Published online Feb 27, 2016. doi: 10.4240/wjgs.v8.i2.106
Peer-review started: April 29, 2015
First decision: August 31, 2015
Revised: December 4, 2015
Accepted: December 13, 2015
Article in press: December 14, 2015
Published online: February 27, 2016
Processing time: 306 Days and 22.1 Hours
Aim of the study is to comprehensively review the latest trends in laparoscopic complete mesocolic excision (CME) with central vascular ligation (CVL) for the multimodal management of right colon cancer. Historical and up-to-date anatomo-embryological concepts are analyzed in detail, focusing on the latest studies of the mesenteric organ, its dissection by mesofascial and retrofascial cleavage planes, and questioning the need for a new terminology in colonic resections. The rationale behind Laparoscopic CME with CVL is thoroughly investigated and explained. Attention is paid to the current surgical techniques and the quality of the surgical specimen, yielded through mesocolic, intramesocolic and muscularis propria plane of surgery. We evaluate the impact on long term oncologic outcome in terms of local recurrence, overall and disease-free survival, according to the plane of resection achieved. Conclusions are drawn on the basis of the available evidence, which suggests a pivotal role of laparoscopic CME with CVL in the multimodal management of right sided colonic cancer: performed in the right mesocolic plane of resection, laparoscopic CME with CVL demonstrates better oncologic results when compared to standard non-mesocolic planes of surgery, with all the advantages of laparoscopic techniques, both in faster recovery and better immunological response. The importance of minimally invasive meso-resectional surgery is thus stressed and highlighted as the new frontier for a modern laparoscopic total right mesocolectomy.
Core tip: Laparoscopic complete mesocolic excision (CME) with central vascular ligation (CVL) is based on resection of the colon within its intact and inviolate mesocolon with high tie ligation, so to improve the quality of the resection specimen produced; up-to-date anatomo-embryological concepts are analyzed in detail, focusing on the latest studies of the mesenteric organ, its dissection by mesofascial and retrofascial cleavage planes, and questioning the need for a new terminology in colonic resections. The rationale behind the CME with CVL is explained and particular attention is paid to the current surgical techniques. The impact on local recurrence, disease-free and overall survival is reviewed. Current literature about laparoscopic CME with CVL demonstrated better quality of the surgical specimen produced and significant survival advantage when compared to standard non-mesocolic resections, stressing the importance of meso-resectional surgery, especially when performed with minimally invasive techniques: higher surgical quality, faster recovery and better immunological response may in fact contribute to better long term oncologic outcome.
- Citation: Siani LM, Garulli G. Laparoscopic complete mesocolic excision with central vascular ligation in right colon cancer: A comprehensive review. World J Gastrointest Surg 2016; 8(2): 106-114
- URL: https://www.wjgnet.com/1948-9366/full/v8/i2/106.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i2.106
At the end of the 19th century, Emil Theodor Kocher[1,2], was the first to theorize oncologic resections based on removal of the involved organ along with its lymphatic drainage; this concept was shortly after substantiated by Miles et al[1] and Jemison et al[2] for rectal and colonic cancer respectively. Yet, the real revolution in oncologic surgery was performed seventy years later by Heald et al[3], who introduced the concept of total excision of the mesorectum (TME), the primitive embryological dorsal mesentery of the rectum: Dissection in the mesorectal plane yields an intact fascial-lined specimen containing all the vasculo-lymphatic pathways and lymph nodes, and reduces the risk of an involved circumferential resection margin (CRM)[3,4]. The embryological right plane of dissection, graded by the pathologist, has been shown to be independently related to the risk of local recurrence, disease free and overall survival[5,6], so to promptly became the central part of any multimodal treatment of rectal cancer[7].
In 2009, Hohenberger et al[8] translated the concept of TME to colonic cancer, noting that traditionally more favorable oncologic results of colon neoplasia was eventually overtaken by rectal cancer: Multimodal strategies, not yet applied to colonic tumors, and a more radical surgical approach performed along embryonic planes of development with higher quality specimens, produce better oncologic outcome; thus, complete mesocolic excision (CME) with central vascular ligation (CVL) was theorized, standardized and eventually validated by several studies[9,10].
The concept of complete excision of the involved organ along with its primitive mesentery, associated to central ligation of the supplying blood vessels, is progressively gaining acceptance as the next step towards a modern surgical oncology; surgical resection of the primitive embryological mesenterium is in fact pivotal for optimal local clearance. The primitive mesenterium is the embryological envelope where the neurolymphovascular structures develop within a double-layered mesenchymal fibrofatty tissue and the initial pathway for cancerous diffusion: Its intact, complete excision is thus essential to clear residual disease in the surgical field, with consequent impact on local control.
Furthermore, CVL allows for an extensive lymph node dissection along the feeding vessels, with significant effect on regional recurrence and systemic dissemination, as shown by improved survival in stage I-III colonic cancers treated with enhanced lymph node harvesting[11,12].
Blending Complete Mesocolic Excision with CVL is thus the logical step in gaining the highest loco-regional control, removing both the intact mesocolon and the apical nodes, with relevant impact on long term outcome. To take advantage of minimally invasive techniques, laparoscopic approach to CME with CVL seems the natural consequence in the evolution of this procedure.
The mesocolon is the adult remnant of the primitive dorsal mesentery[13-19]. In the 5 mm embryo (approximately 32 d), the colon develops within a dorsal mesentery for all its length; an approximately 270° counterclockwise rotation of the primitive mid-gut along the axis of the superior mesenteric artery (SMA) causes the folding of the dorsal mesentery, originating the future mesocolon[13-19].
In 1885, Treves[20] stated that the right mesocolon fuses with the primitive posterior parietal peritoneum, with the consequent obliteration of the space between these embryonic structures. This view of mesenteric obliteration through a process of fusion was than refuted in the early ‘900 by the study of Carl Toldt[21] and Congdon[22], who affirmed that the mesentery of the colon persists in adulthood not only at the level of the transverse and sigmoid colon, but all along its length, being separated from the posterior parietal peritoneum by a loose areolar connective plane referred to as Toldt’s fascia. Later on, Goligher[23] described the possibility of stripping back the colon and its meso towards the midline, restoring the primitive embryological disposition before its rotation, confirming the Toldt’s and Congdon’s remarks.
The increasing focus on the quality of the surgical specimen as an independent variable in the outcome of cancer surgery stresses the need for a more detailed knowledge of the mesocolon anatomy.
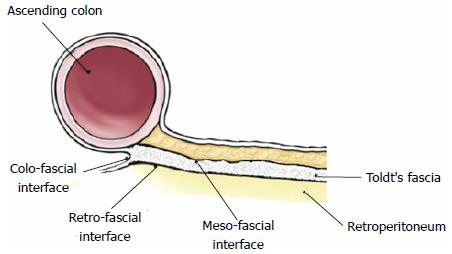
Recent papers demonstrated that the mesocolon persists in adulthood as a distinct anatomic structure, continuous from the ileocecal valve to the rectosigmoid junction, with well defined mesocolic, fascial, and retroperitoneal components and related mesofascial (the apposition between the Toldt’s fascia and the overlaying mesocolon) and retrofascial (the apposition between the Toldt’s fascia and the underlying retroperitoneum) interfaces (Figure 1): These latter are crucial for surgical planes in mesocolic and colonic mobilization[24,25].
Furthermore, recent studies[26,27] investigated the mesocolon by light and electron microscopy: Its structure is homogeneous across all locations and is composed of adipocyte lobules separated by thin fibrous septae layered by mesothelium, with lymphatic channels within this lattice; unexpectedly, a further connective, highly cellular submesothelial layer exists between surface mesothelium and the adipocytes.
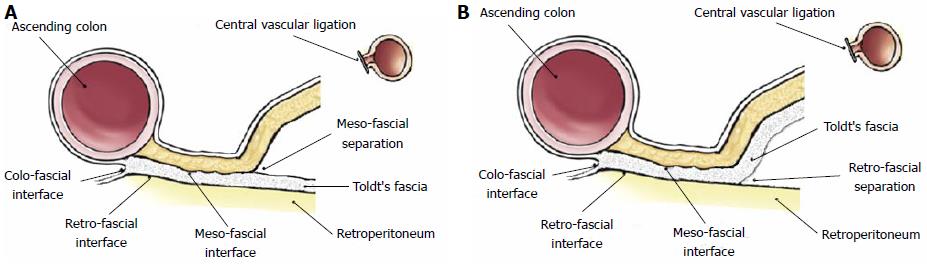
A detailed appraisal of the lymphatic network within the mesocolon by immunohistochemical analysis[28], showed that lymphatic vessels occur within both submesothelial connective tissue and interlobular septations, on average every 0.14 mm and within 0.1 mm from the mesocolic surfaces, generating a rich lymphatic network; the authors stressed that breaching the mesocolic surface extensively disrupts this lymphatic network. In the same study, lymphatic vessels were also identified within the Toldt’s fascia, with no direct communication with those in the mesocolon, and whose clinical significance (independent or integral part of the mesocolon) should be investigated with further dedicated works: In fact, in mesofascial separation, mesocolon and fascia are surgically separated with the Toldt’s fascia left in situ (Figure 2A), whereas in retrofascial separation the mesocolon/fascia complex is separated from the underlying retroperitoneum (Figure 2B); both separations are integral to CME as shown by Hohenberger et al[8], but the exact role of lymphatic channels within the Toldt’s fascia could define only retrofascial separation as an oncologically correct plane of resection.
Some authors[29-32] advocated the need for a new terminology in describing the mesocolon and its related surgical procedures: Visceral and parietal fascia, pre-renal fascia, parietal plane, somatic fascia may ingenerate confusion and should be standardized in the modern view of the mesenteric organ.
A surgical plane is defined as the interface between two contiguous structures, and in resectional colonic surgery the planes are (1) mesofascial; (2) retrofascial; and (3) colofascial, as shown in Figure 1. In keeping with this, a terminology of total or partial right (left) mesocolectomy has been proposed, being more informative than right (left) hemicolectomy or ilecocolic resection because entirely derived for the current anatomical appraisal of the mesenteric organ anatomy.
There are three essential components of CME with CVL: (1) development of a mesofascial or retrofascial plane to mobilize an intact and inviolate mesocolon as an intact package; (2) CVL with high tie to maximize the vertical lymph node dissection (central spread); (3) adequate length of bowel to remove pericolic lymphnodes, maximizing the longitudinal lymphnode harvesting (longitudinal spread).
CME allows for removal of the entire envelope of the primitive dorsal mesentery along the anatomo-embryological avascular cleavage planes, and is therefore fundamental for a true radical R0 resection, as the meso contains the whole potential routes of metastatic spread through lympho-vascular, neuro-perineural and fibro-fatty tissues[8-10]. The mesocolon must be excised as an intact, inviolate package as any breach of its surface and underlying structures threatens the radial margin and disrupts the lymphatic network of the meso-structure with consequent spillage of neoplastic cells within the surgical field, enhancing the risk of local recurrence. This concept stresses further the need for a correct surgical plane of resection to maximize the local clearance, exactly the same way we conceptually perform TME for rectal cancer: To reduce to reduce the risk of an involved CRM and minimize the risk of local failure[7].
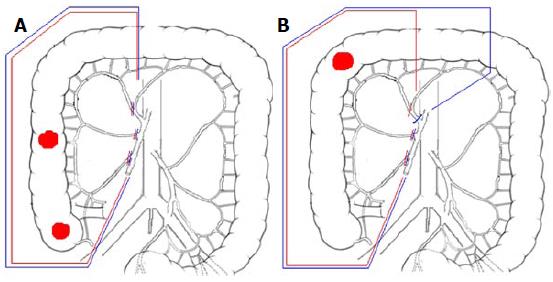
CVL is essential in obtaining an adequate regional control and impact on survival. The latest 2010 JSCCR guidelines recommends D2 dissection for clinically early stages colorectal cancers and D3 dissection for more advanced disease: Impressive results in terms of local recurrence and patients survival have been reported[33,34], also by Western authors who claim CME with CVL for right colonic cancer as oncologically effective as D3 right hemi-colecomy performed in Eastern Countries[8,35,36]. CVL could be crucial in micro-metastatic clearance of central nodes, which are frequently missed by routine histological examination[37], and thus responsible for loco-regional recurrence and systemic dissemination[34]. For cancers located in the hepatic flexure and proximal transverse colon, possibly because of an embryological coalescence of mesenteric fascia, metastatic nodes incidence of about 5% for subpyloric station and about 4% for right gastroepiploic arcade has been reported[38]: Central transection of middle colic vessels, ligation of right gastroepiploic vessels at the origin, 10 to 15 cm of greater omentectomy off the tumor and removal of subpyloric nodes could be beneficial, especially in advanced stages (clinically T3c-d and T4)[8,39], as shown in Figure 3.
Blending CME with CVL is thus the logical step to ensure the best loco-regional control: CME maximizes the local clearance of the surgical field both increasing the chance for an uninvolved CRM and limiting any neoplastic spillage; CVL enhances regional control, removing apical nodes along the surgical trunk of the superior mesenteric vein (SMV), preventing regional recurrence and systemic dissemination: This is probably plausible for cancer without spread beyond the primitive meso-structure, as macroscopic involvement of apical nodes carries a poor outcome, independently from the extension of the surgical resection[40].
Patient is administered general anesthesia and placed in the supine or lithotomy position; a pneumoperitoneum is maintained at 10-12 mmHg using CO2. The first step is always a thorough exploration of the abdominal cavity and peritoneal washing for cytology.
Once created the working space, a medial to lateral technique is generally adopted: The ileocolic vessels are stretched so to delineate the Treves arcade, and peritoneal incision is commenced at the base of the created peritoneal fold; dissection of the anterior peritoneal leaf is performed along the left margin of the SMA, with transection of the ilecocolic and of the inconstant right colic vessels at their roots. An en-bloc lymphadenectomy of the anterior aspect of the SMV from the ileocolic vessels to the gastro-colic trunk of Henle is preformed (D3 lymph node dissection).
The anatomo-embryological plane along the Toldt’s fascia is sharply divided from medial to lateral and from bottom to top along the meso-fascial or retro-fascial plane, sometime mobilizing the duodenum, as suggested by Hohenberger et al[8], but usually dissecting along the plane between the intact dorsal mesocolon of the hepatic flexure and the Fredet’s pre-duodenopancreatic fascia; the meso-fascial or retro-fascial interface must be carefully identified and components separated without breaching of either, respecting the integrity of the right mesocolon and of the retroperitoneal structures such as right ureter and gonadal vessels. The dissection stops at the lateral aspect of the right colon (right lateral peritoneal fold) exposing the colo-fascial interface, which will be separated later.
In case of caecum or ascending colon cancer, the stretched transverse mesocolon is progressively transected with central ligation of the right branch of the middle colic vessels and the colon is stapled 10 cm off the tumor (Total Right Meso-colectomy; Figure 3, blue lines); for hepatic flexure or proximal colon transversum cancers, middle colic and right gastroepiploic vessels are ligated at their roots, subpyloric lymph nodes are removed, 10 to 15 cm of greater omentum off the tumor is excised and colon stapling is carried out just proximal to the splenic flexure (Total Extended Right Meso-colectomy; Figure 3, blue lines).
The hepatic flexure is mobilized by severing the lateral hepatocolic peritoneal fold, with the double components of the superior attachment (right phreno-colic ligament) and the medial attachment (cholecysto-duodeno-colic ligament); division of these peritoneal folds demonstrate the colo-fascial interface at this level, which can be easily mobilized.
The right lateral peritoneal fold and the ileocecal peritoneal folds (caecal ligaments) are progressively severed to obtain complete mobilization of the specimen.
The ileum is stapled at 10-15 cm from the ileocaecal valve and the specimen is extracted within a plastic bag through a protected mini-Pfannestiel incision; side-to-side mechanical intracorporeal or extracorporeal anastomosis is fashioned.
Some authors proposed their experience with modification of the classical approach in CME and CVL: Cho et al[41], adopted a modified CME in respect to 3 major aspects: (1) non performance of kocherization as described in the original paper of Hohenberger et al[8]; (2) clearance of the pre-renal soft tissue behind Gerota’s fascia for T3/4 cancer; and (3) tailored resection of the mesentery and mesocolon according to tumor location.
Feng et al[42] proposed a hybrid medial approach prospectively compared to a completely medial approach: The hybrid approach is based on a first up-to-bottom dissection (section of the gastrocolic ligament and dissection of the middle colic vessels and Henle’s trunk) blending with a subsequent classical medial-to lateral bottom-to-top approach; the study demonstrated less time for CVL and fewer vessel-related complications, especially for the pancreatico-duodenal vessels.
Matsuda et al[43] also stressed a cranio-to-caudal approach for total right meso-colectomy, noting that lymph node dissection around the middle colic vessels is technically demanding and potentially exposed to severe intra-operative bleeding: The author suggests a caudal traction of the mesocolon to detect the origin of the middle colic vessels, maneuver suitable for detecting easily various types of middle colic vein branching and thus reducing the risk of Henle’s trunk and/or pancreatico-duodenal vessels injury.
Laparoscopic CME with CVL, when performed in the right mesocolic plane, produces high quality surgical specimens. A grading system was developed in the CLASICC trial[44], with the aim to compare laparoscopically assisted surgery with open resection for colorectal cancer; it was based on translation of the grading system used in the MRC CR07 trial for rectal cancer[45]: (1) mesocolic plane of resection (“good” plane of surgery; intact, inviolate mesocolon with a smooth peritoneal-lined surface); (2) intramesocolic plane of resection (“moderate” plane of surgery; irregular breaches in the mesocolon, none reaching down to the muscularis propria of the viscus); and (3) Muscularis propria plane (“poor” surgical plane; disruption of the mesocolon down to the muscularis propria).
In the initial study of West et al[46], the mesocolic plane translated into a higher quality of the surgical specimen: Wider cross-sectional tissue around the muscularis propria (mean 2181 ± 895 mm2 compared to muscularis propria plane with a mean of 1447 ± 913 mm2; P = 0.0003), longer distance between the tumor and the mesocolic/retroperitoneal resection margin (44 ± 21 mm vs 21 ± 12 mm for muscularis propria plane, P < 0.0001), longer distance between the tumor and the high vascular tie and greater lymph node yield. The same group, in 2010 compared the quality of specimen between the Erlangen and Leeds experience, by precise tissue morphometry and grading of the surgical plane, concluding that CME with CVL routinely performed in Erlangen yields wider mesocolic area (19657 mm2vs 11829 mm2; P < 0.0001), longer large bowel (median, 314 mm vs 206 mm; P < 0.0001) and ileal (median, 83 mm vs 63 mm; P = 0.003) segment, higher distance between the tumor and the high vascular ties (131 mm vs 90 mm; P < 0.0001) and more lymph nodes harvested (median, 30 vs 18; P < 0.0001), reflecting in higher quality of the surgical specimens and better oncologic outcome[9]. In 2012, CME with CVL was compared to Japanese D3 resection, benchmark for highest survival reported in worldwide literature: Even in this case, CME with CVL showed wider mesocolic area (17957 mm2vs 8309 mm2, P < 0.001), longer bowel segment (324 mm vs 162 mm, P < 0.001) and greater nodal yield (32 vs 18, P < 0.001), but equivalent distance between the tumor and the high vascular tie not statistically different (100 mm vs 99 mm; P = 0.605), translating in similar impressive long term survival[10].
The higher quality of surgical specimen translates in better long term oncologic outcome, with significant impact on local recurrence rate, disease free and overall survival: In the pioneering studies of West[46,9,10], Mesocolic plane of surgery and high tie ligation showed a non-stratified 15% survival advantage at 5 years when compared to non-mesocolic planes of resection; interestingly, the survival boost was even more remarkable in the subset analysis for stage III patients, with an increased survival by 27% at 5 years.
These results were confirmed in subsequent studies comparing the different planes of resection, both in open[8-10,35,36,47] and laparoscopic surgery[40,48-55], reflecting a significant interest for the brilliant results of CME with CVL. Recently, two important studies further substantiated the effect of the correct plane of resection in colonic cancer: A systematic review on 5246 patients revealed a local recurrence rate, 5 years overall and disease free survival of 4.5%, 58.1% and 77.4% respectively[56]; and in 2015, a well structured population-based study by the Danish Colorectal Cancer Group[57] demonstrated a better disease-free survival for patients with stage I-III colonic cancer, suggesting that both laparoscopic and open CME with CVL may significantly improve outcome.
Unfortunately, these numerous studies (the most relevent reported in Table 1) have significant statistical power limitations, being predominately retrospective and non-homogeneous, so that at the moment a definitive high level of evidence cannot be drawn and thus no strong grade of recommendation may be assigned. This highlights the need for sufficiently powered randomized trials, to definitively address the issue and affirm with conclusive evidence that CME with CVL represents the gold standard in the surgical management of (right) colonic cancer.
| Ref. | Plane of surgery | High tie | LN harvesed | R0 | 5y LR | 5y OS | 5y DFS | |||||||
| West et al[9] | Ms | 90% | CVL | 13 cm | CVL | 30 | Ms | 94% | Ms | 4.90% | Ms | 85% | Ms | NR |
| NMs | 40% | Ctl | 9 cm | Ctl | 18 | NMs | 85% | NMs | NR | NMs | 70% | NMs | NR | |
| Hohenberger et al[8] | Ms | 100% | CVL | 13 cm | CVL | 32 | Ms | 97.40% | Ms | 4.90% | Ms | 85% | Ms | NR |
| NMs | 0% | |||||||||||||
| Siani et al[40] | Ms | 65% | CVL | 13 cm | CVL | 30 | Ms | 97% | Ms | NR | Ms | 82.60% | Ms | 73.80% |
| NMs | 35% | Ctl | 9 cm | Ctl | 18 | NMs | 85% | NMs | NR | NMs | 60% | NMs | 59.70% | |
| Kanemitsu et al[34] | Ms | 100% | CVL | NR | CVL | 31 | Ms | NR | Ms | 6% | Ms | 84.50% | Ms | 91.60% |
| NMs | 0% | |||||||||||||
| Liang et al[48] | Ms | 100% | CVL | NR | CVL | 34 ± 8 | Ms | NR | Ms | 2% | Ms | NR | Ms | NR |
| NMs | 0% | |||||||||||||
| Feng et al[53] | Ms | 94% | CVL | NR | CVL | 19 | Ms | NR | Ms | NR | Ms | NR | Ms | NR |
| NMs | 6% | Ctl | NR | Ctl | 14 | NMs | NR | NMs | NR | NMs | NR | NMs | NR | |
| Gouvas et al[55] | Ms | 68.70% | CVL | 8.7 cm | CVL | 33 | Ms | 85.70% | Ms | NR | Ms | NR | Ms | NR |
| NMs | 31.20% | Ctl | NR | Ctl | NR | NMs | NR | NMs | NR | NMs | NR | NMs | NR | |
| Adamina et al[54] | Ms | 100% | CVL | NR | CVL | 22 | Ms | 100% | Ms | NR | Ms | NR | Ms | NR |
| NMs | 0% | |||||||||||||
| Bertelsen et al[57] | Ms | 82% | CVL | NR | CVL | 36 | Ms | 97% | Ms | 11.30% | Ms | 74.90% | Ms | 85.80% |
| NMs | 18% | Ctl | NR | Ctl | 20 | NMs | 95% | NMs | 16.20% | NMs | 69.80% | NMs | 75.90% | |
| Shin et al[51] | Ms | 100% | CVL | NR | CVL | 27.8 ± 13.6 | Ms | NR | Ms | 3.60% | Ms | NR | Ms | 88% |
| NMs | 0% | |||||||||||||
| Bae et al[52] | Ms | 100% | CVL | NR | CVL | 28 | Ms | NR | Ms | NR | Ms | 90.30% | Ms | 83.30% |
| NMs | 0% | |||||||||||||
The current evidence shows the equivalence in terms of tissue morphometry, quality of the surgical specimen and long term oncologic results between laparoscopic and open techniques[39,43,46,52,55-57], but with laparoscopic approach offering all the advantages of minimally invasive surgery, both in faster recovery and in less immunological stress response which could affect long term outcome[58-62].
In the multimodal management of right sided colonic cancer, laparoscopic CME with CVL is progressively gaining a pivotal role on the base of high quality surgical specimen, better local recurrence rate, better 5 years overall and disease-free survival when compared to less radical planes of surgery.
Laparoscopic CME with CVL should be regarded as the new frontier of a modern, meso-resectional oriented surgery, with all the advantages of minimally invasive techniques, which allow for faster recovery and better immunological stress response: Higher quality of yielded surgical specimen, less complications when the laparoscopic procedure is embedded in an Enhanced Recovery After Surgery program[63-72] and better immuno-competence due to less surgical stress[58-62], may thus collectively contribute to better long term oncologic outcome.
Yet, in the absence of high level of evidence which precludes strong grade of recommendation, laparoscopic CME with CVL should be intensely investigated with highly powered, well structured prospective studies, so to define its role in the modern, multimodal management of right colonic cancer.
| 1. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 463] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Jamieson JK, Dobson JF. VII. Lymphatics of the Colon: With Special Reference to the Operative Treatment of Cancer of the Colon. Ann Surg. 1909;50:1077-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1965] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 4. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1932] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 5. | Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 685] [Article Influence: 28.5] [Reference Citation Analysis (9)] |
| 6. | Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 765] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 7. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2068] [Article Influence: 103.4] [Reference Citation Analysis (5)] |
| 8. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-364; discussion 364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1144] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 9. | West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol. 2012;30:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 11. | Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 858] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 12. | Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg. 2006;244:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Banister LH. Anatomy of the large intestine. New York: Churchill Livingstone 1995; 1774-1787. |
| 14. | Brookes M, Zietman A: Alimentary tract. In: Clinical Embryology, a Color Atlas and Text. Florida: CRC press 2000; 148-52. |
| 15. | Jorge JMN, Habr-Gama A. Anatomy and embryology of the colon, rectum and anus. New York: Springer 2007; 1-22. [DOI] [Full Text] |
| 16. | Sadler TW. The digestive system. In: Langman’s Medical Embryology, 9th edition. Lippincott Wilkins and Williams 2003; 285-321. |
| 17. | Fredet P. Peritoine. Morphogenese et morphologie. Fascia d‘accolement. Traite d‘Anatomie Humaine. 1900;4:869-1016. |
| 19. | Cochard LR. The GI system and abdominal wall. Netter’s Atlas of Human Embryology, revised edition. Philadelphia: Saunders 2012; 132-144. |
| 20. | Treves F. Lectures on the Anatomy of the Intestinal Canal and Peritoneum in Man. Br Med J. 1885;1:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Toldt C, Dalla Rosa A. An Atlas of Human Anatomy for Students and Physicians, revised edition. New York: Rebman Company 1919; 407-409. |
| 22. | Congdon ED, Blumberg R, Henry W. Fasciae of fusion and elements of the fused enteric mesenteries in the human adult. Am J Anat. 1942;70:251-279. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Goligher J. Treatment of carcinoma of the colon. London: Balliere Tindall 1984; 485-589. |
| 24. | Culligan K, Coffey JC, Kiran RP, Kalady M, Lavery IC, Remzi FH. The mesocolon: a prospective observational study. Colorectal Dis. 2012;14:421-428; discussion 428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (2)] |
| 25. | Culligan K, Remzi FH, Soop M, Coffey JC. Review of nomenclature in colonic surgery--proposal of a standardised nomenclature based on mesocolic anatomy. Surgeon. 2013;11:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Gao Z, Ye Y, Zhang W, Shen D, Zhong Y, Jiang K, Yang X, Yin M, Liang B, Tian L. An anatomical, histopathological, and molecular biological function study of the fascias posterior to the interperitoneal colon and its associated mesocolon: their relevance to colonic surgery. J Anat. 2013;223:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 27. | Culligan K, Walsh S, Dunne C, Walsh M, Ryan S, Quondamatteo F, Dockery P, Coffey JC. The mesocolon: a histological and electron microscopic characterization of the mesenteric attachment of the colon prior to and after surgical mobilization. Ann Surg. 2014;260:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Culligan K, Sehgal R, Mulligan D, Dunne C, Walsh S, Quondamatteo F, Dockery P, Coffey JC. A detailed appraisal of mesocolic lymphangiology--an immunohistochemical and stereological analysis. J Anat. 2014;225:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Sehgal R, Coffey JC. Historical development of mesenteric anatomy provides a universally applicable anatomic paradigm for complete/total mesocolic excision. Gastroenterol Rep (Oxf). 2014;2:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 30. | Coffey JC, Sehgal R, Culligan K, Dunne C, McGrath D, Lawes N, Walsh D. Terminology and nomenclature in colonic surgery: universal application of a rule-based approach derived from updates on mesenteric anatomy. Tech Coloproctol. 2014;18:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 31. | Sehgal R, Coffey JC. The development of consensus for complete mesocolic excision (CME) should commence with standardisation of anatomy and related terminology. Int J Colorectal Dis. 2014;29:763-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Sehgal R, Coffey JC. Standardization of the nomenclature based on contemporary mesocolic anatomy is paramount prior to performing a complete mesocolic excision. Int J Colorectal Dis. 2014;29:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Lee SD, Lim SB. D3 lymphadenectomy using a medial to lateral approach for curable right-sided colon cancer. Int J Colorectal Dis. 2009;24:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Kanemitsu Y, Komori K, Kimura K, Kato T. D3 Lymph Node Dissection in Right Hemicolectomy with a No-touch Isolation Technique in Patients With Colon Cancer. Dis Colon Rectum. 2013;56:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | West NP, Sutton KM, Ingeholm P, Hagemann-Madsen RH, Hohenberger W, Quirke P. Improving the quality of colon cancer surgery through a surgical education program. Dis Colon Rectum. 2010;53:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Bertelsen CA, Bols B, Ingeholm P, Jansen JE, Neuenschwander AU, Vilandt J. Can the quality of colonic surgery be improved by standardization of surgical technique with complete mesocolic excision? Colorectal Dis. 2011;13:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | van der Zaag ES, Bouma WH, Tanis PJ, Ubbink DT, Bemelman WA, Buskens CJ. Systematic review of sentinel lymph node mapping procedure in colorectal cancer. Ann Surg Oncol. 2012;19:3449-3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Toyota S, Ohta H, Anazawa S. Rationale for extent of lymph node dissection for right colon cancer. Dis Colon Rectum. 1995;38:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 39. | Søndenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler H, Brown G, Tudyka V, D’Hoore A, Kennedy RH. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery: proceedings of a consensus conference. Int J Colorectal Dis. 2014;29:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 40. | Siani LM, Pulica C. Laparoscopic complete mesocolic excision with central vascular ligation in right colon cancer: Long-term oncologic outcome between mesocolic and non-mesocolic planes of surgery. Scand J Surg. 2015;104:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Cho MS, Baek SJ, Hur H, Soh Min B, Baik SH, Kyu Kim N. Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: long-term outcomes and prognostic factors. Ann Surg. 2015;261:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Feng B, Ling TL, Lu AG, Wang ML, Ma JJ, Li JW, Zang L, Sun J, Zheng MH. Completely medial versus hybrid medial approach for laparoscopic complete mesocolic excision in right hemicolon cancer. Surg Endosc. 2014;28:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Matsuda T, Iwasaki T, Mitsutsuji M, Hirata K, Maekawa Y, Tanaka T, Shimada E, Kakeji Y. Cranial-to-caudal approach for radical lymph node dissection along the surgical trunk in laparoscopic right hemicolectomy. Surg Endosc. 2015;29:1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2325] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 45. | Quirke P, Sebag-Montefiore D, Steele R, Khanna S, Monson J, Holliday A, Thompson L, Griffiths G, Stephens R for the NCRI colorectal cancer study group and CR0 7 participants. Local recurrence after rectal cancer resection is strongly related to the plane of surgical dissection and is further reduced by preoperative short course radiotherapy. Preliminary results of the MRC CR07 trial. J Clin Oncol. 2006;24:A3512. |
| 46. | West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol. 2008;9:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 47. | Siani LM, Pulica C. Stage I-IIIC right colonic cancer treated with complete mesocolic excision and central vascular ligation: quality of surgical specimen and long term oncologic outcome according to the plane of surgery. Minerva Chir. 2014;69:199-208. [PubMed] |
| 48. | Liang JT, Lai HS, Huang J, Sun CT. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc. 2015;29:2394-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Mori S, Baba K, Yanagi M, Kita Y, Yanagita S, Uchikado Y, Arigami T, Uenosono Y, Okumura H, Nakajo A. Laparoscopic complete mesocolic excision with radical lymph node dissection along the surgical trunk for right colon cancer. Surg Endosc. 2015;29:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Kang J, Kim IK, Kang SI, Sohn SK, Lee KY. Laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc. 2014;28:2747-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Shin JW, Amar AH, Kim SH, Kwak JM, Baek SJ, Cho JS, Kim J. Complete mesocolic excision with D3 lymph node dissection in laparoscopic colectomy for stages II and III colon cancer: long-term oncologic outcomes in 168 patients. Tech Coloproctol. 2014;18:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol. 2014;21:2288-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 53. | Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP. Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc. 2012;26:3669-3675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Adamina M, Manwaring ML, Park KJ, Delaney CP. Laparoscopic complete mesocolic excision for right colon cancer. Surg Endosc. 2012;26:2976-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Gouvas N, Pechlivanides G, Zervakis N, Kafousi M, Xynos E. Complete mesocolic excision in colon cancer surgery: a comparison between open and laparoscopic approach. Colorectal Dis. 2012;14:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Kontovounisios C, Kinross J, Tan E, Brown G, Rasheed S, Tekkis P. Complete mesocolic excision in colorectal cancer: a systematic review. Colorectal Dis. 2015;17:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 58. | Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, Li J. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL, de Lange-de Klerk ES, Bonjer HJ, Bemelman WA, Cuesta MA. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 61. | Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ, vd Peet DL, vd Tol MP, Bonjer HJ, Cuesta MA. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011;26:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Sylla P, Kirman I, Whelan RL. Immunological advantages of advanced laparoscopy. Surg Clin North Am. 2005;85:1-18, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1813] [Article Influence: 62.5] [Reference Citation Analysis (4)] |
| 64. | Lovely JK, Maxson PM, Jacob AK, Cima RR, Horlocker TT, Hebl JR, Harmsen WS, Huebner M, Larson DW. Case-matched series of enhanced versus standard recovery pathway in minimally invasive colorectal surgery. Br J Surg. 2012;99:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Ramírez JM, Blasco JA, Roig JV, Maeso-Martínez S, Casal JE, Esteban F, Lic DC. Enhanced recovery in colorectal surgery: a multicentre study. BMC Surg. 2011;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, MacFie J, Liberman AS, Soop M. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 474] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 67. | Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, Macfie J, Liberman AS, Soop M. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37:259-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 868] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 68. | Wilmore DW. From Cuthbertson to fast-track surgery: 70 years of progress in reducing stress in surgical patients. Ann Surg. 2002;236:643-648. [PubMed] |
| 69. | Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 787] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 70. | Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, Gouma DJ, Bemelman WA. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 475] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 71. | Walter CJ, Collin J, Dumville JC, Drew PJ, Monson JR. Enhanced recovery in colorectal resections: a systematic review and meta-analysis. Colorectal Dis. 2009;11:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis. 2009;24:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chow CFK, Garg P, Mayol J, Yoshimatsu K S- Editor: Kong JX L- Editor: A E- Editor: Wu HL