Published online Jul 27, 2015. doi: 10.4240/wjgs.v7.i7.123
Peer-review started: February 27, 2015
First decision: April 27, 2015
Revised: May 26, 2015
Accepted: June 9, 2015
Article in press: June 11, 2015
Published online: July 27, 2015
Processing time: 150 Days and 12.2 Hours
We report two cases of delayed esophageal perforation occurring with endoscopic submucosal dissection. Our cases involved delayed perforation after 10 d in case 1 and after 6 d in case 2. Both cases were related to solid food. We performed subtotal esophagectomy with gastric tube reconstruction of the esophagus via the subcutaneous route anterior to the thoracic wall without conservative treatment because both cases involved chest pain and major leakage of food into the mediastinum. Postoperative complications were a local factor (including suture failure and esophageal stricture) in case 1, and we performed endoscopic balloon dilatation five times for esophageal stricture. There was no intrathoracic and mediastinal infection in either case. Surgical treatment for delayed esophageal perforation can be performed safely and surely if diagnosis and assessment are not delayed.
Core tip: Patients with early esophageal cancer often experience endoscopic submucosal dissection with complications, including bleeding, perforation, and stenosis. Although most cases are successfully treated conservatively, perforation is a life-threatening complication and can require surgical intervention. In our cases, we performed surgery approximately three hours after the patients’ complaints. Postoperative complications were a local factor (including wound infection and esophageal stricture) and did not include intrathoracic and mediastinal infection. Surgical treatment for delayed esophageal perforation can be performed safely and surely if diagnosis and assessment are not delayed.
- Citation: Matsuda Y, Kataoka N, Yamaguchi T, Tomita M, Sakamoto K, Makimoto S. Delayed esophageal perforation occurring with endoscopic submucosal dissection: A report of two cases. World J Gastrointest Surg 2015; 7(7): 123-127
- URL: https://www.wjgnet.com/1948-9366/full/v7/i7/123.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i7.123
Patients with early esophageal cancer often undergo endoscopic submucosal dissection (ESD) as a standard treatment. ESD for the esophagus has a high rate of complete resection and a low complication rate[1]. However, performing this procedure on the esophagus is technically difficult because the wall of the esophagus is thin. The risk is particularly high for superficially spreading esophageal cancer. The complications of ESD include bleeding, perforation, and stenosis. The most frequent ESD complication is stenosis (11.6%). Another complication, perforation, is not uncommon (5.0%), and is life threatening. These complications can be successfully treated without surgery in most cases[2]. However, delayed perforation is rare and can require surgical intervention. In this report, we describe our experience with the surgical treatment of delayed esophageal perforation after ESD, and we discuss management strategies.
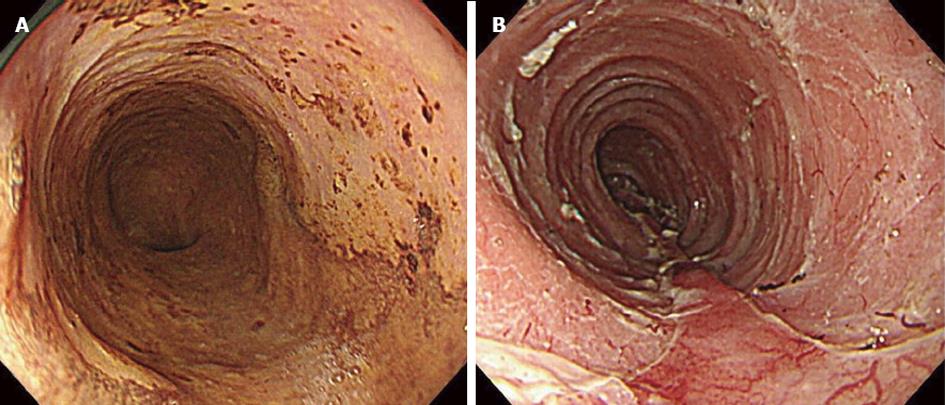
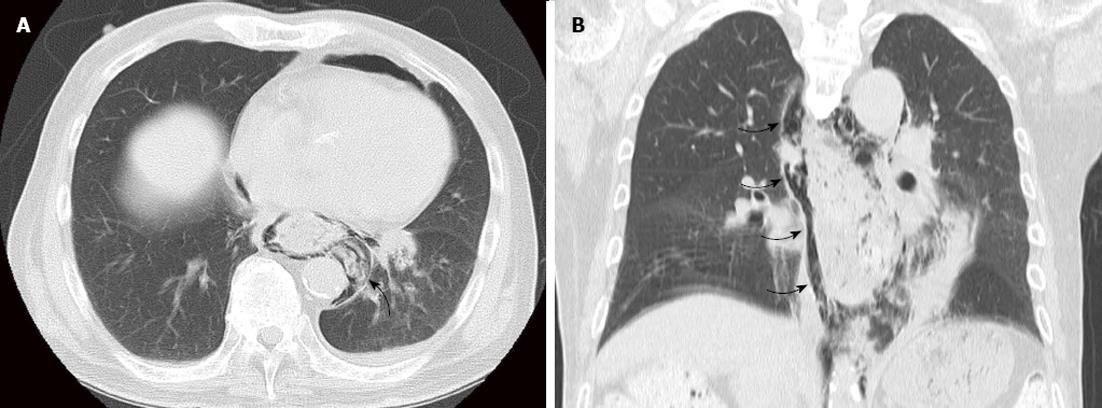
Case 1 was an 83-year-old man with diabetes mellitus who complained of heartburn. Esophagogastroduodenoscopy (EGD) was performed, and middle thoracic esophageal cancer was found (Type 0-IIc). The location was 25-28 cm from the incisor teeth, and the lesion covered three-quarters of the circumference (Figure 1). The depth of tumor invasion indicated that the tumor was in contact with or invaded the muscularis mucosa (M3), as revealed by endoscopic diagnosis. Enhanced computed tomography (CT) did not show a main lesion or lymph node metastases (cT1aN0M0 cStageI). We chose to perform ESD, and after resection of the lesion, we injected a steroid (triamcinolone acetonide; Kenacort®-A, Bristol-Myers Squibb, New York, NY, United States) into an artificial ulcer (Figure 1). The histopathological findings were 45 cm × 34 mm, SCC, pT1a-LPM, pHM (2 mm), pVM0, INFa, ly0, v0, and CurA. On day 6 after ESD, the patient began to eat a meal, and esophageal obstruction was suspected due to a complaint of a feeling of blockage while swallowing solid food. Although we performed EGD, stenosis was not found, and the resected area was cured. On day 10, the patient had sudden chest pain during dinner. Enhanced CT showed food residue in his mediastinum, and we diagnosed perforation of the esophagus (Figure 2). After approximately 3 h, we performed subtotal esophagectomy with gastric tube reconstruction of the esophagus via the subcutaneous route anterior to the thoracic wall without lymph node dissection. The operative duration was 385 min. The blood loss was 1040 cc (including pleural effusion), and 4 units of red cell concentrate were administered. The perforation extended from the right side to the posterior wall of the esophagus at the inferior mediastinum. The postoperative complications were wound infection and esophageal stricture. The patient did not have an intrathoracic and mediastinal infection. We performed endoscopic balloon dilatation (EBD) for esophageal stricture five times, and the patient was discharged 88 d after surgery.
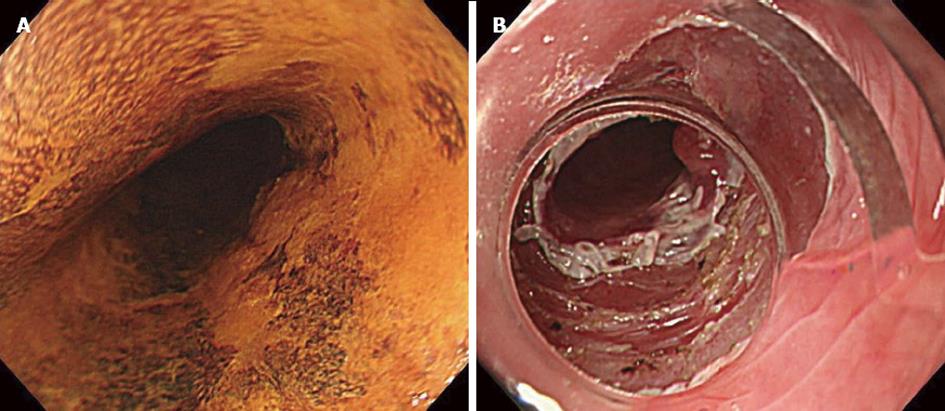
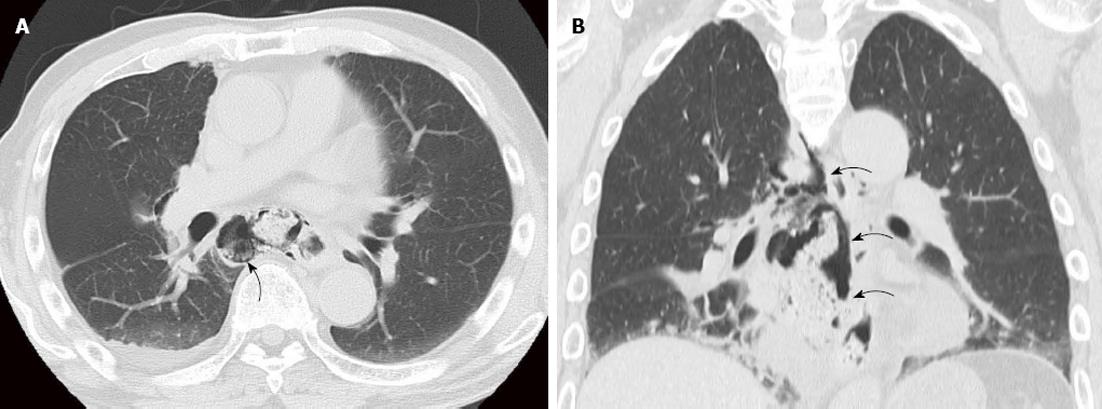
Case 2 was a 75-year-old man who had experienced ESD three times for upper and middle thoracic esophageal cancer over three years. Ten months after the last ESD, postoperative follow-up found middle thoracic esophageal cancer (Type 0-IIb). The location was 25-28 cm from the incisor teeth, and the extent of the lesion ranged over half of its circumference. The depth of tumor invasion was intraepithelial (M1) as revealed by endoscopic diagnosis. Enhanced CT did not show a main lesion or lymph node metastases (cT1aN0M0 cStageI). Although we performed ESD, we could not completely resect the lesion due to marked fibrosis (Figure 3). On postoperative day 3, the patient ate a meal and had no symptoms. On day 6, the patient had sudden chest pain during breakfast. Enhanced CT showed food residue in his mediastinum, and we diagnosed perforation of the esophagus (Figure 4). After approximately 3 h, we performed subtotal esophagectomy with gastric tube reconstruction of the esophagus via the subcutaneous route anterior to the thoracic wall with lymph node dissection. The operative duration was 390 min. The blood loss was 270 cc, and no transfusion was administered. The perforation involved the right side of the esophagus below the tracheal bifurcation. Histological examination revealed IIc, 4 mm × 4 mm, SCC, pT1a-EP, ly0, v0, pPM0 (100 mm), and pDM0 (150 mm). There were no postoperative complications in the hospital. The patient did not have an intrathoracic and mediastinal infection and was discharged 47 d after surgery.
ESD is a popular endoscopic procedure for the stomach and colon. ESD in the esophagus is accompanied by technical difficulties. Recently, the application of ESD for esophageal lesions has been reported. In the 2007 guidelines of the Japanese Esophageal Society, the absolute indications for this procedure were M1 and tumors invading the lamina propria (M2) that spread to less than two-thirds of their circumference. The relative indication for this procedure was M3 without clinical lymph node involvement[3]. In case 1, the depth of tumor invasion was M3, and the lymph nodes had no metastases. We selected ESD because the patient was elderly. In case 2, we performed ESD because the depth of tumor invasion was M1, and the lymph nodes had no metastases.
The complications of ESD in the esophagus are bleeding, perforation, and stenosis. The most common complication is stenosis, which is reported in 11.6% of cases[2]. Near-circumferential lesions are a risk factor for stenosis, which has been reported in 45% of such cases[4]. Takeuchi et al[5] reported that a steroid injected into the remaining submucosal layer of the post-ESD ulcer base was very effective at preventing postoperative stricture after esophageal ESD.
The rate of perforation is low (5.0%) and is caused by perioperative perforation of the ESD and dilation of esophageal strictures[2,6]. Delayed perforation has been reported following the injection of steroids into the deeper layer of the ulcer base and food bolus obstruction[5,7]. Tumor size was not shown to influence the incidence of perforation[1]. In our cases, both perforations were related to solid food. Additionally, a steroid might have been involved in case 1, and fibrosis from a past ESD might have been involved in case 2.
Most perforation cases are treated conservatively, and surgery is rare[2]. Conservative treatment can be selected in cases of effective endoscopic clipping, non-severe mediastinitis related to minor leaks, and stable vital signs. When conservative treatment (such as fasting, intravenous administrations of antibiotics, and drainage) has been ineffective for several days, surgical treatment should be selected[8,9]. Otherwise, the patient is at a risk. Lee et al[10] reported that their patient with a perforation developed unstable vital signs during endoscopic clipping, which resulted in an urgent operation. We selected surgery from the outset because the reported chest pain was strong, and enhanced CT showed major leakage of food into the mediastinum. There was no delay in diagnosis or in treatment in either case. Although the surgery was an invasive treatment, postoperative complications were the only local factor (wound infection and esophageal stricture), and the patients’ general conditions were stable after surgery. Conservative treatment should not be performed without safety and surety because of the attendant risks[8-10].
In conclusion, surgical treatment for delayed esophageal perforation occurring with ESD can be performed safely when diagnosis and assessment are not delayed.
Case 1 was an 83-year-old man with diabetes mellitus who complained of heartburn. Case 2 was a 75-year-old man who had experienced endoscopic submucosal dissection (ESD) three times for upper and middle thoracic esophageal cancer over three years.
Delayed esophageal perforation occurring with endoscopic submucosal dissection.
Mediastinitis, empyema, pneumonia.
Tumor makers were within normal limits.
Computed tomography scan did not show a main lesion or lymph node metastases (cT1aN0M0 cStageI).
In case 1, the histopathological findings were 45 mm × 34 mm, SCC, pT1a-LPM, pHM (2 mm), pVM0, INFa, ly0, v0, and CurA. In case 2, histological examination revealed IIc, 4 mm × 4 mm, SCC, pT1a-EP, ly0, v0, pPM0 (100 mm), and pDM0 (150 mm).
The authors performed sub total esophagectomy with gastric tube reconstruction of the esophagus via the subcutaneous route anterior to the thoracic wall.
Delayed esophageal perforation occurring with endoscopic submucosal dissection is rare.
In the 2007 guidelines of the Japanese Esophageal Society, the absolute indications for this procedure were M1 and tumors invading the lamina propria (M2) that spread to less than two-thirds of their circumference.
Food bolus obstruction may be related to delayed esophageal perforation occurring with ESD.
This is a nice case report on the subject of esophageal perforation after endoscopic submucosal dissection.
| 1. | Sun F, Yuan P, Chen T, Hu J. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. J Cardiothorac Surg. 2014;9:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci. 2014;59:1862-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Asano M. Endoscopic submucosal dissection and surgical treatment for gastrointestinal cancer. World J Gastrointest Endosc. 2012;4:438-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Tang B, Bai JY, Zhao XY, Fan CQ, Yang X, Deng L, Yang SM, Yu J. Endoscopic submucosal dissection for superficial esophageal cancer with near-circumferential lesions: our experience with 40 patients. Surg Endosc. 2015;29:2141-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Takeuchi M, Hashimoto S, Kobayashi M, Sato Y, Narisawa R, Aoyagi Y. Prevention of subsequent stricture formation after esophageal endoscopic submucosal dissection. Japanese Gastroenterol Endosc. 2011;53:3064. [DOI] [Full Text] |
| 6. | Takahashi H, Arimura Y, Okahara S, Uchida S, Ishigaki S, Tsukagoshi H, Shinomura Y, Hosokawa M. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy. 2011;43:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Sato H, Inoue H, Ikeda H, Grace R Santi E, Yoshida A, Onimaru M, Kudo S. Clinical experience of esophageal perforation occurring with endoscopic submucosal dissection. Dis Esophagus. 2014;27:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-264, 264.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (5)] |
| 9. | Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, Koch S, Houcke P, Cellier C, Heresbach D. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Lee CT, Chang CY, Tai CM, Wang WL, Tseng CH, Hwang JC, Lin JT. Endoscopic submucosal dissection for early esophageal neoplasia: a single center experience in South Taiwan. J Formos Med Assoc. 2012;111:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
P- Reviewer: Chen YJ, Medina-Franco H S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/