©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 78-82
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.78
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.78
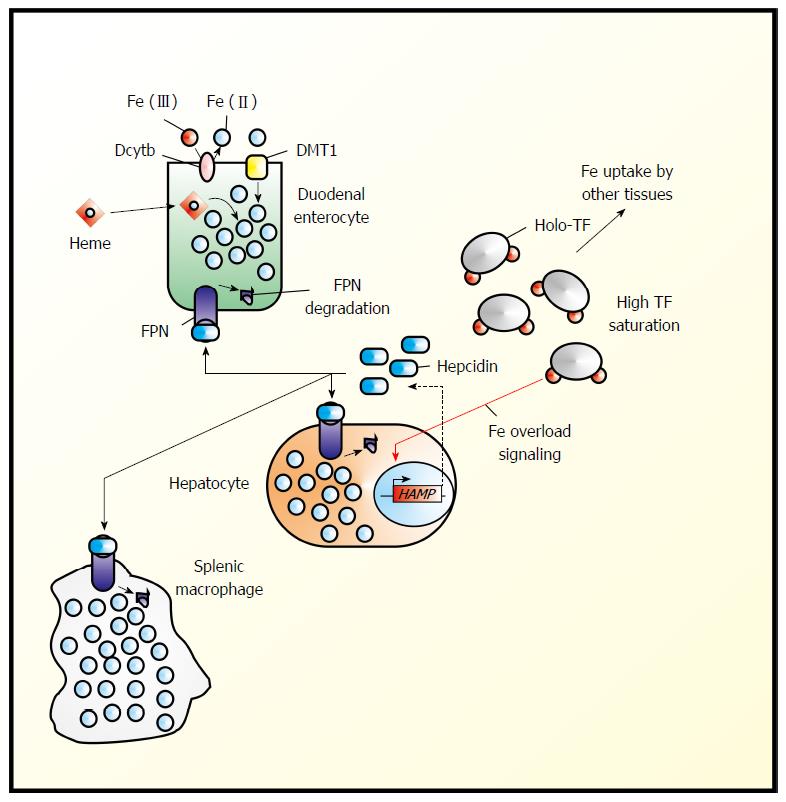
Figure 1 Hepcidin in the regulation of systemic iron homeostasis.
Systemic iron (Fe) homeostasis is primarily regulated by the hepcidin-ferroportin (FPN1) axis. Duodenal enterocytes import dietary Fe from several sources including heme Fe and non-heme Fe, which typically must be reduced at the level of the apical membrane by chemical reductants such as ascorbate[26,27] or by plasma membrane oxidoreductases (e.g., Dcytb), The reduced Fe then enters a common intracellular pool of Fe. “Sensing” the level of transferrin (TF) saturation and levels of Fe stores under conditions of Fe overload, hepatocytes up-regulate the expression of hepcidin, which is then released into the plasma where it can then bind to ferroportin (FPN1), thereby triggering FPN1 internalization and proteosomal degradation[1].
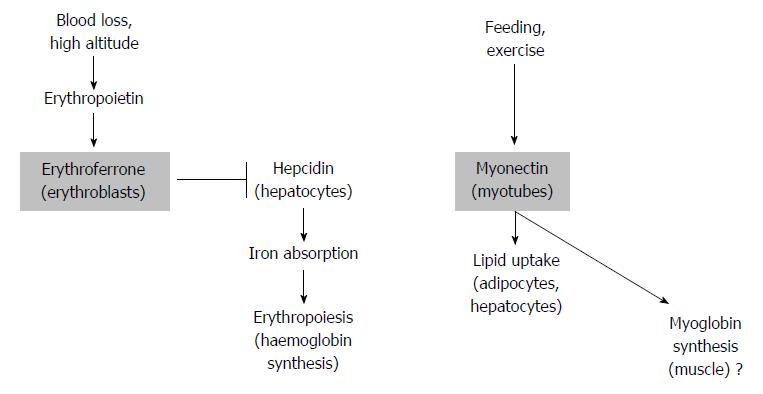
Figure 2 Two potential actions of erythroferrone.
After blood loss, stress erythropoiesis is triggered by erythropoietin. This results in erythroferrone release from erythroblasts and in turn down-regulation of hepcidin. As a result, iron absorption and hemoglobin synthesis and erythropoiesis are increased (left). In response to feeding and exercise, myonectin (erythroferrone) produced by myotubes triggers fatty acid uptake by adipocytes and hepatocytes (right). Considering the function of erythroferrone in erythropoiesis, regulation of myoglobin is suggested to be a likely consequence of myonectin release (far right).
- Citation: Lawen A. Is erythroferrone finally the long sought-after systemic erythroid regulator of iron? World J Biol Chem 2015; 6(3): 78-82
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/78.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.78