Published online Jan 27, 2021. doi: 10.4331/wjbc.v12.i1.1
Peer-review started: June 24, 2020
First decision: November 4, 2020
Revised: November 17, 2020
Accepted: December 30, 2020
Article in press: December 30, 2020
Published online: January 27, 2021
Processing time: 204 Days and 18 Hours
Matrix metalloproteinases (MMPs), including MMP-9, are an integral part of the immune response and are upregulated in response to a variety of stimuli. New details continue to emerge concerning the mechanistic and regulatory pathways that mediate MMP-9 secretion. There is significant evidence for regulation of inflammation by dimethyl sulfoxide (DMSO) and 3',5'-cyclic adenosine monophosphate (cAMP), thus investigation of how these two molecules may regulate both MMP-9 and tumor necrosis factor α (TNFα) secretion by human monocytes was of high interest. The hypothesis tested in this study was that DMSO and cAMP regulate MMP-9 and TNFα secretion by distinct mechanisms.
To investigate the regulation of lipopolysaccharide (LPS)-stimulated MMP-9 and tumor necrosis factor α secretion in THP-1 human monocytes by dimethyl sulfoxide and cAMP.
The paper describes a basic research study using THP-1 human monocyte cells. All experiments were conducted at the University of Missouri-St. Louis in the Department of Chemistry and Biochemistry. Human monocyte cells were grown, cultured, and prepared for experiments in the University of Missouri-St. Louis Cell Culture Facility as per accepted guidelines. Cells were treated with LPS for selected exposure times and the conditioned medium was collected for analysis of MMP-9 and TNFα production. Inhibitors including DMSO, cAMP regulators, and anti-TNFα antibody were added to the cells prior to LPS treatment. MMP-9 secretion was analyzed by gel electrophoresis/western blot and quantitated by ImageJ software. TNFα secretion was analyzed by enzyme-linked immuno sorbent assay. All data is presented as the average and standard error for at least 3 trials. Statistical analysis was done using a two-tailed paired Student t-test. P values less than 0.05 were considered significant and designated as such in the Figures. LPS and cAMP regulators were from Sigma-Aldrich, MMP-9 standard and antibody and TNFα antibodies were from R&D Systems, and amyloid-β peptide was from rPeptide.
In our investigation of MMP-9 secretion from THP-1 human monocytes, we made the following findings. Inclusion of DMSO in the cell treatment inhibited LPS-induced MMP-9, but not TNFα, secretion. Inclusion of DMSO in the cell treatment at different concentrations inhibited LPS-induced MMP-9 secretion in a dose-dependent fashion. A cell-permeable cAMP analog, dibutyryl cAMP, inhibited both LPS-induced MMP-9 and TNFα secretion. Pretreatment of the cells with the adenylyl cyclase activator forskolin inhibited LPS-induced MMP-9 and TNFα secretion. Pretreatment of the cells with the general cAMP phosphodiesterase inhibitor IBMX reduced LPS-induced MMP-9 and TNFα in a dose-dependent fashion. Pre-treatment of monocytes with an anti-TNFα antibody blocked LPS-induced MMP-9 and TNFα secretion. Amyloid-β peptide induced MMP-9 secretion, which occurred much later than TNFα secretion. The latter two findings strongly suggested an upstream role for TNFα in mediating LPS-stimulate MMP-9 secretion.
The cumulative data indicated that MMP-9 secretion was a distinct process from TNFα secretion and occurred downstream. First, DMSO inhibited MMP-9, but not TNFα, suggesting that the MMP-9 secretion process was selectively altered. Second, cAMP inhibited both MMP-9 and TNFα with a similar potency, but at different monocyte cell exposure time points. The pattern of cAMP inhibition for these two molecules suggested that MMP-9 secretion lies downstream of TNFα and that TNFα may a key component of the pathway leading to MMP-9 secretion. This temporal relationship fit a model whereby early TNFα secretion directly led to later MMP-9 secretion. Lastly, antibody-blocking of TNFα diminished MMP-9 secretion, suggesting a direct link between TNFα secretion and MMP-9 secretion.
Core Tip: This article describes the stimulation of matrix metalloproteinase-9 (MMP-9) and tumor necrosis factor α (TNFα) secretion from human monocyte cells by lipopolysaccharide, and the inhibition of this process by dimethyl sulfoxide and increased intracellular levels of 3',5'-cyclic adenosine monophosphate. The experimental findings demonstrated that dimethyl sulfoxide concentration must be carefully controlled in inflammatory studies, that MMP-9 secretion occurred downstream from TNFα secretion and that the two processes were distinct, and that TNFα is an integral component of the pathway leading to MMP-9 secretion.
- Citation: Denner DR, Udan-Johns ML, Nichols MR. Inhibition of matrix metalloproteinase-9 secretion by dimethyl sulfoxide and cyclic adenosine monophosphate in human monocytes. World J Biol Chem 2021; 12(1): 1-14
- URL: https://www.wjgnet.com/1949-8454/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v12.i1.1
Matrix-metalloproteinases (MMPs) are zinc-dependent extracellular matrix-degrading enzymes that have a key role in a wide variety of physiological[1] and pathological[2,3] processes. Physiological processes include embryonic development, morphogenesis, angiogenesis, tissue repair, and immune responses while pathological processes include arthritis, cancer, neurological disease, breakdown of the blood–brain barrier, and cerebral hemorrhage. MMP subgroups are comprised of collagenases, gelatinases, stromalysins, and membrane-type and their activity is controlled by gene expression, zymogen activation, and endogenous tissue inhibitors[4,5]. MMP-1, MMP-8, MMP-13, and MMP-18 are collagenases that cleave interstitial collagen type I, II, and III and digest some extracellular matrix and non-extracellular matrix proteins. MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are gelatinases that degrade both collagens and gelatins[3].
MMPs have been described as a type of inflammatory cytokine since they are significantly expressed, secreted, and activated upon stimulation of inflammatory cells by various endogenous or exogenous factors[2]. MMP-9 is an important MMP in myeloid/monocyte cells and substantial work has been done on understanding the types of stimuli and mechanistic pathways that lead to MMP-9 secretion. Stimulation of inflammatory cells with lipopolysaccharide (LPS), cytokines, amino acids, and protein aggregates such as amyloid-β (Aβ) triggers signaling pathways that involve nuclear factor κB, protein kinase Cα, extracellular signal-regulated kinase 1 and 2 and mitogen-activated kinase p38, and lead to MMP-9 secretion[6-9]. MMP-9 secretion, and inflammation in general, can be regulated in a number of ways and there have been previous reports of regulation by both dimethyl sulfoxide (DMSO)[9-11] and the intracellular signaling molecule 3',5'-cyclic adenosine monophosphate (cAMP)[7].
DMSO is commonly used as a vehicle for delivering hydrophobic compounds, such as enzyme inhibitors or signaling regulators to cells, thus its role as an inflammatory regulator is of significant interest. DMSO inhibited production of MMP-9 in TNFα-stimulated human keratinocytes at concentrations of 0.75% and higher[9]. DMSO also decreased interleukin-1β (IL-1β)-induced interleukin-6 and macrophage chemoattractant protein-1 secretions in a dose-dependent manner in differentiated-Caco-2 cells[10]. Suppression of many pro-inflammatory cytokines/chemokines by DMSO in Escherichia coli- and herpes simplex virus-1-stimulated whole human blood has also been demonstrated[11]. Cyclic AMP is a critical signaling molecule and is involved in a myriad of physiological cellular processes including regulation of inflammatory processes. Support for this regulation includes the observation that adenosine dose-dependently decreased MMP-9 secretion in isolated human neutrophils regardless of whether the cells were stimulated by N-formylmethionyl-leucyl-phenylalanine, LPS, or H2O2[7]. Adenosine increased intracellular cAMP levels and it was concluded that the effect was mediated through a cAMP-mediated
MMP-9 and TNFα are important markers and mediators of inflammatory processes in immune cells. In this report we sought to further examine temporal and regulatory mechanisms of MMP-9 secretion in THP-1 human monocytes after stimulation with LPS. Specifically, dose-dependent regulation of MMP-9 and TNFα by the aprotic solvent DMSO and the intracellular signaling molecule cAMP. The findings demonstrated distinct regulatory effects by DMSO and cAMP on MMP-9 and TNFα secretion. These distinct, but significant and efficacious, effects by DMSO and cAMP revealed a probable role for TNFα in the pathway leading to MMP-9 secretion.
THP-1 monocytic cells were obtained from ATCC (Manassas, VA, United States) and maintained in RPMI-1640 culture medium (HyClone, Logan, UT, United States) containing 2 mmol/L L-glutamine, 25 mmol/L HEPES, 1.5 g/L sodium bicarbonate, 10% fetal bovine serum (HyClone), 50 U/mL penicillin, 50 mg/mL streptomycin (HyClone), and 50 mmol/L β-mercaptoethanol at 37 °C in 5% CO2. For cell assays, THP-1 cells were centrifuged at 500 g for 10 min, washed, and resuspended in reduced fetal calf serum (2%) growth medium. Cell concentrations were adjusted to 1.0 × 106 cells/mL and 0.3 mL (3.0 × 105 cells) was added to individual wells of a 48-well sterile culture plate. For experiments evaluating the regulatory effect of DMSO on LPS-stimulated MMP-9 and TNFα secretion, suspension THP-1 monocyte cells were treated with DMSO (Thermo Fisher Scientific) at concentrations ranging from 0.016%-2% for 5 min prior to subsequent treatment with 1 μg/mL Escherichia coli bacterial 026.B6 LPS (Sigma-Aldrich, St. Louis). Cells were incubated for 72 h at 37 °C in 5% CO2. DMSO concentrations were varied from 0.016%-2% to evaluate the effect on cell response and specific concentrations for each experiment is detailed in each figure legend. Control cell treatments without LPS but in the presence of DMSO were done for all studies. For experiments assessing the regulatory role of cAMP on LPS-stimulated MMP-9 and TNFα secretion, THP-1 monocytes were treated with cAMP regulators dibutyryl 3',5'-cyclic adenosine monophosphate (Bt2-cAMP), forskolin (Fsk), and 3-isobutyl-1-methylxanthine (IBMX) for 5 min prior to subsequent treatment with 1 μg/mL LPS. Cells were incubated for 6-72 h at 37 °C in 5% CO2. In one experiment, cells were stimulated with 0.3 μg/mL LPS. Concentrations or concentration ranges used for the regulators were 0.3 mmol/L for Bt2-cAMP, 0.01-0.1 mmol/L for Fsk, and 0.01-0.3 mmol/L for IBMX. Control cell treatments without LPS but in the presence of cAMP regulators were done for all studies. For experiments assessing the effect of an anti-human TNFα monoclonal antibody (MAB610, R&D Systems, Minneapolis, MN, United States) on LPS-stimulated MMP-9 and TNFα secretion, THP-1 monocytes were treated with the antibody for 5 min prior to subsequent treatment with 1 μg/mL LPS. Cells were incubated for 48 h at 37 °C in 5% CO2. Control cell treatments with or without LPS and/or in the presence or absence of the antibody, were done for these studies. After all treatments, cells were removed from each well, centrifuged at 2500 g for 10 min, and the supernatant was collected and frozen at −20 °C for subsequent analysis.
Supernatants from individual wells of treated THP-1 monocytes were mixed 1:1 with Laemmli SDS sample buffer (Bio-Rad) containing 5% β-mercaptoethanol. Samples and 10 μL recombinant human MMP-9 western blotting standard (WBC018, R&D Systems, Minneapolis, MN, United States) were heated at 95 °C for 5 min and separated on 7.5% Tris-HCl gels (Ready Gel, Bio-Rad) under denaturing conditions (25 mmol/L Tris, 192 mmol/L glycine, 0.1% SDS at pH 8.3) using a Mini Protean 3 Cell (Bio-Rad). Pre-stained SDS-PAGE standards (BioRad) were loaded directly into gel wells. Gels were transferred to Immobilon-P PVDF membrane (Millipore) in transfer buffer containing 25 mmol/L Tris base, 192 mmol/L glycine, and 10% methanol at pH 8.3 using a Tank VEP-2 electroblotting system (Owl Separation Systems). Following protein transfer, the membrane was blocked for 1 h at 25 °C with PBS containing 0.2% Tween 20 (PBST) supplemented with 5% nonfat dry milk, and probed for 1 h at 25 °C with 1 μg/mL goat anti-human MMP-9 antibody (AB911, R&D Systems) in PBST containing 1% milk. After washing membrane 3× in PBST, the membrane was incubated with a 1:1000 dilution of goat anti-mouse IgG-HRP secondary antibody (R&D systems) in PBST containing 1% milk for 1 h at 25 °C. Protein detection was accomplished using ECL Western Blotting Substrate reagents (Pierce) and exposure to film (Kodak). Densitometry of immunoblot TIFF images was done using ImageJ 1.52a.
Measurement of secreted TNFα in the supernatants was determined by enzyme-linked immuno sorbent assay (ELISA). Briefly, 0.1 mL of 4 mg/mL monoclonal anti-human TNFα capture antibody (MAB610, R&D Systems) was added to 96-well plates for overnight incubation at room temperature. Wells were washed with PBS (HyClone) containing 0.05% Tween-20 and blocked with 0.3 mL PBS containing 1% bull serum albumin (BSA), 5% sucrose and 0.05% NaN3 for 1 h at room temperature. After washing, successive additions of 0.05 mL samples or standards (2 h), 0.1 mL biotinylated polyclonal anti-human TNFα detection antibody (BAF210, R&D Systems) in 20 mmol/L Tris with 150 mmol/L NaCl and 0.1% BSA (2 h), 0.1 mL streptavidin-HRP (R&D Systems) diluted 200 times with PBS containing 1% BSA (20 min), and 0.1 mL of equal volumes of 3,3',5,5'-tetramethylbenzidine and hydrogen peroxide (KPL, Gaithersburg, MD, United States) (30 min). The reaction was stopped by the addition of 1% H2SO4 solution. The optical density of each sample was analyzed at 450 nm with a reference reading at 630 nm using a SpectraMax 340 absorbance platereader (Molecular Devices, Union City, CA, United States). A standard curve was constructed by sequential dilution of a TNFα standard from 2000-15 pg/mL. The concentration of TNFα in the experimental samples was calculated from the standard curve
Amyloid-β peptide (Aβ42) (rPeptide, Bogarth, GA, United States) was dissolved in 100% hexafluoroisopropanol (Sigma) for 1 h, aliquotted into sterile microcentrifuge tubes, dried in a vacuum centrifuge, and stored at -20 °C. Prior to cell treatment the lyophilized peptides were resuspended in sterile water to 100 μmol/L peptide concentration and incubated at 4 °C for 24 h. Cells were exposed to a final concentration of 17 μmol/L Aβ42 for selected times. For experiments conducted on Aβ42-stimulated MMP-9 and TNFα secretion, THP-1 monocytes were treated with Aβ42 at a final concentration of 17 μmol/L, and incubated for 6, 24, or 48 h at 37 °C in 5% CO2. Control cell treatments without Aβ42 were done with the same volume of sterile water.
Two-tailed paired Student t-tests were performed for experiments when applicable to determine the confidence limit at which two measurements were statistically different. Analysis was applied to each data set and P values less than 0.05 were considered significant and are designated in the Figures with a superscript a (a) for (P < 0.05) or superscript b (b) for (P < 0.01).
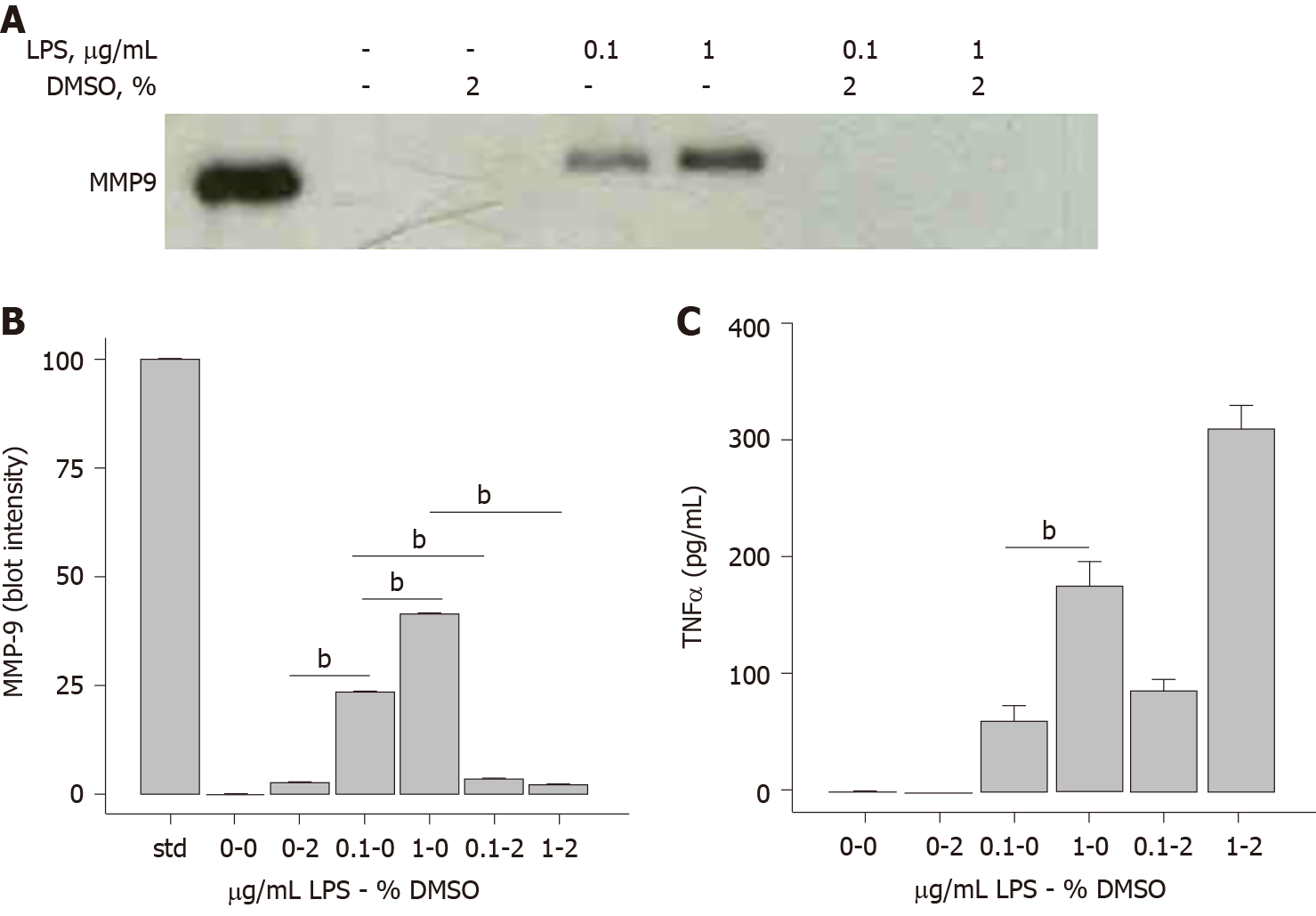
In the course of investigating MMP-9 secretion by THP-1 human monocytes after exposure to LPS, it was observed that DMSO had an inhibitory effect on this process. Western blot analysis showed that MMP-9 secretion was sensitive to LPS concentration, in that 1 μg/mL LPS triggered greater MMP-9 protein secretion compared to 0.1 μg/mL LPS (Figure 1A). The inclusion of 2% DMSO almost completely abolished the release of MMP-9 from THP-1 cells after stimulation with LPS at either concentration (Figure 1A). Densitometry analysis of the immunoblot is provided in Figure 1B. TNFα is a classical proinflammatory cytokine and LPS-induced TNFα secretion in immune cells has been well-studied. Surprisingly, this process was not inhibited by DMSO. The same samples in Figure 1A were analyzed for TNFα levels by ELISA. As expected, 1 μg/mL LPS triggered greater TNFα secretion compared to 0.1 μg/mL in THP-1 cells (Figure 1C). 2% DMSO did not diminish the LPS-induced TNFα response indicating a MMP-9-selective effect by DMSO.
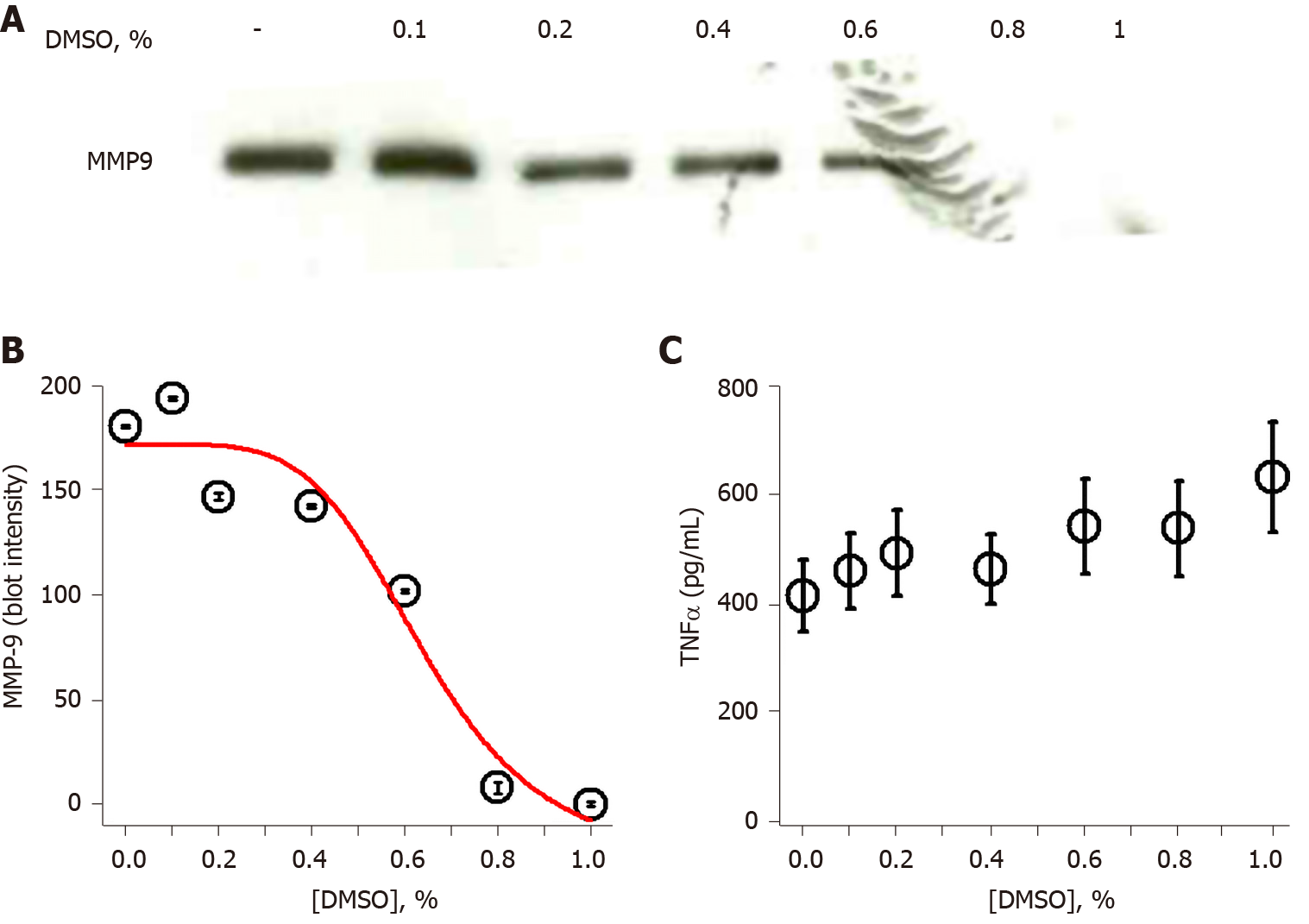
The observation that DMSO inhibited MMP-9 secretion in THP-1 monocytes prompted additional investigation of concentration dependence. THP-1 cells were exposed to increasing concentrations of DMSO prior to stimulation with 1 μg/mL LPS. MMP-9 secretion, measured by immunoblot, was slightly diminished at 0.2% DMSO and almost completely inhibited at 1% DMSO (Figure 2A). Densitometry analysis of the Figure 2A immunoblot allowed non-linear curve fitting of the dose-dependent DMSO effect on secreted MMP-9. The inhibition curve fit yielded an IC50 value of 0.64% DMSO (Figure 2B). Analysis of the same LPS/DMSO samples for TNFα levels again showed no inhibitory effect of DMSO on LPS-stimulated TNFα secretion (Figure 2C).
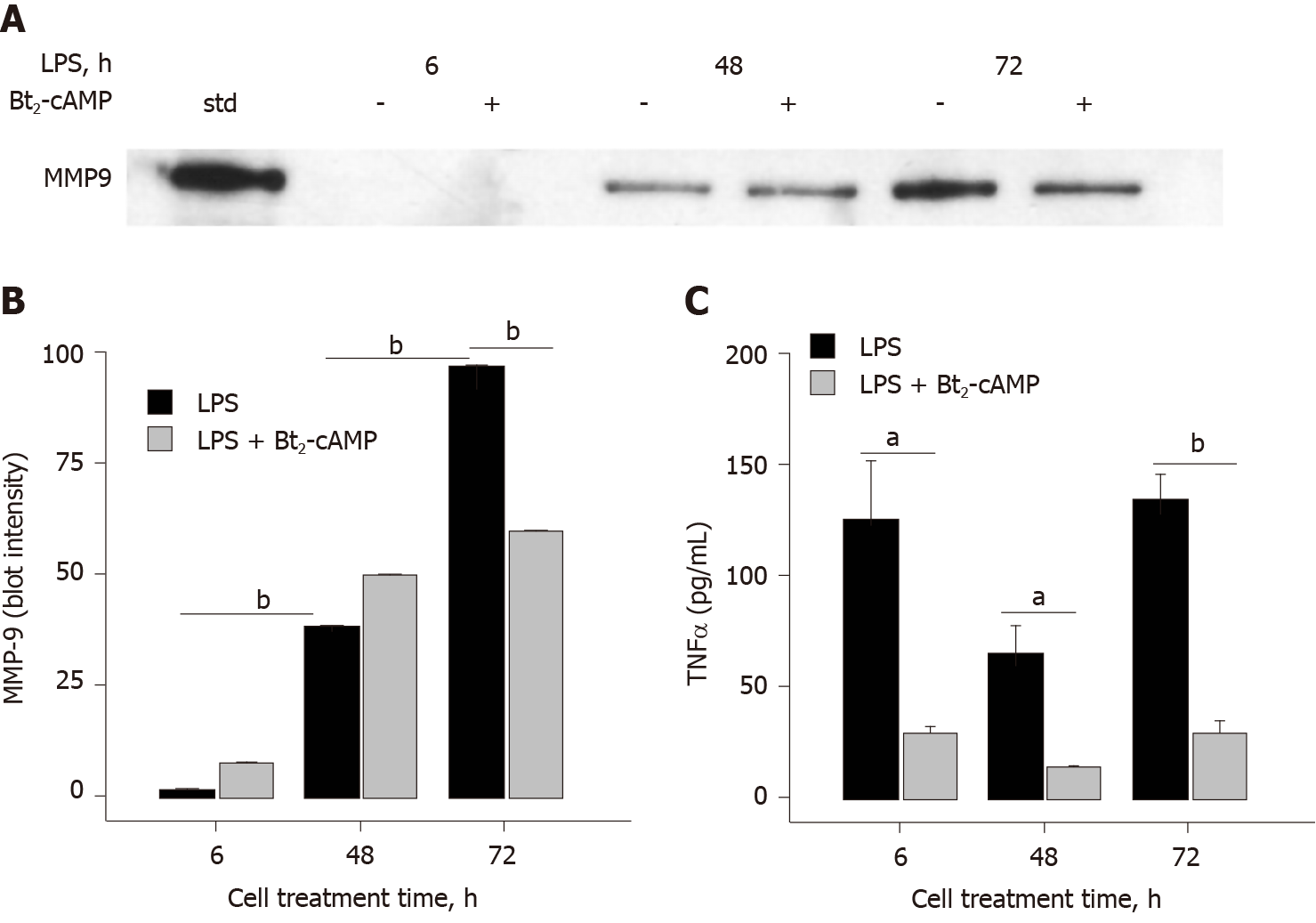
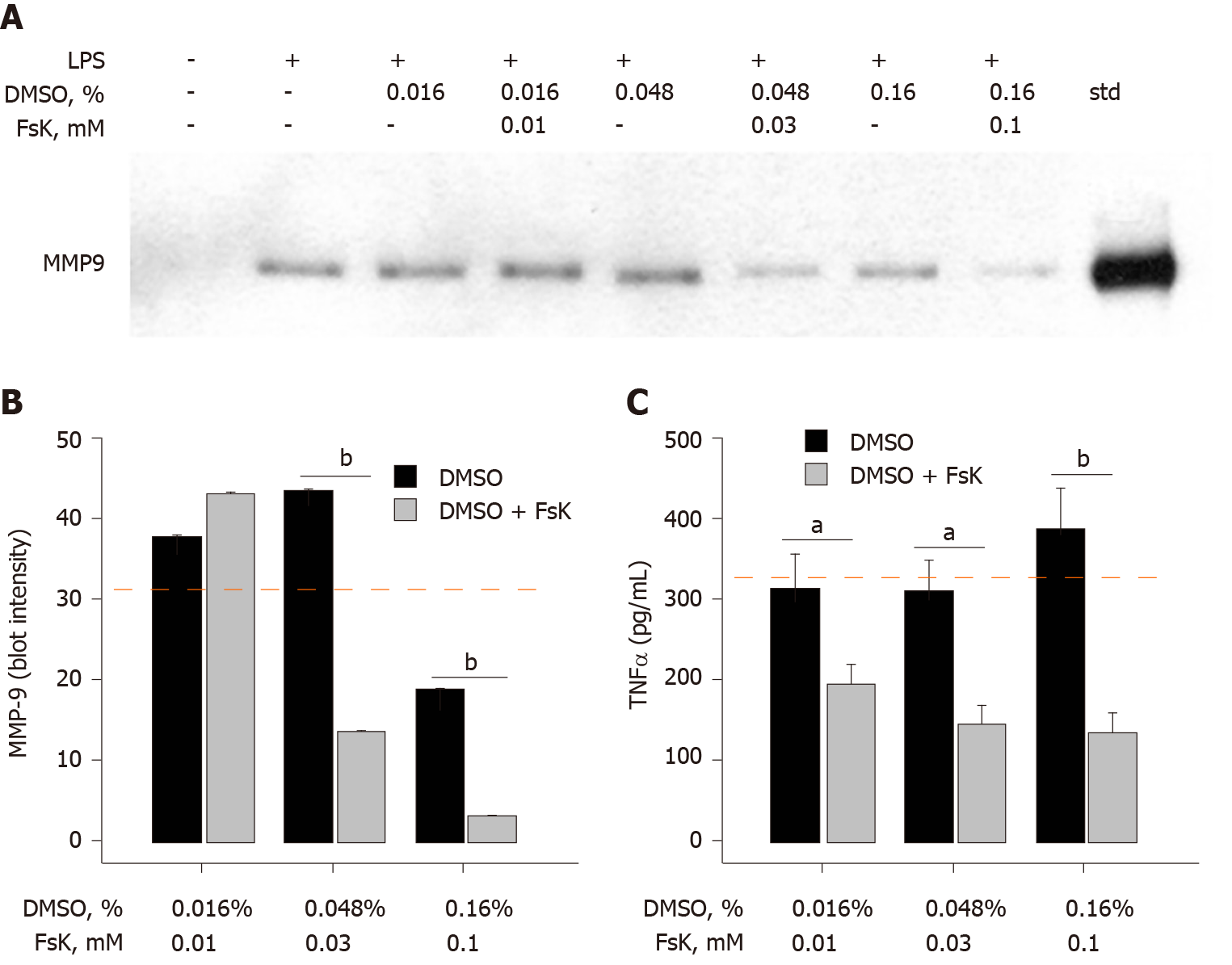
Addition of Bt2-cAMP, a cell-permeable cAMP analog that mimics the activity of cAMP[12], to THP-1 monocytes prior to LPS treatment resulted in an inhibition of MMP-9 secretion (40%), but only at the later cell-exposure time points (Figure 3A and B). The effect of Bt2-cAMP on TNFα was very different in that TNFα secretion was significantly inhibited (> 77%) at earlier and later time points. Fsk, an adenylyl cyclase activator that can also raise intracellular cAMP levels[13], was tested for its ability to alter LPS-induced MMP-9 and TNFα secretion. Due to the hydrophobic nature of Fsk, DMSO was used as a vehicle in this 72 h LPS cell treatment experiment. The DMSO concentration was carefully determined and controlled to fully ascertain the effect of Fsk. DMSO did not inhibit LPS-induced MMP-9 secretion at 0.016% and 0.048%, but 0.16% DMSO did inhibit 50% of the control LPS monocyte treatment (Figure 4A and B). Fsk potently inhibited MMP-9 secretion far in excess of any DMSO inhibition that was observed at 30 μmol/L and 100 μmol/L, but not 10 μmol/L, Fsk (Figure 4A and B). As in the earlier data, DMSO did not inhibit LPS-induced TNFα secretion (Figure 4C). However, Fsk dose-dependently inhibited TNFα secretion, with 39% inhibition at 10 μmol/L, 54% inhibition at 30 μmol/L, and 66% inhibition at 100 μmol/L (Figure 4C). The Fsk inhibition values were determined relative to the corresponding DMSO-treated THP-1 monocytes. Fsk also inhibited LPS-induced TNFα secretion at an early time point (6 h), with 57% and 47% inhibition observed at 30 μmol/L and 100 μmol/L respectively (data not shown).
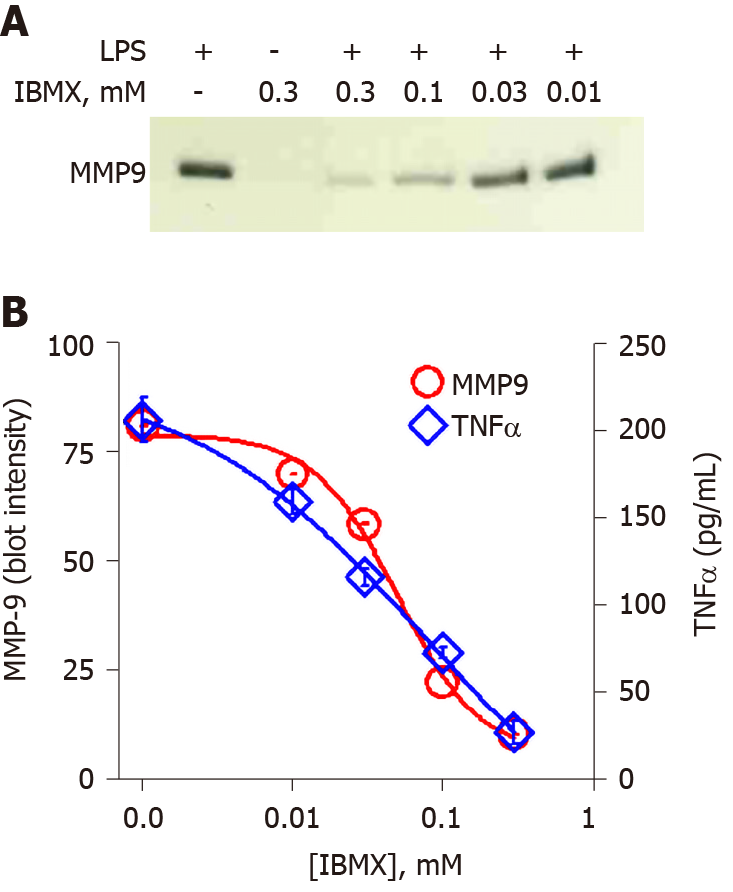
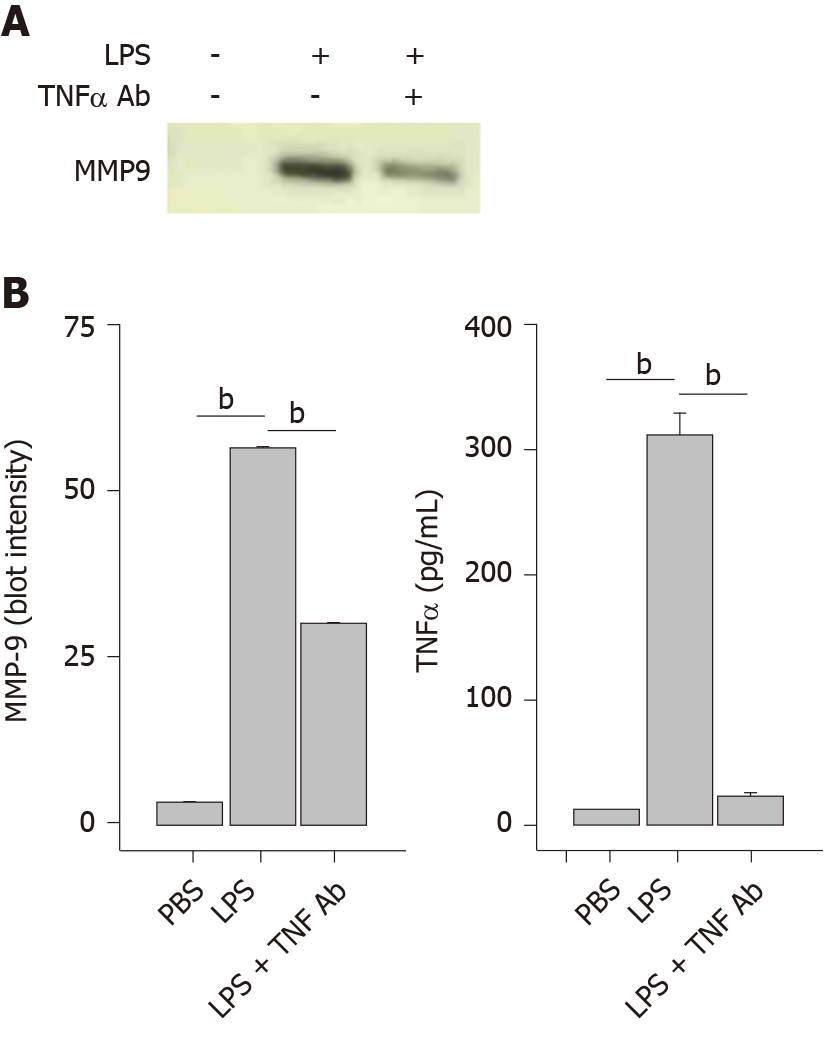
In order to increase intracellular cAMP levels by a different pathway, the cell-permeable general cAMP phosphodiesterase (PDE) inhibitor IBMX was tested. Treatment of THP-1 monocytes with a concentration range of IBMX prior to application of LPS produced a dose-dependent inhibition of MMP-9 secretion (Figure 5A). Quantitation of the immunoblot by densitometry yielded an inhibition plot that was then analyzed by nonlinear curve fitting. The fit produced an IC50 value of 0.05 mmol/L for IBMX inhibition of MMP-9 secretion (Figure 5B, circles). The effect of IBMX on TNFα secretion was also assessed in the same supernatant samples by ELISA. A very similar dose-dependent inhibition was observed for TNFα as for MMP-9 with an IC50 value of 0.08 mmol/L (Figure 5B, diamonds). The temporal and connected relationship between LPS-induced secretion of TNFα and MMP-9 strongly suggested that TNFα played a contributory role in MMP-9 secretion. This idea was tested by the inclusion of an anti-TNFα antibody prior to LPS treatment of THP-1 monocytes. The presence of the TNFα antibody reduced the secretion of MMP-9 when analyzed by immunoblot (Figure 6A). Quantitative analysis by densitometry showed 47% inhibition of MMP-9 by blocking TNFα (Figure 6B, left plot). As expected, the presence of the TNFα antibody significantly reduced TNFα levels in the cell supernatant (Figure 6B, right plot).
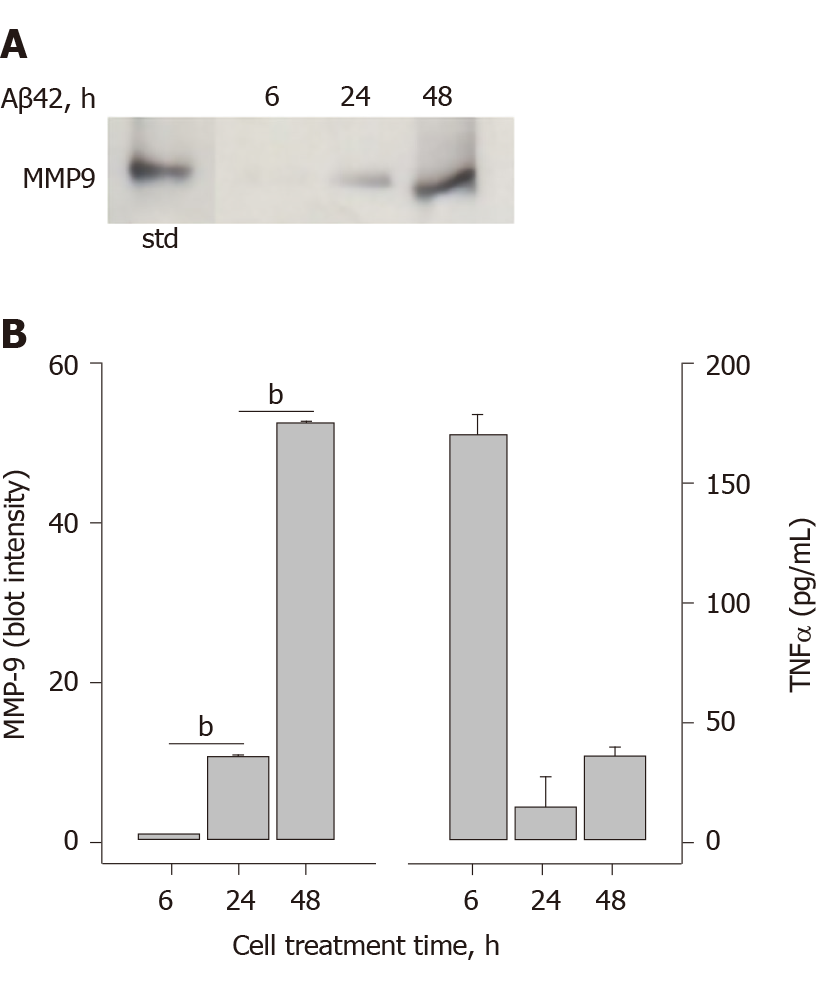
In an effort to examine MMP-9 and TNFα secretion by a cell-stimulator other than LPS, the ability of Aβ42 oligomers to trigger THP-1 monocyte MMP-9 secretion was examined. Hexafluoroisopropanol-treated and vacuum-dried Aβ42 was reconstituted in sterile H2O and incubated for 24 h at 4 °C to allow oligomer formation. THP-1 cells exposed to the Aβ42 oligomers began to secrete MMP-9 at 24 h of treatment, with a much larger level by 48 h (Figure 7A). Comparison of the immunoblot densitometry quantitation and ELISA measurement of secreted TNFα indicated that maximum TNFα secretion occurred prior to MMP-9 secretion (Figure 7B). The temporal relationship between to Aβ42 oligomer-induced TNFα secretion and MMP-9 secretion was similar to that observed by LPS. Significant analysis was performed to rule out any LPS contamination within the Aβ42 samples and delineate differences between LPS and Aβ42 stimulation of monocytes. These analytical measures were described in a previous publication[14]. The two pathogens, one exogenous (LPS) and one endogenous (Aβ), both trigger pathways leading to MMP-9 secretion.
LPS triggers Toll-like receptor 4 (TLR4) activation and downstream signaling pathways that include myeloid differentiation protein 88 and NF-kB-mediated transcription and expression of innate immune genes such as TNFα and IL-1β[15-17]. LPS also activates extracellular signal-regulated kinases 1/2, mitogen-activated kinase p38, mitochondrial reactive oxygen species, and MMP-9 expression[6,18].
DMSO has long been recognized to have anti-inflammatory and analgesic properties[19]. Although controversial, it has been used in a variety of formulations for the treatment of interstitial cystitis, cutaneous scleroderma, and wound healing, with an additional potential as an anti-viral[19]. Furthermore, as a polar, aprotic solvent miscible with water, DMSO is widely used as a vehicle for hydrophobic compounds in cellular assays. Here we show that application of low concentrations (0.2%-0.8%) of DMSO to human monocytes inhibited the secretion of MMP-9. The mechanism by which the inhibition occurred was not a generalized process as DMSO did not inhibit secretion of TNFα, another inflammatory product and mediator. Cyclic AMP, on the other hand, inhibited both TNFα and MMP-9 secretion. The manner in which cAMP was generated in the monocytes did not influence the inhibitory outcome, whether by direct application of a cell-permeable cAMP analog, cAMP synthesis by activation of adenylyl cyclase, or boosting cAMP levels by inhibition of PDE.
Our findings indicated that DMSO concentration should be restricted to 0.4% or below to avoid inadvertent inhibition of MMP-9 secretion. Furthermore, there are specific distinctions in the mechanistic pathways by which MMP-9 and TNFα are secreted, and the inhibitory effect of DMSO on MMP-9 secretion reveals one or more areas of these mechanistic distinctions. Our DMSO inhibitory observations and effective concentration were consistent with other studies. Majtan and Majtan[9] previously demonstrated DMSO inhibition of MMP-9 transcription and synthesis in TNFα-treated human keratinocytes[9]. We expanded our investigation by stimulating monocytes further upstream with LPS and comparing both DMSO and cAMP regulation of TNFα and MMP-9 secretion. The findings showed that DMSO did not alter LPS-induced TNFα levels indicating a distinctive effect for MMP-9. Previous studies have shown DMSO inhibition of MMP-2 in human aorta endothelial cells at slightly higher concentrations of 1%-3%[20]. For other inflammatory molecules, Elisia et al[11] found that 0.5%-2% DMSO inhibited expression of numerous cytokines in bacterial-stimulated whole blood samples and in the joints of arthritic mice[11]. The findings on TNFα were mixed, with some inhibition observed at 2% DMSO in whole blood, but no inhibition in the arthritic mouse paws. Hollebeeck et al[10] reported DMSO inhibition of the intestinal Caco-2 cell inflammatory response in the range of 0.1%-0.5%. The cells were stimulated with IL-1β or a proinflammatory cocktail consisting of IL-1β, TNFα, interferon-γ and LPS. Differential effects by DMSO were observed, whereby DMSO inhibited transcription of IL-6, IL-1α, IL-1β, and cyclooxygenase-2, but not IL-8 or TNFα.
Cyclic-AMP is a ubiquitous second messenger molecule that can modulate a number of processes by direct binding to molecular targets or by activation of cAMP-dependent protein kinase (PKA) and subsequent downstream phosphorylation. Adenylyl cyclase and PDE tightly control cAMP levels by regulating synthesis and degradation respectively. Cyclic AMP is well-recognized as an inflammation modifier in a variety of cell types and an important therapeutic target (reviewed in[21,22]). Attenuation of LPS-induced TNFα production by agents that raise cAMP levels was specifically demonstrated in monocyte/macrophage cells[15,23]. Furthermore, elevation of cAMP levels in neutrophils inhibited MMP-9 secretion and implicated cAMP-dependent PKA in the process[7].
The mechanism by which cAMP regulates cytokine pathways has been widely researched and there are multiple points at which this occurs. Two of these areas in monocytes/macrophages include the orphan nuclear receptor Nr4a1 (Nur77) and the nuclear factor κB transcriptional complex. Elevated cAMP levels induce Nur77 expression leading to repression of numerous inflammatory genes reviewed in[24]. Either incomplete or excessive phosphorylation of cAMP response element binding protein can suppress TNFα transcription[25]. PKA can also regulate cAMP response element binding protein/deoxyribonucleic acid binding in ganglioside and plasminogen-induced microglial cytokine production[26]. It has been proposed for some time that cAMP has a role as an inflammation gate-keeper[23]. Our results were consistent with previous findings and showed that increased cAMP levels attenuated LPS-induced MMP-9 and TNFα secretion.
Several aspects of our data indicated that MMP-9 secretion was a distinct process from TNFα secretion and occurred downstream. First, DMSO inhibited MMP-9, but not TNFα, suggesting that the MMP-9 secretion process was selectively altered. Second, cAMP inhibited both MMP-9 and TNFα with a similar potency, but at different monocyte cell exposure time points. The pattern of cAMP inhibition for these two molecules suggested that MMP-9 secretion lies downstream of TNFα and that TNFα may a key component of the pathway leading to MMP-9 secretion. This temporal relationship fit a model whereby early TNFα secretion directly led to later MMP-9 secretion. Lastly, antibody-blocking of TNFα diminished MMP-9 secretion, suggesting a direct link between TNFα secretion and MMP-9 secretion.
Bacterial LPS stimulation of human monocytic cells has been well-studied and evokes a classical innate immune response to exogenous pathogens via TLR4. It is now widely-recognized that endogenous pathogens, such as Aβ in Alzheimer’s disease, can trigger similar pathways via TLR4 and other TLRs[14,27-29]. Stimulation of MMP-9 secretion by Aβ42 has been shown previously in THP-1 monocytes[30], murine microglia[31], rat astrocytes[32], and human retinal pigment epithelial cells[33]. Furthermore, MMP-9 levels are significantly elevated in plasma from Alzheimer’s disease patients[34] and in 5xFAD/MBP−/− transgenic mice[35]. Our findings show that Aβ42 oligomer-induced MMP-9 secretion appears to follow the same temporal pathway as LPS in that significant MMP-9 levels were not observed until later cell exposure time points and well after TNFα release. The LPS and Aβ42 results fit a mechanism whereby induced TNFα secretion and subsequent autocrine stimulation of TNF receptor type 1 leads to MMP-9 production and secretion[36].
The cumulative data indicated that MMP-9 secretion was a distinct process from TNFα secretion and occurred downstream. First, DMSO inhibited MMP-9, but not TNFα, suggesting that the MMP-9 secretion process was selectively altered. Second, cAMP inhibited both MMP-9 and TNFα with a similar potency, but at different monocyte cell exposure time points. The pattern of cAMP inhibition for these two molecules suggested that MMP-9 secretion lies downstream of TNFα and that TNFα may a key component of the pathway leading to MMP-9 secretion. This temporal relationship fit a model whereby early TNFα secretion directly led to later MMP-9 secretion. Lastly, antibody-blocking of TNFα diminished MMP-9 secretion, suggesting a direct link between TNFα secretion and MMP-9 secretion.
Matrix metalloproteinases (MMPs), including MMP-9, are an integral part of the immune response and are upregulated in response to a variety of stimuli. New details continue to emerge concerning the mechanistic and regulatory pathways that mediate MMP-9 secretion. There is significant evidence for regulation of inflammation by dimethyl sulfoxide (DMSO) and 3',5'-cyclic adenosine monophosphate (cAMP), thus investigation of how these two molecules may regulate both MMP-9 and tumor necrosis factor α (TNFα) secretion by human monocytes was of high interest. The hypothesis tested in this study was that DMSO and cAMP regulate MMP-9 and TNFα secretion by distinct mechanisms.
The objective of this study was further examine temporal and regulatory mechanisms of MMP-9 secretion in THP-1 human monocytes after stimulation with lipopolysaccharide (LPS). Specifically, dose-dependent regulation of MMP-9 and TNFα by the aprotic solvent DMSO and the intracellular signaling molecule cAMP.
The objective of this study was further examine temporal and regulatory mechanisms of MMP-9 secretion in THP-1 human monocytes after stimulation with LPS. Specifically, dose-dependent regulation of MMP-9 and TNFα by the aprotic solvent DMSO and the intracellular signaling molecule cAMP.
The paper describes a basic research study using THP-1 human monocyte cells. All experiments were conducted at the University of Missouri-St. Louis in the Department of Chemistry and Biochemistry. Human monocyte cells were grown, cultured, and prepared for experiments in the University of Missouri-St. Louis Cell Culture Facility as per accepted guidelines. Cells were treated with LPS for selected exposure times and the conditioned medium was collected for analysis of MMP-9 and TNFα production. Inhibitors including DMSO, cAMP regulators, and anti-TNFα antibody were added to the cells prior to LPS treatment. MMP-9 secretion was analyzed by gel electrophoresis/western blot and quantitated by ImageJ software. TNFα secretion was analyzed by enzyme-linked immuno sorbent assay. All data is presented as the average and standard error for at least 3 trials. Statistical analysis was done using a two-tailed paired Student t-test P values less than 0.05 were considered significant and designated as such with an asterisk in the figures (P < 0.05). LPS and cAMP regulators were from Sigma-Aldrich, MMP-9 standard and antibody and TNFα antibodies were from R&D Systems, and amyloid-β peptide was from rPeptide.
In our investigation of MMP-9 secretion from THP-1 human monocytes, we made the following findings. Inclusion of DMSO in the cell treatment inhibited LPS-induced MMP-9, but not TNFα, secretion. Inclusion of DMSO in the cell treatment at different concentrations inhibited LPS-induced MMP-9 secretion in a dose-dependent fashion. A cell-permeable cAMP analog, dibutyryl cAMP, inhibited both LPS-induced MMP-9 and TNFα secretion. Pretreatment of the cells with the adenylyl cyclase activator forskolin inhibited LPS-induced MMP-9 and TNFα secretion. Pretreatment of the cells with the general cAMP phosphodiesterase inhibitor reduced LPS-induced MMP-9 and TNFα in a dose-dependent fashion. Pre-treatment of monocytes with an anti-TNFα antibody blocked LPS-induced MMP-9 and TNFα secretion. Amyloid-β peptide-induced MMP-9 secretion and occurred much later than TNFα secretion. The latter two findings strongly suggested an upstream role for TNFα in mediating LPS-stimulate MMP-9 secretion.
The cumulative data indicated that MMP-9 secretion was a distinct process from TNFα secretion and occurred downstream. First, DMSO inhibited MMP-9, but not TNFα, suggesting that the MMP-9 secretion process was selectively altered. Second, cAMP inhibited both MMP-9 and TNFα with a similar potency, but at different monocyte cell exposure time points. The pattern of cAMP inhibition for these two molecules suggested that MMP-9 secretion lies downstream of TNFα and that TNFα may a key component of the pathway leading to MMP-9 secretion. This temporal relationship fit a model whereby early TNFα secretion directly led to later MMP-9 secretion. Lastly, antibody-blocking of TNFα diminished MMP-9 secretion, suggesting a direct link between TNFα secretion and MMP-9 secretion.
Regulation of MMP-9 is a significant important in many disease processes including arthritis and Alzheimer’s disease. Further understanding of the pathways leading to MMP-9 secretion and regulation of this process will be important for managing human health.
| 1. | Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 616] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 2. | Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Zipfel P, Rochais C, Baranger K, Rivera S, Dallemagne P. Matrix Metalloproteinases as New Targets in Alzheimer's Disease: Opportunities and Challenges. J Med Chem. 2020;63:10705-10725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Laronha H, Caldeira J. Structure and Function of Human Matrix Metalloproteinases. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 399] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 5. | Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 6. | Lai WC, Zhou M, Shankavaram U, Peng G, Wahl LM. Differential regulation of lipopolysaccharide-induced monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Immunol. 2003;170:6244-6249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Ernens I, Rouy D, Velot E, Devaux Y, Wagner DR. Adenosine inhibits matrix metalloproteinase-9 secretion by neutrophils: implication of A2a receptor and cAMP/PKA/Ca2+ pathway. Circ Res. 2006;99:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Shin Y, Yoon SH, Choe EY, Cho SH, Woo CH, Rho JY, Kim JH. PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp Mol Med. 2007;39:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Majtan J, Majtan V. Dimethyl sulfoxide attenuates TNF-α-induced production of MMP-9 in human keratinocytes. J Toxicol Environ Health A. 2011;74:1319-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Hollebeeck S, Raas T, Piront N, Schneider YJ, Toussaint O, Larondelle Y, During A. Dimethyl sulfoxide (DMSO) attenuates the inflammatory response in the in vitro intestinal Caco-2 cell model. Toxicol Lett. 2011;206:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Elisia I, Nakamura H, Lam V, Hofs E, Cederberg R, Cait J, Hughes MR, Lee L, Jia W, Adomat HH, Guns ES, McNagny KM, Samudio I, Krystal G. DMSO Represses Inflammatory Cytokine Production from Human Blood Cells and Reduces Autoimmune Arthritis. PLoS One. 2016;11:e0152538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Kaukel E, Hilz H. Permeation of dibutyryl cAMP into HeLa cells and its convesion to monobutyryl cAMP. Biochem Biophys Res Commun. 1972;46:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Peters JR, Nambi P, Sibley DR, Lefkowitz RJ. Enhanced adenylate cyclase activity of turkey erythrocytes following treatment with beta-adrenergic receptor antagonists. Eur J Pharmacol. 1984;107:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Abeta(1-42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271:20828-20835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 292] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Woo CH, Lim JH, Kim JH. Lipopolysaccharide induces matrix metalloproteinase-9 expression via a mitochondrial reactive oxygen species-p38 kinase-activator protein-1 pathway in Raw 264.7 cells. J Immunol. 2004;173:6973-6980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Capriotti K, Capriotti JA. Dimethyl sulfoxide: history, chemistry, and clinical utility in dermatology. J Clin Aesthet Dermatol. 2012;5:24-26. [PubMed] |
| 20. | Koizumi K, Tsutsumi Y, Yoshioka Y, Watanabe M, Okamoto T, Mukai Y, Nakagawa S, Mayumi T. Anti-angiogenic effects of dimethyl sulfoxide on endothelial cells. Biol Pharm Bull. 2003;26:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Raker VK, Becker C, Steinbrink K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front Immunol. 2016;7:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 22. | Pearse DD, Hughes ZA. PDE4B as a microglia target to reduce neuroinflammation. Glia. 2016;64:1698-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci. 2002;99:7628-7633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 350] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Chong YH, Shin YJ, Suh YH. Cyclic AMP inhibition of tumor necrosis factor alpha production induced by amyloidogenic C-terminal peptide of Alzheimer's amyloid precursor protein in macrophages: involvement of multiple intracellular pathways and cyclic AMP response element binding protein. Mol Pharmacol. 2003;63:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Min KJ, Yang MS, Jou I, Joe EH. Protein kinase A mediates microglial activation induced by plasminogen and gangliosides. Exp Mol Med. 2004;36:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol. 2008;181:7254-7262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982-11992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 469] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 29. | Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 Ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1163] [Cited by in RCA: 1212] [Article Influence: 75.8] [Reference Citation Analysis (13)] |
| 30. | Chong YH, Sung JH, Shin SA, Chung JH, Suh YH. Effects of the beta-amyloid and carboxyl-terminal fragment of Alzheimer's amyloid precursor protein on the production of the tumor necrosis factor-alpha and matrix metalloproteinase-9 by human monocytic THP-1. J Biol Chem. 2001;276:23511-23517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Park SY, Park TG, Lee SJ, Bae YS, Ko MJ, Choi YW. α-Iso-cubebenol inhibits inflammation-mediated neurotoxicity and amyloid beta 1-42 fibril-induced microglial activation. J Pharm Pharmacol. 2014;66:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Deb S, Wenjun Zhang J, Gottschall PE. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain Res. 2003;970:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Cao L, Liu C, Wang F, Wang H. SIRT1 negatively regulates amyloid-beta-induced inflammation via the NF-κB pathway. Braz J Med Biol Res. 2013;46:659-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, Cudkowicz ME, Beal MF. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer's disease. Neurochem Int. 2003;43:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Ou-Yang MH, Van Nostrand WE. The absence of myelin basic protein promotes neuroinflammation and reduces amyloid β-protein accumulation in Tg-5xFAD mice. J Neuroinflammation. 2013;10:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Heidinger M, Kolb H, Krell HW, Jochum M, Ries C. Modulation of autocrine TNF-alpha-stimulated matrix metalloproteinase 9 (MMP-9) expression by mitogen-activated protein kinases in THP-1 monocytic cells. Biol Chem. 2006;387:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Immunology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, Rangel-Corona R S-Editor: Zhang H L-Editor: A P-Editor: Ma YJ