomgren K, Lindholm D. NF-kappaB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur J Neurosci. 2009;30:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. 2002;277:35920-35931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Lubin FD, Ren Y, Xu X, Anderson AE. Nuclear factor-kappa B regulates seizure threshold and gene transcription following convulsant stimulation. J Neurochem. 2007;103:1381-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60:1022-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (7)] |
| 48. | Koppel I, Aid-Pavlidis T, Jaanson K, Sepp M, Pruunsild P, Palm K, Timmusk T. Tissue-specific and neural activity-regulated expression of human BDNF gene in BAC transgenic mice. BMC Neurosci. 2009;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Pruunsild P, Sepp M, Orav E, Koppel I, Timmusk T. Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J Neurosci. 2011;31:3295-3308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 50. | Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 955] [Article Influence: 41.5] [Reference Citation Analysis (6)] |
| 51. | Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341-348. [PubMed] |
| 52. | Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1494] [Cited by in RCA: 1413] [Article Influence: 78.5] [Reference Citation Analysis (9)] |
| 53. | Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 777] [Cited by in RCA: 722] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 54. | Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222-238. [PubMed] |
| 55. | Farhadi HF, Mowla SJ, Petrecca K, Morris SJ, Seidah NG, Murphy RA. Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J Neurosci. 2000;20:4059-4068. [PubMed] |
| 56. | Adachi N, Kohara K, Tsumoto T. Difference in trafficking of brain-derived neurotrophic factor between axons and dendrites of cortical neurons, revealed by live-cell imaging. BMC Neurosci. 2005;6:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257-269. [PubMed] |
| 58. | Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245-255. [PubMed] |
| 59. | Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156-6166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 60. | Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185-14198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJ, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 62. | Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 276] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 63. | Horton AC, Rácz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757-771. [PubMed] |
| 64. | Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23:6188-6199. [PubMed] |
| 65. | Park JJ, Cawley NX, Loh YP. A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol Cell Neurosci. 2008;39:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Kwinter DM, Lo K, Mafi P, Silverman MA. Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience. 2009;162:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Edelmann E, Lessmann V, Brigadski T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology. 2014;76 Pt C:610-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 68. | Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res. 2009;65:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 69. | Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887-5897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 407] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 70. | Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350-10364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci. 2009;12:767-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 72. | Sadakata T, Furuichi T. Developmentally regulated Ca2+-dependent activator protein for secretion 2 (CAPS2) is involved in BDNF secretion and is associated with autism susceptibility. Cerebellum. 2009;8:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Shinoda Y, Sadakata T, Nakao K, Katoh-Semba R, Kinameri E, Furuya A, Yanagawa Y, Hirase H, Furuichi T. Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc Natl Acad Sci USA. 2011;108:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chrétien M, Murphy RA. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951-960. [PubMed] |
| 75. | Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 879] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 76. | Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 278] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 77. | Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1258] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 78. | Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 628] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 79. | Barker PA. Whither proBDNF? Nat Neurosci. 2009;12:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Shelly M, Cancedda L, Lim BK, Popescu AT, Cheng PL, Gao H, Poo MM. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011;71:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 81. | Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci USA. 2011;108:18430-18435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 82. | Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 735] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 83. | Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275-279. [PubMed] |
| 84. | Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 464] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 85. | Wirth MJ, Brun A, Grabert J, Patz S, Wahle P. Accelerated dendritic development of rat cortical pyramidal cells and interneurons after biolistic transfection with BDNF and NT4/5. Development. 2003;130:5827-5838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Miller FD, Kaplan DR. Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol. 2003;13:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 87. | Dijkhuizen PA, Ghosh A. BDNF regulates primary dendrite formation in cortical neurons via the PI3-kinase and MAP kinase signaling pathways. J Neurobiol. 2005;62:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 88. | Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 89. | Vicario-Abejón C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci. 2002;3:965-974. [PubMed] |
| 90. | Martínez A, Alcántara S, Borrell V, Del Río JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336-7350. [PubMed] |
| 91. | Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093-1101. [PubMed] |
| 92. | Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285-4298. [PubMed] |
| 93. | Vicario-Abejón C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256-7271. [PubMed] |
| 94. | Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249-4258. [PubMed] |
| 95. | Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 933] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 96. | Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234-7244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Abidin I, Eysel UT, Lessmann V, Mittmann T. Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. J Physiol. 2008;586:1885-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Marty S, Wehrlé R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087-8095. [PubMed] |
| 100. | Seil FJ, Drake-Baumann R. TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis. J Neurosci. 2000;20:5367-5373. [PubMed] |
| 101. | Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 603] [Article Influence: 33.5] [Reference Citation Analysis (10)] |
| 102. | Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 103. | Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 104. | Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol. 1997;498:153-164. [PubMed] |
| 105. | Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport. 1994;6:21-25. [PubMed] |
| 106. | Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074-8077. [PubMed] |
| 107. | Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci USA. 1997;94:8191-8195. [PubMed] |
| 108. | Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 229] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Lesser SS, Sherwood NT, Lo DC. Neurotrophins differentially regulate voltage-gated ion channels. Mol Cell Neurosci. 1997;10:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J Physiol. 2002;542:413-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 280] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 112. | Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 113. | Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 114. | Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 115. | Itami C, Kimura F, Kohno T, Matsuoka M, Ichikawa M, Tsumoto T, Nakamura S. Brain-derived neurotrophic factor-dependent unmasking of “silent” synapses in the developing mouse barrel cortex. Proc Natl Acad Sci USA. 2003;100:13069-13074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 116. | Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383-3386. [PubMed] |
| 117. | Bolton MM, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221-3232. [PubMed] |
| 118. | Baldelli P, Novara M, Carabelli V, Hernández-Guijo JM, Carbone E. BDNF up-regulates evoked GABAergic transmission in developing hippocampus by potentiating presynaptic N- and P/Q-type Ca2+ channels signalling. Eur J Neurosci. 2002;16:2297-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 120. | Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959-2966. [PubMed] |
| 121. | Brünig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 122. | Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722-8732. [PubMed] |
| 123. | Mizoguchi Y, Ishibashi H, Nabekura J. The action of BDNF on GABA(A) currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J Physiol. 2003;548:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 124. | Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331-356. [PubMed] |
| 125. | Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2625] [Cited by in RCA: 2890] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 126. | Panja D, Bramham CR. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology. 2014;76 Pt C:664-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 127. | Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 993] [Article Influence: 33.1] [Reference Citation Analysis (6)] |
| 128. | Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972-4983. [PubMed] |
| 129. | Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 546] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 130. | Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 637] [Article Influence: 23.6] [Reference Citation Analysis (9)] |
| 131. | Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 132. | Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA. 2002;99:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 599] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 133. | Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989-9999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 134. | Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, Collier DA. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 135. | Ho BC, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 136. | Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, Pendleton N, Horan M, Payton A. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 137. | Molendijk ML, Bus BA, Spinhoven P, Kaimatzoglou A, Oude Voshaar RC, Penninx BW, van IJzendoorn MH, Elzinga BM. A systematic review and meta-analysis on the association between BDNF val(66)met and hippocampal volume--a genuine effect or a winners curse? Am J Med Genet B Neuropsychiatr Genet. 2012;159B:731-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 138. | Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1059] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 139. | Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. 2010;30:8866-8870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 140. | Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 734] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 141. | Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. BDNF genotype potentially modifying the association between incident stroke and depression. Neurobiol Aging. 2008;29:789-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 142. | Gratacòs M, González JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. 2007;61:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 143. | Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3037] [Cited by in RCA: 3346] [Article Influence: 139.4] [Reference Citation Analysis (9)] |
| 144. | Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239-259. [PubMed] |
| 145. | Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci Lett. 1994;174:67-72. [PubMed] |
| 146. | Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 147. | Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695-702. [PubMed] |
| 148. | Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res. 1997;49:71-81. [PubMed] |
| 149. | Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res. 2000;76:347-354. [PubMed] |
| 150. | Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 420] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 151. | Narisawa-Saito M, Wakabayashi K, Tsuji S, Takahashi H, Nawa H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport. 1996;7:2925-2928. [PubMed] |
| 152. | Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E, Koehler N, Bartels M. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 153. | Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 792] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 154. | Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050-4058. [PubMed] |
| 155. | Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, Francis BM, Mount HT, Mufson EJ, Salehi A, Fahnestock M. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2009;29:9321-9329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 156. | Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 157. | Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594-13599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 688] [Cited by in RCA: 654] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 158. | Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA. 1988;85:5733-5737. [PubMed] |
| 159. | Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856-860. [PubMed] |
| 160. | Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76-83. [PubMed] |
| 161. | Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294-330. [PubMed] |
| 162. | Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18:225-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 163. | Seo H, Sonntag KC, Isacson O. Generalized brain and skin proteasome inhibition in Huntington’s disease. Ann Neurol. 2004;56:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 164. | Ferrer I, Goutan E, Marín C, Rey MJ, Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 256] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 165. | Ciammola A, Sassone J, Cannella M, Calza S, Poletti B, Frati L, Squitieri F, Silani V. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 166. | Zuccato C, Liber D, Ramos C, Tarditi A, Rigamonti D, Tartari M, Valenza M, Cattaneo E. Progressive loss of BDNF in a mouse model of Huntington’s disease and rescue by BDNF delivery. Pharmacol Res. 2005;52:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 167. | Giralt A, Carretón O, Lao-Peregrin C, Martín ED, Alberch J. Conditional BDNF release under pathological conditions improves Huntington’s disease pathology by delaying neuronal dysfunction. Mol Neurodegener. 2011;6:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 168. | Xie Y, Hayden MR, Xu B. BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci. 2010;30:14708-14718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 169. | Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, Hannan AJ. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 170. | Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA, Duan W. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington’s disease mouse model. Exp Neurol. 2008;210:154-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 171. | Jiang M, Peng Q, Liu X, Jin J, Hou Z, Zhang J, Mori S, Ross CA, Ye K, Duan W. Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntington’s disease. Hum Mol Genet. 2013;22:2462-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 172. | Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250-4258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 319] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 173. | Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, Beal MF, Jones L, Kooperberg C, Olson JM. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27:11758-11768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 174. | Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, Schulze TG, Deschner M, Schmäl C, Höfels S. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. 2005;58:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 175. | Kishikawa S, Li JL, Gillis T, Hakky MM, Warby S, Hayden M, MacDonald ME, Myers RH, Gusella JF. Brain-derived neurotrophic factor does not influence age at neurologic onset of Huntington’s disease. Neurobiol Dis. 2006;24:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 176. | Mai M, Akkad AD, Wieczorek S, Saft C, Andrich J, Kraus PH, Epplen JT, Arning L. No association between polymorphisms in the BDNF gene and age at onset in Huntington disease. BMC Med Genet. 2006;7:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 177. | Metzger S, Bauer P, Tomiuk J, Laccone F, Didonato S, Gellera C, Mariotti C, Lange HW, Weirich-Schwaiger H, Wenning GK. Genetic analysis of candidate genes modifying the age-at-onset in Huntington’s disease. Hum Genet. 2006;120:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 178. | Alberch J, López M, Badenas C, Carrasco JL, Milà M, Muñoz E, Canals JM. Association between BDNF Val66Met polymorphism and age at onset in Huntington disease. Neurology. 2005;65:964-965. [PubMed] |
| 179. | del Toro D, Canals JM, Ginés S, Kojima M, Egea G, Alberch J. Mutant huntingtin impairs the post-Golgi trafficking of brain-derived neurotrophic factor but not its Val66Met polymorphism. J Neurosci. 2006;26:12748-12757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 180. | Numakawa T, Yokomaku D, Richards M, Hori H, Adachi N, Kunugi H. Functional interactions between steroid hormones and neurotrophin BDNF. World J Biol Chem. 2010;1:133-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 181. | Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2529] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 182. | Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7:231-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 183. | Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 184. | Favalli G, Li J, Belmonte-de-Abreu P, Wong AH, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 185. | Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 638] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 186. | Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29-37. [PubMed] |
| 187. | Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 188. | Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 189. | Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, Lee SW, Yoon D, Han C, Kim DJ. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 190. | Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143-148. [PubMed] |
| 191. | Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70-75. [PubMed] |
| 192. | Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 928] [Cited by in RCA: 911] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 193. | Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260-265. [PubMed] |
| 194. | Bayer TA, Schramm M, Feldmann N, Knable MB, Falkai P. Antidepressant drug exposure is associated with mRNA levels of tyrosine receptor kinase B in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 195. | Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 196. | Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768-1777. [PubMed] |
| 197. | Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Toné S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997;28:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 198. | Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 199. | Barbany G, Persson H. Regulation of Neurotrophin mRNA Expression in the Rat Brain by Glucocorticoids. Eur J Neurosci. 1992;4:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 200. | Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 279] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 201. | Jacobsen JP, Mørk A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006;1110:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 202. | Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539-7547. [PubMed] |
| 203. | Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 281] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 204. | Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 205. | Coppell AL, Pei Q, Zetterström TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 206. | De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, Murray TK, Gaillard JP, Deville C, Xhenseval V. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 207. | Rogóz Z, Skuza G, Legutko B. Repeated treatment with mirtazepine induces brain-derived neurotrophic factor gene expression in rats. J Physiol Pharmacol. 2005;56:661-671. [PubMed] |
| 208. | Angelucci F, Aloe L, Jiménez-Vasquez P, Mathé AA. Electroconvulsive stimuli alter the regional concentrations of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in adult rat brain. J ECT. 2002;18:138-143. [PubMed] |
| 209. | Smith MA, Zhang LX, Lyons WE, Mamounas LA. Anterograde transport of endogenous brain-derived neurotrophic factor in hippocampal mossy fibers. Neuroreport. 1997;8:1829-1834. [PubMed] |
| 210. | Altar CA, Whitehead RE, Chen R, Wörtwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703-709. [PubMed] |
| 211. | Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, Bukhman YV, Young TA, Charles V, Palfreyman MG. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24:2667-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 250] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 212. | Liu X, Qi Q, Xiao G, Li J, Luo HR, Ye K. O-methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity. Pharmacology. 2013;91:185-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 213. | Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav. 1997;56:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 633] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 214. | Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349-357. [PubMed] |
| 215. | Rantamäki T, Vesa L, Antila H, Di Lieto A, Tammela P, Schmitt A, Lesch KP, Rios M, Castrén E. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS One. 2011;6:e20567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 216. | Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vásquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 217. | de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 3264] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 218. | Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1420] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 219. | Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci USA. 2009;106:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 220. | Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci USA. 2008;105:4862-4867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 221. | Lambert WM, Xu CF, Neubert TA, Chao MV, Garabedian MJ, Jeanneteau FD. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol Cell Biol. 2013;33:3700-3714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 222. | Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2013;239:173-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 223. | Available from: http://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml. |
| 224. | Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 725] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 225. | Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 226. | Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1202] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 227. | Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609-620, 544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 228. | Vasic N, Connemann BJ, Wolf RC, Tumani H, Brettschneider J. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262:375-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 229. | Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 230. | Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K, Koizumi S, Wakabayashi K, Takahashi H, Someya T. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry. 2000;5:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 231. | Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P, Thome J. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52:79-86. [PubMed] |
| 232. | Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 421] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 233. | Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 234. | Issa G, Wilson C, Terry AV, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis. 2010;39:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 235. | Grillo RW, Ottoni GL, Leke R, Souza DO, Portela LV, Lara DR. Reduced serum BDNF levels in schizophrenic patients on clozapine or typical antipsychotics. J Psychiatr Res. 2007;41:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 236. | Lee AH, Lange C, Ricken R, Hellweg R, Lang UE. Reduced brain-derived neurotrophic factor serum concentrations in acute schizophrenic patients increase during antipsychotic treatment. J Clin Psychopharmacol. 2011;31:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 237. | Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 361] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 238. | Snigdha S, Neill JC, McLean SL, Shemar GK, Cruise L, Shahid M, Henry B. Phencyclidine (PCP)-induced disruption in cognitive performance is gender-specific and associated with a reduction in brain-derived neurotrophic factor (BDNF) in specific regions of the female rat brain. J Mol Neurosci. 2011;43:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 239. | Fumagalli F, Molteni R, Roceri M, Bedogni F, Santero R, Fossati C, Gennarelli M, Racagni G, Riva MA. Effect of antipsychotic drugs on brain-derived neurotrophic factor expression under reduced N-methyl-D-aspartate receptor activity. J Neurosci Res. 2003;72:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 240. | Ashe PC, Chlan-Fourney J, Juorio AV, Li XM. Brain-derived neurotrophic factor (BDNF) mRNA in rats with neonatal ibotenic acid lesions of the ventral hippocampus. Brain Res. 2002;956:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 241. | Angelucci F, Mathé AA, Aloe L. Brain-derived neurotrophic factor and tyrosine kinase receptor TrkB in rat brain are significantly altered after haloperidol and risperidone administration. J Neurosci Res. 2000;60:783-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 242. | Parikh V, Khan MM, Mahadik SP. Olanzapine counteracts reduction of brain-derived neurotrophic factor and TrkB receptors in rat hippocampus produced by haloperidol. Neurosci Lett. 2004;356:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 243. | Pillai A, Terry AV, Mahadik SP. Differential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampus. Schizophr Res. 2006;82:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 244. | Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101-106. [PubMed] |
| 245. | Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301-1308. [PubMed] |
| 246. | Takahashi M, Kakita A, Futamura T, Watanabe Y, Mizuno M, Sakimura K, Castren E, Nabeshima T, Someya T, Nawa H. Sustained brain-derived neurotrophic factor up-regulation and sensorimotor gating abnormality induced by postnatal exposure to phencyclidine: comparison with adult treatment. J Neurochem. 2006;99:770-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 247. | Hock C, Heese K, Müller-Spahn F, Hulette C, Rosenberg C, Otten U. Decreased trkA neurotrophin receptor expression in the parietal cortex of patients with Alzheimer’s disease. Neurosci Lett. 1998;241:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 248. | Gervasoni N, Aubry JM, Bondolfi G, Osiek C, Schwald M, Bertschy G, Karege F. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 249. | Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;27:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 200] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 250. | Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry. 2004;55:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 251. | Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 252. | Xiu MH, Hui L, Dang YF, Hou TD, Zhang CX, Zheng YL, Chen da C, Kosten TR, Zhang XY. Decreased serum BDNF levels in chronic institutionalized schizophrenia on long-term treatment with typical and atypical antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 253. | Hung YY, Huang TL. Lower serum tropomyosin receptor kinase B levels in patients with schizophrenia. Biomed J. 2013;36:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 254. | Pillai A. Decreased expression of Sprouty2 in the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder: a correlation with BDNF expression. PLoS One. 2008;3:e1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 255. | Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 256. | Wong J, Rothmond DA, Webster MJ, Weickert CS. Increases in two truncated TrkB isoforms in the prefrontal cortex of people with schizophrenia. Schizophr Bull. 2013;39:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 257. | Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, Nawa H. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 258. | Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 259. | Palomino A, Vallejo-Illarramendi A, González-Pinto A, Aldama A, González-Gómez C, Mosquera F, González-García G, Matute C. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr Res. 2006;86:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 260. | Jindal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naïve first episode schizophrenia. Schizophr Res. 2010;119:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 261. | Iritani S, Niizato K, Nawa H, Ikeda K, Emson PC. Immunohistochemical study of brain-derived neurotrophic factor and its receptor, TrkB, in the hippocampal formation of schizophrenic brains. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 262. | Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li XM. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
P- Reviewer: Chakrabarti, S, Forero DA S- Editor: Song XX L- Editor: A E- Editor: Wu HL