©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 130-140
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.130
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.130
Figure 1 Schematic representation of the kallikrein-kinin system.
Bradykinin and Lys-bradykinin (BK), generated by the action of plasma or tissue kallikrein on the precursor (high or low molecular weight kininogen) are the main bradykinin and its receptor (B2R) agonists. These peptides can be converted to B1R agonists after removal of C-terminal-Arg. Both peptidases, membrane-bound carboxypeptidase M, linked to B1R at the C-terminal domain or the soluble carboxypeptidase N are able to remove Arg from the C-terminal portion of BK. B2R is constitutively expressed, showing physiological effects such as vasodilation, transient nitric oxide (NO) production by endothelial nitric oxide synthase (eNOS), whereas B1R expression is induced by injury or inflammatory conditions, with long-lasting NO production, resulting in a neurotoxic environment with reactive oxygen species (ROS) production and increased release of glutamate with excitoxicity-induced neuronal death.
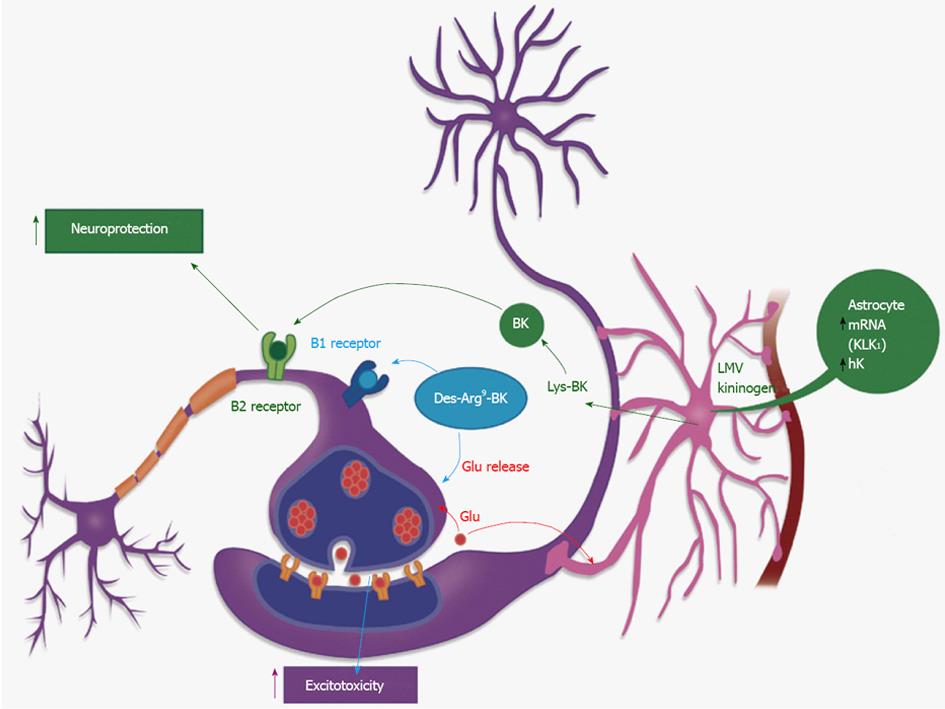
Figure 2 Cross-talk between glial and neural cells related to the kallikrein-kinin system.
An adaptation based on the image found at the following site: http://learn.genetics.utah.edu/units/addiction/reward/images/neuronsAstrocyte.jpg. Kallikrein 1 (KK1) in the hippocampus, acts on its main substrate, the low molecular weight kininogen, to release Lys-bradykinin (BK) which can be hydrolyzed to BK, Des-Arg9BK or des-Arg10-Lys-BK by kininases, localized in astrocytes or at the extracellular matrix. These short-living peptides will act on the neuronal surface: binding to kinin B1R they will induce an increase in glutamate release, thus increasing neuronal excitability. Acting on kinin B2R these peptides will produce neuroprotection[42-45].
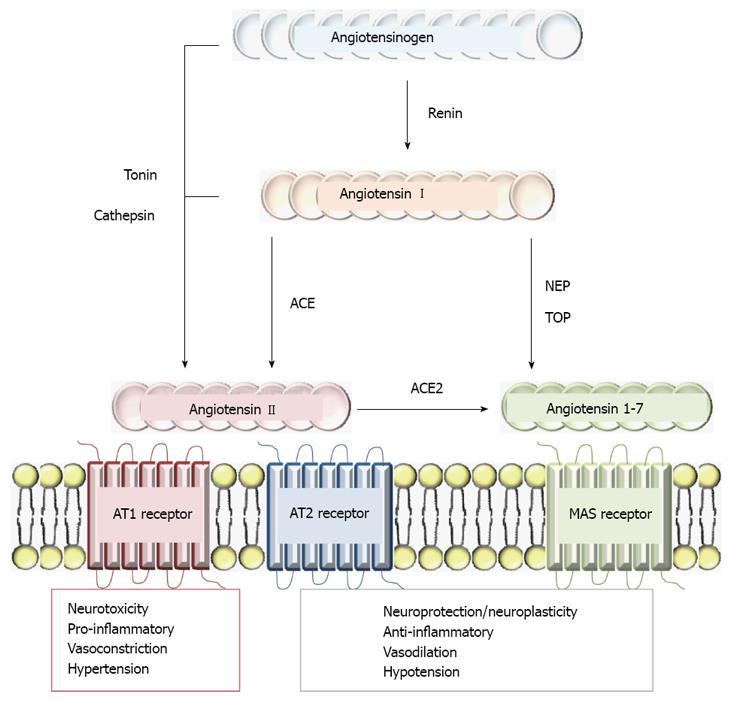
Figure 3 Schematic representation of the renin-angiotensin system and its physiopathological effects.
Ang II may be generated in the brain via the classical pathway, through renin and angiotensin converting enzyme (ACE) action (through Ang I cleavage) or can be directly released from angiotensinogen by cathepsin G or tonin actions. Ang1-7 is active in several organs including the brain and several endopeptidases such as thimet oligopeptidase (TOP) or neutral endopeptidases (NEP) may metabolize Ang I, generating Ang1-7. AngII may also be hydrolyzed by ACE2 to generate Ang1-7. Binding to Ang II type 1 receptor (AT1R), Ang II stimulates vasoconstriction, aldosterone and steroid hormones release, which are involved in sodium reabsorption and water retention. AT1R activity is also related to hypertension, heart dysfunction, brain ischemia, abnormal stress responses, blood-brain barrier breakdown and inflammation. The second receptor involved in Ang II activity is AT2R and is expressed during fetal development, decreasing after birth and remaining at a low concentration during adulthood. It has been linked to cell proliferation, differentiation, apoptosis and the regeneration of several tissues. Ang1-7 is a Mas receptor agonist, which is related to neuronal plasticity and changes in cellular phenotype that are produced by neuronal activity such as synaptic rearrangements and mossy fiber sprouting in the hippocampus.
Figure 4 Schematic representation of the role of angiotensin converting enzyme in the renin-angiotensin and kallikrein-kinin systems.
A: Conversion of AngI into AngII by angiotensin converting enzyme (ACE); B: Bradykinin (BK) degradation by ACE. Physiological effects on the renin-angiotensin system mediated by Ang II type 1 receptor (AT1R) include: vasoconstriction, neuroinflammation, and increased sympathetic nerve activity. Those mediated by Ang II type 2 receptor (AT2R) include cell differentiation and vasodilation. The effects on the kallikrein-kinin system, mediated by kinins, bradykinin and their receptor (B2R) also include vasodilation and hypotension, via the release of nitric oxide (NO), prostacyclins and endothelium-derived hyperpolarizing factor (EDHF). It is important to emphasize that in human pathological conditions, the use of ACE inhibitors results in downregulation of AngII production. In this sense, the kallikrein-kinin system is upregulated and the physiological effects of kinins are potentiated, as all kinin-related peptides are less hydrolyzed by ACE inhibition.
- Citation: Naffah-Mazzacoratti MDG, Gouveia TLF, Simões PSR, Perosa SR. What have we learned about the kallikrein-kinin and renin-angiotensin systems in neurological disorders? World J Biol Chem 2014; 5(2): 130-140
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/130.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.130