Published online Dec 27, 2017. doi: 10.4240/wjgs.v9.i12.281
Peer-review started: August 8, 2017
First decision: September 7, 2017
Revised: September 18, 2017
Accepted: November 25, 2017
Article in press: November 25, 2017
Published online: December 27, 2017
Processing time: 141 Days and 22.9 Hours
We review 6 cases of diaphragmatic perforation, with and without herniation, treated in our institution. All patients with diaphragmatic perforation underwent radiofrequency ablation (RFA) treatments for hepatocellular carcinoma (HCC) performed at Kurume University Hospital and Tobata Kyoritsu Hospital. We investigated the clinical profiles of the 6 patients between January 2003 and December 2013. We further describe the clinical presentation, diagnosis, and treatment of diaphragmatic perforation. The change in the volume of liver and the change in the Child-Pugh score from just after the RFA to the onset of perforation was evaluated using a paired t-test. At the time of perforation, 4 patients had herniation of the viscera, while the other 2 patients had no herniation. The majority of ablated tumors were located adjacent to the diaphragm, in segments 4, 6, and 8. The average interval from RFA to the onset of perforation was 12.8 mo (range, 6-21 mo). The median Child-Pugh score at the onset of perforation (8.2) was significantly higher compared to the median Child-Pugh score just after RFA (6.5) (P = 0.031). All patients underwent laparotomy and direct suture of the diaphragm defect, with uneventful post-surgical recovery. Diaphragmatic perforation after RFA is not a matter that can be ignored. Clinicians should carefully address this complication by performing RFA for HCC adjacent to diaphragm.
Core tip: Diaphragmatic perforation after radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) has been rarely described in the literature; however, it is one of the most serious complications. We conducted a retrospective analysis of 6 cases of diaphragmatic perforation after RFA, and considered the following 3 causative factors for this complication: Location, thermal damage, and liver cirrhosis. Moreover, we found that this complication tends to develop late after RFA. We propose that diaphragmatic perforation after RFA is a rare complication. Clinicians should take steps to prevent thermal injury to the diaphragm by performing RFA for HCC adjacent to the diaphragm and carefully follow up after RFA.
- Citation: Nagasu S, Okuda K, Kuromatsu R, Nomura Y, Torimura T, Akagi Y. Surgically treated diaphragmatic perforation after radiofrequency ablation for hepatocellular carcinoma. World J Gastrointest Surg 2017; 9(12): 281-287
- URL: https://www.wjgnet.com/1948-9366/full/v9/i12/281.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i12.281
Radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) is a minimally invasive treatment commonly used for unresectable primary and metastatic hepatic tumors. Although studies have provided evidence of the safety of RFA, including a low rate of mortality and of major complication[1-4]. Numerous studies have reported complications associated with RFA. Mulier et al[1] calculated a complication rate of 8.9% and a mortality rate of 0.5%, with only 5 cases (0.1%) of injury to the diaphragm described. Curley et al[2] classified complications after hepatic RFA into early complications (within 30 d), including death, abscess at the RFA lesion, and hemorrhage, as well as late complications (more than 30 d after operation), including biliary fistula, hepatic insufficiency, and pleural effusion. They reported a rate of early complications of 7.1% and of late complications of 2.4%. However, they did not describe any occurrence of injury to the diaphragm. In the previous literature only 12 cases of diaphragm perforation with herniation and 3 cases of without herniation after hepatic RFA have been reported[5-19]. Yet, over the last decade, we have encountered 6 cases of late-onset perforation of the diaphragm, with and without herniation, after hepatic RFA, requiring surgical treatment. The etiology of the perforation of the diaphragm might be collateral thermal damage to the diaphragm during RFA. However, the clinical course of diaphragm perforation and herniation has not been sufficiently clarified. Therefore, the aims of our case report were to describe the clinical presentation, diagnosis, and treatment of our 6 cases of diaphragm perforation, with and without herniation, after RFA.
The study protocol was approved by the Institutional Review Board of Kurume University, Japan (No. 14113). All participants provided informed, written consent. Six patients were diagnosed with a perforation of the diaphragm after RFA for HCC, with a concomitant diaphragm herniation identified in 4 of the 6 patients. All patients underwent surgical treatment of the perforation, and herniation when present, at the division of Hepatobiliary Pancreatic Surgery of the Department of Surgery, Kurume University Hospital. All patients treated with RFA for HCC from January 2003 and December 2013 were evaluated for this study to define complications that happened within 6 mo after RFA (late-onset). Initial RFA treatments were performed at two different institutions: the Department of Gastrointestinal Medicine, Kurume University Hospital, and the Department of Surgery, Tobata Kyoritsu Hospital.
The total number of the patients who underwent RFA during this period was 1427 patients, who carried 2134 tumors. In 1 of our 6 cases, RFA was performed using a cluster cool tip electrode for ablation (Cool-Tip Radiofrequency System, Radionics2, Cosman Medical; RF 3000, Boston Scientific), with return electrodes applied to the patient’s legs. For the other 5 cases, RFA was performed using monopolar internally cooled electrodes, (Radionics, Cosman Medical). Expandable needles (LeVeen needle, Boston Scientific) were used to position the electrode on the target tissue in 5 of the 6 cases.
Under local anesthesia, the needle electrode was inserted percutaneously in 5 cases, and placed at the target tissue under ultrasonography guidance. In the remaining case, the needle electrode was inserted with the patient under general anesthesia and placed at the target tissue using a transthoracic approach via an artificial pneumothorax, under computed tomography (CT) guidance. No evidence of excessive bleeding at the needle insertion site was observed in any of the cases.
Follow-up CT was performed one week after RFA (“just after RFA”), with subsequent CT follow-up conducted every 6-12 mo. Blood tests, including assessment of tumor markers, were performed every 3 mo.
A dynamic CT was performed in all cases at the onset of perforation, using a 256 slice multi-detector computed tomography scanners (Brilliance iCT, PHILIPS/Aquilion, TOSHIBA) according to a standard protocol. Oyparomin or Iopaque (Fujiyakuhin Co., Saitama) was used as the contrast medium for CT imaging. The contrast medium was injected via peripheral intravenous administration using a power injector at a rate of 3 to 4 mL/s, with a total dosage of 1.5 mL/kg calculated from the patient’s body weight. The change in the volume of the liver was measured from the dynamic CT images using a commercially available workstation (Synaps Vincent, Fujifilm Co. Kanagawa).
The change in the volume of liver and in the Child-Pugh score from just after the RFA to the onset of perforation was evaluated using a paired t-test analysis. A P value < 0.05 was considered statistically significant. Statistical analysis was performed using JMP 11.0.0 (SAS: Roppongi, Minatoku, Tokyo, Japan).
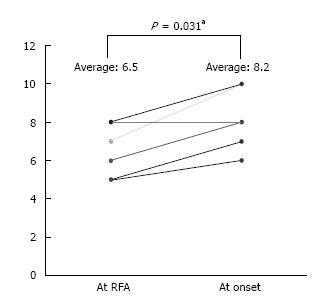
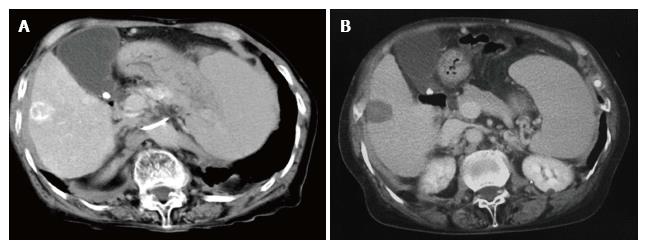
The clinical profiles of all patients are summarized in Table 1 (Table 1). A perforation of the diaphragm developed in 6 patients, 3 men and 3 women, 49 to 79 years old. All patients had underlying liver cirrhosis, with two cases belonging to each of the cirrhosis Child-Pugh classes A, B, or C. The median Child-Pugh score just after RFA was 6.5, with a significant increase to 8.2 at the onset of perforation (P = 0.031; Figure 1). The tumors treated by RFA were single lesions; 21 to 31 mm in diameter; located in liver segments 4, 6, or 8; and adjacent to the diaphragm (Figure 2 A-B: Case 4). At the time of perforation, 4 patients had a perforation with herniated viscera, with the other 2 patients having a perforation without herniation. The interval between RFA and onset of perforation ranged from 6 mo to 21 mo. Three patients had a history of long standing refractory pleural effusion prior to the perforation.
| Case | Age/sex | Tumor location/size (mm) | Time from RFA to DP/DH (mo) | Underlying liver disease/CP sore | Previous intractable pleural effusion | Herniation viscera | Symptom | Treatment for DP/DH | Prognosis after DP/DH treatment |
| 1 | 49/M | S4/17 | 17 | Alcoholic-LC | Absent | Absent | Absent | Surgical repair (laparotomy) | 2 yr |
| Child A | alive | ||||||||
| 2 | 79/F | S8/19 | 9 | HCV-LC | Present | Present (small intestine) | Abdominal pain | Surgical repair (laparotomy) | 3 yr |
| Child B | alive | ||||||||
| 3 | 68/M | S8/26 | 21 | HCV-LC | Present | Present (mesenteric fat) | Abdominal pain | Surgical repair (laparotomy) | 6 mo |
| Child C | died by LF | ||||||||
| 4 | 70/F | S6/23 | 8 | HCV-LC | Present | Present (large intestine) | Dyspnea | Surgical repair and colectomy (laparotomy) | 4 yr |
| Child C | died by LF | ||||||||
| 5 | 65/M | S8/21 | 16 | HCV-LC | Absent | Present (Large intestine) | Abdominal pain | Surgical repair (laparotomy) | 2 yr |
| Child B | died by LF | ||||||||
| 6 | 76/F | S8/20 | 6 | HCV-LC | Absent | Absent | Absent | Surgical repair (laparotomy) | 4 yr |
| Child A | alive |
Four cases with the herniation had symptoms, such as upper abdominal pain and dyspnea, but the case without herniation did not have symptoms. Symptom onset in cases with symptoms was sudden, which did not prevent progress. Meanwhile, 2 cases (Cases 1 and 6) were asymptomatic and were diagnosed at that time of operation of recurrent HCC incidentally.
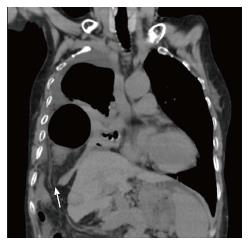
In 4 cases presenting with clinical symptoms, a right diaphragm defect, with and without herniated viscera in the right pleural cavity, was identified on coronal dynamic CT image (Figure 3: Case 4). The herniated viscera included the small intestine in 3 cases and the large intestine in 1 case. All cases were diagnosed with liver cirrhosis based on serum chemistry and CT findings of the morphological features of the liver and spleen. Table 2 shows findings of CT at just after RFA and at the onset (Table 2). At the onset of perforation, disintegration of the diaphragm (4 of 6 cases) and pleural effusion (5 of 6 cases) were visible on CT imaging. However, characteristic findings of diaphragm injury were not visible on CT images obtained just after RFA. Liver volume at the onset of perforation was decreased from at just after RFA volume in 5 of the 6 cases, although this difference was not statistically significant (P = 0.138; Table 3).
| Case | Just after RFA | At onset | ||||||
| Disintegration of diaphragm | Thickening of diaphragm | Ascites | Pleural effusion | Disintegration of diaphragm | Thickening of diaphragm | Ascites | Pleural effusion | |
| 1 | No | No | No | No | No | No | No | No |
| 2 | No | No | No | Yes | Yes | Yes | Yes | Yes |
| 3 | No | No | No | Yes | Yes | No | Yes | Yes |
| 4 | No | No | No | Yes | Yes | No | Yes | Yes |
| 5 | No | No | No | No | Yes | No | No | Yes |
| 6 | No | No | No | No | No | No | Yes | Yes |
| Case | Just after RFA (mL) | At onset (mL) |
| 1 | 1005 | 1055 |
| 2 | ||
| 3 | 653- | 539 |
| 4 | 1130 | 893 |
| 5 | 971 | 946 |
| 6 | 987 | 866 |
| Median | 987 | 893 |
Relevant parameters of RFA procedures are summarized in Table 4. All cases underwent RFA with the electrode inserted via an intercostal approach. The peak power attained was 80 W, and the temperature of the ablated tissue was increased to 68 °C-95 °C. Total irradiation time ranged between 10 and 28 min. Dynamic CT performed just after RFA identified viability of a part of the HCC in 3 cases. Among these 3 patients, 2 underwent additional RFA using the same technique on the viable part of the tumor, with the other patient undergoing real-time CT guided RFA under pneumothorax.
| Case | Anesthesia | Guidance | Approach | Electrode | Number of session | Max power (W) | Max Temperature (ºC) | Additional RFA | Irradiation duration (min) |
| 1 | Local | US | Intercostal | Single cool-tip | 1 | 50 | 76 | Yes | 10 |
| 2 | Local | US | Intercostal | Single cool-tip | 1 | 60 | 84 | Yes | 11 |
| 3 | General | CT | Intercostal | Expansion-type | 8 | 80 | No | 28 | |
| 4 | Local | US | Intercostal | Single cool-tip | 2 | 80 | 86 | No | 16 |
| 5 | Local | US | Intercostal | Single cool-tip | 1 | 50 | 87 | No | 11 |
| 6 | Local | US | Intercostal | Single cool-tip | 2 | 80 | 95 | Yes | 21 |
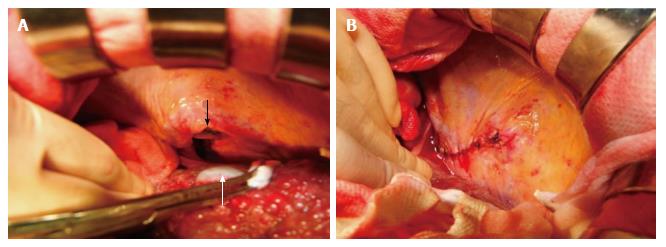
All cases of diaphragm rupture were treated by surgical laparotomy and simple suture of the diaphragm defect (Figure 4 A-B: Case 2). In case 4, resection of the incarcerated large intestine was also performed. All cases had an uneventful postoperative course. Three patients died of hepatic deterioration due to advanced cirrhosis at 6, 24, and 48 mo postoperatively, respectively.
The mechanism of diaphragm perforation after RFA has not been clarified. In our cases, the RFA needle electrode did not penetrate the diaphragm directly except in one case in which RFA was performed under CT guidance using a transthoracic approach via an artificial pneumothorax. Therefore, mechanical damage caused by the needle itself may not completely explain diaphragmatic injury. Considering the clinical profiles of our cases, there are 3 causative factors of diaphragm perforation after RFA: The location of the targeted lesions, collateral thermal injury during RFA, and the advanced cirrhosis status.
Collateral thermal damage to the diaphragm during RFA to these target areas adjacent to the diaphragm is common. In previous clinical case series, the targeted tumor was usually located adjacent to the diaphragm, in liver segments 7, 8, or 5[13]. Head et al[20] reported injury to the diaphragm in 5 of 29 patients (17%) who underwent ablation of hepatic tumors adjacent to the diaphragm. In our cases, all tumors that were treated by RFA were located adjacent to the diaphragm.
The thermal damage to the diaphragm may result in an inflammatory response, leading to fibrosis that could ultimately weaken the muscle fibers of the diaphragm and cause a late-onset defect[10,17]. Poor liver function might prevent the injured tissue from healing adequately, with complications, such as ascites and pleural effusion, thereby further contributing to tissue damage[5].
In this study, we found that the median Child-Pugh score at the onset was significantly higher than at just after RFA. As liver function gradually turns worse, the restoration for the diaphragmatic inflammatory change delays, and it is thought that it leads to diaphragmatic perforation.
Furthermore, one of the complications of aggravated liver function is Chilaiditi’s syndrome. Moaven et al[21] reported that the incidence of Chilaiditi’s syndrome inevitably increases in patients with cirrhosis due to atrophy of the right lobe of the liver, which creates space between the diaphragm and the liver. In our study, progressive atrophy of the liver was identified, on sequential dynamic CT after RFA, in 4 of 5 cases. Therefore, it is plausible that this atrophy of the liver was one of the factors contributing to the development of perforation and herniation of the diaphragm.
In the absence of characteristic symptoms of injury to the diaphragm and the relatively long interval between RFA and the onset of the perforation, it is difficult to predict and diagnose a late-onset diaphragm perforation caused by RFA. In this study, we experienced sudden symptom onset after more than 6 mo. Head et al[20] indicated that thickening of the diaphragm and localized fluid collection on post-ablation (just before perforation) CT scan were the most common imaging findings related to diaphragm damage. However, as in our cases, there may not be symptoms and CT findings specialized in diaphragm perforation at just RFA.
Development of intractable pleural effusion during the follow up period after RFA is another possible sign of diaphragm perforation[16,22]. In our cases, intractable plural effusion before the onset of diaphragmatic herniation was present in 3 of our 6 cases. Ascites following liver cirrhosis might have collected in the plural cavity through a defect in the diaphragm. In cases of intractable pleural effusion in which no defect of the diaphragm is detected by CT and ultrasonography, it would be helpful to perform a dual scope thoracoscopy or peritoneoscopy[22].
Diaphragm perforation and herniation, particularly with symptoms, must be surgically repaired as much as possible. In our experience, when there is not ileus, intestinal necrosis and breathing disorder, it is not necessary to hurry. Although the majority of our patients had advanced liver cirrhosis, prompt and appropriate surgical treatment was safe and effective, with patients recovering rapidly and uneventfully after surgery.
In summary, diaphragmatic herniation consequent to thermal injury of RFA is a rare complication, but it is not a matter that can be ignored in the management of HCC. In performing RFA for liver tumors located adjacent to the diaphragm, clinicians must devise methods for avoiding thermal injury of the diaphragm and regularly monitor the integrity of the diaphragm to achieve early diagnosis of defects over a long-term postoperative follow up.
In the case of diaphragmatic perforation with herniation after radiofrequency ablation (RFA), symptoms, such as upper abdominal pain or dyspnea, develop suddenly, while in the case of perforation without herniation, there may be no symptoms.
Diaphragmatic perforation with or without herniation after radiofrequency ablation for hepatocellular carcinoma.
In case of acute onset, it is necessary to distinguish from acute abdomen and respiratory failure and the history of RFA for hepatocellular carcinoma located adjacent to the diaphragm and computed tomography (CT) findings would be helpful to diagnose.
In the case of diaphragmatic perforation with and without herniation after RFA, liver function, such as Child-Pugh score, may decline in many cases.
In the case of diaphragmatic perforation with herniation after RFA, a right diaphragm defect and herniated viscera in the right pleural cavity is identified on coronal dynamic CT image.
There were no pathological findings as all cases may undergo direct discontinued sutures without trimming in this study.
Diaphragm perforation and herniation, particularly with symptoms, must be surgically repaired as much as possible, but when there is not ileus, intestinal necrosis and breathing disorder, it is not necessary to hurry.
In performing RFA for liver tumors located adjacent to the diaphragm, clinicians must devise methods for avoiding thermal injury of the diaphragm and regularly monitor the integrity of the diaphragm to achieve early diagnosis of defects over a long-term postoperative follow up.
We greatly thank Dr. Hidehiro Sato and Dr. Masafumi Yasunaga for advice on experimental design. We also thank Ms. Miwa Sakai for analyzing the data of a large number of patients.
| 1. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 2. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. [PubMed] |
| 3. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 935] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 4. | Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123-134; discussion 134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Koda M, Ueki M, Maeda N, Murawaki Y. Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;180:1561-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Shibuya A, Nakazawa T, Saigenji K, Furuta K, Matsunaga K. Diaphragmatic hernia after radiofrequency ablation therapy for hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186:S241-S243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | di Francesco F, di Sandro S, Doria C, Ramirez C, Iaria M, Navarro V, Silvestry S, Needleman L, Frank A. Diaphragmatic hernia occurring 15 months after percutaneous radiofrequency ablation of a hepatocellular cancer. Am Surg. 2008;74:129-132. [PubMed] |
| 8. | Nawa T, Mochizuki K, Yakushijin T, Hamano M, Itose I, Egawa S, Nishida T, Tsutsui S, Hiramatsu N, Kanto T. [A patient who developed diaphragmatic hernia 20 months after percutaneous radiofrequency ablation for hepatocellular carcinoma]. Nihon Shokakibyo Gakkai Zasshi. 2010;107:1167-1174. [PubMed] |
| 9. | Yamagami T, Yoshimatsu R, Matsushima S, Tanaka O, Miura H, Nishimura T. Diaphragmatic hernia after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2011;34 Suppl 2:S175-S177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Singh M, Singh G, Pandey A, Cha CH, Kulkarni S. Laparoscopic repair of iatrogenic diaphragmatic hernia following radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011;41:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Boissier F, Labbé V, Marchetti G, Valade S, Djibré M. Acute respiratory distress and shock secondary to complicated diaphragmatic hernia. Intensive Care Med. 2011;37:725-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Kim JS, Kim HS, Myung DS, Lee GH, Park KJ, Cho SB, Joo YE, Choi SK. A case of diaphragmatic hernia induced by radiofrequency ablation for hepatocellular carcinoma. Korean J Gastroenterol. 2013;62:174-178. [PubMed] |
| 13. | Zhou M, He H, Cai H, Chen H, Hu Y, Shu Z, Deng Y. Diaphragmatic perforation with colonic herniation due to hepatic radiofrequency ablation: A case report and review of the literature. Oncol Lett. 2013;6:1719-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Nomura R, Tokumura H, Furihata M. Laparoscopic repair of a diaphragmatic hernia associated with radiofrequency ablation for hepatocellular carcinoma: lessons from a case and the review of the literature. Int Surg. 2014;99:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Nakamura T, Masuda K, Thethi RS, Sako H, Yoh T, Nakao T, Yoshimura N. Successful surgical rescue of delayed onset diaphragmatic hernia following radiofrequency ablation for hepatocellular carcinoma. Ulus Travma Acil Cerrahi Derg. 2014;20:295-299. [PubMed] |
| 16. | Kanso F, Nahon P, Blaison D, Trinchet JC, Beaugrand M, Seror O, Martinod E. Diaphragmatic necrosis after radiofrequency ablation of hepatocellular carcinoma: a successful surgical repair. Clin Res Hepatol Gastroenterol. 2013;37:e59-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Lin MW, Lee JM. Video-assisted thoracoscopic surgery for diaphragmatic defect complication with refractory hydrothorax related to radiofrequency ablation. J Formos Med Assoc. 2010;109:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Thiemann M, Benhidjeb T, Anders S, Gebauer B, Strik MW. Hepato-pericardial fistula following radiofrequency ablation (RFA) for liver metastasis: a case report and review of the literature. Langenbecks Arch Surg. 2008;393:1013-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Kobayashi T, Katsumi S, Wada Y, Horiuchi S, Takano J, Ando Y, Omura K, Takeda T, Watanabe S, Saito K. [A case of hepatocellular carcinoma complicated by pleural effusion mixed with bile after radiofrequency ablation]. Nihon Shokakibyo Gakkai Zasshi. 2014;111:1128-1134. [PubMed] |
| 20. | Head HW, Dodd GD 3rd, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. 2007;243:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Moaven O, Hodin RA. Chilaiditi syndrome: a rare entity with important differential diagnoses. Gastroenterol Hepatol (NY). 2012;8:276-278. [PubMed] |
| 22. | Kang CM, Ko HK, Song SY, Kim KS, Choi JS, Lee WJ, Kim BR. Multimedia manuscript. Dual-scope guided (simultaneous thoraco-laparoscopic) transthoracic transdiaphragmatic intraoperative radiofrequency ablation for hepatocellular carcinoma located beneath the diaphragm. Surg Endosc. 2008;22:541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kai K, Qin JM, Sun XT S- Editor: Cui LJ L- Editor: A E- Editor: Lu YJ