Published online Apr 27, 2015. doi: 10.4240/wjgs.v7.i4.60
Peer-review started: July 18, 2014
First decision: December 17, 2014
Revised: January 21, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: April 27, 2015
Processing time: 264 Days and 20 Hours
Pancreatic neoplasms producing exclusively glucagon associated with glucagon cell hyperplasia of the islets and not related to hereditary endocrine syndromes have been recently described. They represent a novel entity within the panel of non-syndromic disorders associated with hyperglucagonemia. This case report describes a 36-year-old female with a 10 years history of non-specific abdominal pain. No underlying cause was evident despite extensive diagnostic work-up. More recently she was diagnosed with gall bladder stones. Abdominal ultrasound, computerised tomography and magnetic resonance imaging revealed no pathologic findings apart from cholelithiasis. Endoscopic ultrasound revealed a 5.5 mm pancreatic lesion. Fine needle aspiration showed cells focally expressing chromogranin, suggestive but not diagnostic of a low grade neuroendocrine tumor. OctreoScan® was negative. Serum glucagon was elevated to 66 pmol/L (normal: 0-50 pmol/L). Other gut hormones, chromogranin A and chromogranin B were normal. Cholecystectomy and enucleation of the pancreatic lesion were undertaken. Postoperatively, abdominal symptoms resolved and serum glucagon dropped to 7 pmol/L. Although H and E staining confirmed normal pancreatic tissue, immunohistochemistry was initially thought to be suggestive of alpha cell hyperplasia. A count of glucagon positive cells from 5 islets, compared to 5 islets from 5 normal pancreata indicated that islet size and glucagon cell ratios were increased, however still within the wide range of normal physiological findings. Glucagon receptor gene (GCGR) sequencing revealed a heterozygous deletion, K349_G359del and 4 missense mutations. This case may potentially represent a progenitor stage of glucagon cell adenomatosis with hyperglucagonemia in the absence of glucagonoma syndrome. The identification of novel GCGR mutations suggests that these may represent the underlying cause of this condition.
Core tip: We identify novel mutations in the glucagon receptor gene in a patient with hyperglucagonemia but no glucagonoma syndrome. Physicians dealing with pancreatic disorders should be aware of this unusual condition.
- Citation: Miller HC, Kidd M, Modlin IM, Cohen P, Dina R, Drymousis P, Vlavianos P, Klöppel G, Frilling A. Glucagon receptor gene mutations with hyperglucagonemia but without the glucagonoma syndrome. World J Gastrointest Surg 2015; 7(4): 60-66
- URL: https://www.wjgnet.com/1948-9366/full/v7/i4/60.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i4.60
Glucagon cell adenomatosis has been reported by Henopp et al[1] as an independent previously unrecognised disease of the endocrine pancreas. Multiple pancreatic neoplasms exclusively producing glucagon, associated with glucagon cell hyperplasia of the islets and unrelated to multiple endocrine neoplasia (MEN) type 1 (MEN 1), p27 MEN or von Hippel-Lindau (VHL) syndromes, are the hallmarks of the condition[2]. To date very few such patients have been reported[1,3,4].
Most patients present with abdominal pain and increased serum glucagon levels but fail to exhibit the characteristics of the glucagonoma syndrome (necrolytic migratory erythema, diabetes mellitus, stomatitis and weight loss)[5]. While macroscopic tumors are evident on imaging in some, numerous microadenomas scattered throughout the pancreas and enlarged islets are the findings in others[1,3,4]. Malignancy has not been identified in any cases reported to date. The underlying cause of glucagon cell hyperplasia and consequent development of glucagon cell neoplasia without the glucagonoma syndrome remains unknown. Yu et al[3], Zhou et al[6] have proposed that malfunction of the glucagon receptor (GCGR) and/or glucagon may be responsible for the disease after detection of a homozygous missense mutation, c.256C>T (P86S) in the GCGR of a patient.
We present another example of hyperglucagonemia without morphological evidence of neoplasia or the glucagonoma syndrome in which we identified GCGR mutations which may represent the underlying pathogenic cause of the condition.
A 36 years old Caucasian female with no previous medical or known family history was referred to us in 2011 with a 10 year history of non-specific diffuse abdominal pain. She repeatedly underwent complete gastrointestinal diagnostic work-up over a period of 8 years which revealed no pathologic results. In 2009, she had been diagnosed with cholelithiasis on abdominal ultrasound. Upon referral to our centre in 2011, extensive investigations including upper and lower intestinal endoscopy, computerised tomography and magnetic resonance imaging (MRI) were carried out. Apart from the previously diagnosed cholelithiasis, no other pathology was evident. Endoscopic ultrasound (EUS) confirmed calculi in the gallbladder and a mild dilatation of the distal common bile duct. In addition, a 5.5 mm hypoechoic lesion with irregular margins was detected in the pancreatic tail. Fine needle aspiration (FNA) revealed cells focally expressing chromogranin A. The features were suggestive but not diagnostic of a low grade neuroendocrine tumor. Somatostatin receptor scintigraphy showed no foci of increased uptake. While serum gastrin, vasoactive intestinal polypeptide, somatostatin, and pancreatic polypeptide were within the normal range, glucagon was elevated to 66 pmol/L (normal: 0-50 pmol/L). Serum fasting and postprandial glucose was normal. Neuroendocrine tumor markers chromogranin A and chromogranin B were not elevated. At laparotomy, a sub-centimeter lobulated lesion was found at the inferior margin of the pancreatic tail corresponding with the lesion identified on EUS. No further lesions were identified in the remaining pancreas after meticulous bimanual exploration and intraoperative ultrasound. There were no enlarged peripancreatic lymph nodes. The pancreatic tail lesion was enucleated and cholecystectomy performed. A grade 1 pancreatic fistula developed postoperatively and resolved within 2 wk. The further course was uneventful and the patient was entirely asymptomatic. Moreover, she reported that the abdominal pain she experienced over the last decade had completely disappeared. Serum glucagon was assessed 1 mo postoperatively after the pancreatic morphology returned to normal on imaging. It was found to have decreased to 7 pmol/L. Serum glucagon was monitored at regular intervals (see Table 1). At the last follow-up, 31 mo after surgery, the patient remained asymptomatic with a normal MRI result, serum glucagon was 10 pmol/L and insulin was within the normal range.
| Time | Serum glucagon (pmol/L) (normal range: 0-50 pmol/L) |
| Pre-surgery | |
| 2 mo | 66 |
| Post-surgery | |
| 1 mo | 7 |
| 5 mo | 28 |
| 6 mo | 6 |
| 17 mo | 15 |
| 20 mo | 29 |
| 31 mo | 10 |
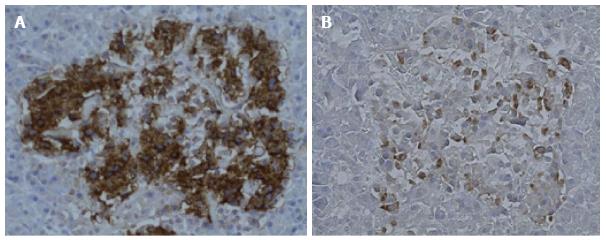
Histology (H and E) showed features of normal pancreatic tissue. Immunohistochemical examination for glucagon and insulin was undertaken using the technique of Henopp et al[1]. Approximately 20% of the islet cells were glucagon positive and 80% insulin positive. Glucagon cell hyperplasia was initially considered (Figure 1). In order to investigate this further, glucagon cell counts were done with 5 islets from 5 normal pancreatic controls and compared to 5 islets from the patient (Table 2). The counts showed that the average islet size and the average number of glucagon positive cells per islet were increased in the patient, however still within the wide range observed in normal pancreatic tissue.
A peripheral blood sample was obtained from the patient, her daughter and a healthy individual as a normal control (informed consent obtained). Genomic DNA (gDNA) was extracted using the DNeasy Blood and Tissue Kit according to the protocol (Qiagen, catalogue number: 9506). Polymerase chain reaction (PCR) amplification of exons 2-13 (and most of exon 14) of GCGR and the intron:exon borders was carried out using previously described primers[6]. Purified PCR products were sequenced by the W.M. Keck Biotechnology Resource Laboratory at Yale University, New Haven, United States using an automated Applied Biosystems 373A Stretch DNA sequencer (Perkin-Elmer, Norwalk, United States). PCR products were sequenced using forward primers. If ambiguous peaks were evident, the sequence was confirmed with the reverse primers[7]. Bioedit software was used to analyse the sequencing results[8]. Sequencing products were compared to the control sample and the national centre for biotechnology information (NCBI) reference sequences for the human GCGR, DNA (NG_016409.1), mRNA (NM_000160.3) and protein (NP_000151.1).
MEN1 sequencing was carried out on gDNA from the peripheral blood. PCR amplification of exons 2-10 of MEN1 was undertaken using previously described primers[9,10]. The DNA extraction, sequencing and analysis were carried out using the same technique as for GCGR. The reference sequence used was NCBI GenBank: U93237.1.
VHL sequencing was carried out on gDNA from the peripheral blood. PCR amplification of exons 1-3 of VHL was undertaken using primers previously described[11]. The DNA extraction, sequencing and analysis were carried out using the same technique as for GCGR. The reference sequence used was NCBI GenBank: NM_000551.3.
A heterozygous deletion of 33 nucleotides in exon 11 of the GCGR was detected. This corresponded to a K349_G359del in the GCGR with the loss of the following 11 amino acids, KSTLTLIPLLG. There were also 5 heterozygous point mutations including 4 missense mutations, E362K, V368L, K381E, S389N and 1 synonymous mutation (Figure 2). There were no mutations in MEN1 or VHL. No mutations were detectable in the daughter.
This case report represents the second case of hyperglucagonemia which has been associated with a specific genetic lesion in the GCGR. The case could potentially represent a progenitor stage of an entity leading to glucagon cell adenomatosis.
To date 8 individuals exhibiting characteristics of glucagon cell adenomatosis with hyperglucagonemia but without glucagonoma syndrome have been reported in the literature[1,3,4,6,12,13]. It is a matter of debate whether all cases cited completely fulfil the criteria of glucagon cell adenomatosis as defined by Henopp et al[1]. For example, the individual described by Yu et al[3] had not only raised serum glucagon levels but also pathologic values of pancreatic polypeptide. The patient reported by Balas et al[13] in 1988 had normal serum glucagon levels; however immunohistological findings in the resected pancreas were consistent with glucagon cell adenomatosis. In our patient, although the morphology of the resected pancreatic islets was within the broad range of findings reported in unaffected pancreata, we speculate that a cluster of hyperfunctioning cells might potentially be responsible for the development of hyperglucagonemia. Functional studies would be needed to confirm this theory.
The majority of individuals had glucagon cell adenomatosis, but were asymptomatic with respect to evidence of the glucagonoma syndrome. The results of imaging ranged from no pathologic findings to diffuse pancreatic enlargement associated with multiple tumors of various sizes. Abdominal pain is present in most individuals as was the case in our patient (Table 3). While the case we present exhibited normal uptake on somatostatin receptor scintigraphy, diffusely increased uptake was reported on OctreoScan® in a patient with diffuse pancreatic enlargement and multiple tumors by Henopp et al[1]. In our patient the positive staining for chromogranin on FNA was thought to be suggestive of a neuroendocrine tumor. This might reflect the small number of cells obtained from the FNA, with a sampling error leading to a higher proportion of chromogranin positive cells (e.g., if FNA sampling comprised an islet). In comparison to two reported cases which had highly elevated serum glucagon levels, our patient had only slightly increased serum glucagon (Table 3). The lack of standardised serum glucagon reporting in the majority of cases and the small number of patients means it is difficult to tell if the levels in our patient were truly lower than average.
| Martignoni et al[4] | Henopp et al[1] (patient 2) | Yu et al[3], Zhou et al[6] | Present case | |
| Patient | 54, M | 43, F | 60, F | 36, F |
| Origin | - | - | Persian | Caucasian |
| Clinical symptoms | Abdominal pain Diarrhea1 | Abdominal pain | Abdominal pain Constipation | Abdominal pain |
| Serum Glucagon (pmol/L) | Elevated | Elevated (25-fold)2 | 170113 | 66 |
| Imaging | Negative | Positive | Positive | Negative (positive on EUS) |
| OctreoScan® | Negative | - | Negative | Negative |
| Localization | No focal abnormality | Tail | Uncinate | Tail |
| Pancreatic pathology | α-cell hyperplasia nesidioblastosis | α-cell hyperplasia, large cystic tumor and small solid tumors, multiple microadenomas | α-cell hyperplasia non-functioning pancreatic NET microglucagonoma microadenoma | Normal pancreatic morphology on standard H and E staining |
| GCGR | - | - | Homozygous gDNA point mutation | Heterozygous gDNA deletion 5 point mutations |
| Other Genes | - | Negative for MEN1/VHL gDNA mutations | - | Negative for MEN1/VHL gDNA mutations |
| Relatives GCGR | - | - | Brother Negative | Daughter Negative |
The majority of previously reported patients demonstrated numerous microadenomas expressing almost exclusively glucagon and/or glucagon cell hyperplasia. This observation prompted Henopp et al[1,14] to postulate that diffuse glucagon cell hyperplasia might represent a precursor from of glucagon cell neoplasia. In the case described by Yu et al[3], 60%-80% of the hyperplastic islet cells stained positive for glucagon but negative for insulin. A similar trend was noted by Henopp et al[1]. In our patient, the pancreatic morphology was unusual, nevertheless still within the wide range of physiological findings. Approximately 20% of the islet cells expressed glucagon while 80% expressed insulin. Based on this observation and only mildly increased serum glucagon, we hypothesize that the disease might have been diagnosed at a very early stage prior to evidence of hyperplasic transformation and development of overt morphological evidence of neoplasia/s. While a subcentimeter nodule at the pancreatic tail was evident on EUS and confirmed intraoperatively, standard histology showed regular findings. This scenario resembles a report by Martignoni et al[4] of hyperglucagonemia but no microadenomas.
Both of the two previously reported patients for whom follow-up data was available showed increased serum glucagon levels after pancreatic resection in the presence of negative imaging results[1,3]. These findings underline the presumption of disease persistence. Our patient however, had normal serum glucagon levels at 31 mo after surgery (10 pmol/L) (Table 1). Due to the genetic predisposition of the disease we cannot exclude the possibility that at some point in the future the disease may recur therefore our patient requires life-long follow up. Any future increases in serum glucagon levels could potentially represent the emergence of alpha cell hyperfunction consistent with the concept of a residual genomic lesion representing a diffuse alpha cell abnormality in the remaining pancreatic islets.
The GCGR is a member of the class B G protein-coupled receptor family, glucagon binding triggers downstream signalling, allowing glucagon to regulate blood glucose levels by stimulating glycogenolysis[15,16]. The knockout mouse for GCGR expresses high glucagon levels associated with pancreatic enlargement, glucagon cell adenomatosis and microglucagonomas or glucagonomas at 10-12 mo when compared to their heterozygous littermates[17,18]. Based on these observations, Yu et al[3,6] sequenced GCGR and the glucagon gene in their patient with hyperglucagonemia, alpha cell hyperplasia and microglucagonoma. They detected a homozygous c.256C>T (P86S) mutation in GCGR resulting in lower binding affinity of GCGR P86S to glucagon and hypothesized that this mutation was responsible for the alpha cell hyperplasia and hyperglucagonemia. They showed in vitro that the GCGR P86S localized to the plasma membrane but bound glucagon with less avidity than wild type GCGR; a greater glucagon concentration was thus needed to trigger downstream signalling via adenylate cyclase activation[6]. Neuroendocrine cells undergoing hyperplastic changes is particularly relevant for MEN1 conditions however they probably also occur in sporadic cases. Very recently Klöppel et al[14] identified 3 further patients with germline GCGR mutations and glucagon cell adenomatosis unrelated to MEN1 or VHL syndromes. The genetic lesions present in the GCGR were not described, however a further 3 patients had glucagon cell adenomatosis in the absence of any GCGR mutation[14].
Our case represents the second case with genetic lesions described in the GCGR associated with hyperglucagonemia in the absence of the glucagonoma syndrome. The heterozygous K349_G359del and E362K, V368L, K381E, S389N mutations could potentially represent a loss of function mutation in the GCGR. Functional studies would be needed to show if these mutations might be the cause of the hyperglucagonemia observed in our patient. All mutations were in exon 11 towards the C terminal end of GCGR. The point mutations appear to represent rather conservative amino acid changes in terms of hydrophobicity. Lysine and glutamate have a positively and a negatively charged R group respectively and the serine to asparagine change represents an alteration from a hydroxyl R group to a carboxamide R group. Site directed mutagenesis studies have noted that D385 is relevant to the specificity of glucagon/GCGR binding[19]. Since this residue is close to the K381E mutation site and adjacent to the glucagon binding site, the alteration in R group may affect glucagon binding. However in the absence of high resolution crystal structure data for the human glucagon receptor (except for the extracellular N terminal domain) and site directed mutagenesis studies for these sites, the effects of these genetic changes cannot be directly inferred[15].
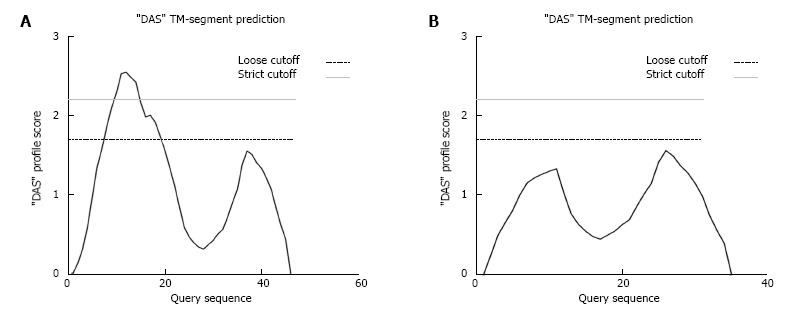
The K349_G359del falls within the 6th transmembrane domain of GCGR, therefore the 11 amino acid deletion could prevent GCGR from inserting into the plasma membrane. This would prevent GCGR binding to glucagon[20]. In structural studies where COS-1 cells were transfected with the rat glucagon receptor gene, truncation mutants lacking any of the different transmembrane domains, were not localized to the plasma membrane suggesting that all 7 transmembrane domains are needed for correct membrane insertion[21]. The K349_G359del mutation is predicted by membrane topology prediction software to prevent the GCGR from properly inserting into the plasma membrane[22] (Figure 3). If this was the case, then the GCGR would be miss-localized preventing glucagon binding. This would however need to be confirmed by in vitro protein localization studies and assays to check glucagon binding efficiency in the presence of the deletion. In addition, since the mutation in the GCGR is heterozygous, there would still be a normal gene copy present which might allow sufficient glucagon signalling via the remaining receptors to give normal function. However, the clinical pathology evident in the presence of hyperglucagonemia seems to suggest that this may not be the case.
The phenotype could potentially represent incomplete dominance leading to the modest elevation of serum glucagon in our patient. Alternatively, it is possible that this individual might have a second mutation in the other copy of the GCGR within some of the pancreatic alpha cells which could potentially be causing them to become hyperfunctional.
It has been previously suggested that incretin treatment is associated with the development of alpha cell hyperplasia since pancreata from autopsies of incretin treated persons exhibit alpha cell hyperplasia (and beta cell hyperplasia) and some had glucagon expressing microadenomas[23,24]. A possibility exists that as incretin usage increases, alpha cell hyperplasia may become more prevalent.
In conclusion, we have identified a novel heterozygous K349_G359 deletion and 4 missense mutations in the GCGR which appear to be associated with hyperglucagonemia without the glucagonoma syndrome. Physicians dealing with pancreatic disorders should be aware of this very unusual condition. Further study leading to a better understanding of this disease entity would be of benefit to patients. The further usage of GCGR sequencing in such individuals should be undertaken to provide additional information on the breadth of the spectrum of mutational abnormalities associated with alpha cell transformation and excess glucagon production.
36 years old patient with a 10 year history of non-specific diffuse abdominal pain.
A sub-centimeter lobulated lesion was found at the inferior margin of the pancreatic tail, no further lesions were identified in the remaining pancreas after meticulous bimanual exploration and intraoperative ultrasound.
Fine needle aspiration revealed cells focally expressing CgA. The features were suggestive but not diagnostic of a low grade neuroendocrine tumor.
Serum glucagon was elevated to 66 pmol/L (normal: 0-50 pmol/L). Other gut hormones were within the normal range.
Endoscopic ultrasound identified a 5.5 mm hypoechoic lesion with irregular margins in the pancreatic tail.
Histology (H and E) showed features of normal pancreatic tissue. Glucagon cell hyperplasia was initially considered based on glucagon immunohistochemistry. Further investigation revealed that the average islet size and the average number of glucagon positive cells per islet were increased in the patient, however still within the wide range observed in normal pancreatic tissue.
At laparotomy, a sub-centimeter lobulated lesion was found at the inferior margin of the pancreatic tail and was enucleated.
This is a very rare disease entity. Genetic lesions in the glucagon receptor (GCGR) have only been described in one individual in the literature in the context of glucagon cell adenomatosis with hyperglucagonemia but without glucagonoma syndrome. Several additional cases exhibiting the characteristics of glucagon cell adenomatosis with hyperglucagonemia but without glucagonoma syndrome have been published however their GCGR mutation status remains unknown.
The authors have identified novel GCGR mutations which appear to be associated with hyperglucagonemia without the glucagonoma syndrome. Physicians dealing with pancreatic disorders should be aware of this very unusual condition.
This is an interesting case of an entity not described before.
| 1. | Henopp T, Anlauf M, Schmitt A, Schlenger R, Zalatnai A, Couvelard A, Ruszniewski P, Schaps KP, Jonkers YM, Speel EJ. Glucagon cell adenomatosis: a newly recognized disease of the endocrine pancreas. J Clin Endocrinol Metab. 2009;94:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA. 2006;103:15558-15563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 404] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Yu R, Nissen NN, Dhall D, Heaney AP. Nesidioblastosis and hyperplasia of alpha cells, microglucagonoma, and nonfunctioning islet cell tumor of the pancreas: review of the literature. Pancreas. 2008;36:428-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Martignoni ME, Kated H, Stiegler M, Büchler MW, Friess H, Zimmermann A, Schirp U, Nitzsche EU. Nesidioblastosis with glucagon-reactive islet cell hyperplasia: a case report. Pancreas. 2003;26:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ. Glucagonoma and the glucagonoma syndrome - cumulative experience with an elusive endocrine tumour. Clin Endocrinol (Oxf). 2011;74:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Zhou C, Dhall D, Nissen NN, Chen CR, Yu R. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, alpha cell hyperplasia, and islet cell tumor. Pancreas. 2009;38:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer. 2005;103:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95-98. |
| 9. | Mayr B, Apenberg S, Rothämel T, von zur Mühlen A, Brabant G. Menin mutations in patients with multiple endocrine neoplasia type 1. Eur J Endocrinol. 1997;137:684-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Wang EH, Ebrahimi SA, Wu AY, Kashefi C, Passaro E, Sawicki MP. Mutation of the MENIN gene in sporadic pancreatic endocrine tumors. Cancer Res. 1998;58:4417-4420. [PubMed] |
| 11. | Huang Y, Zhou D, Liu J, Zhou P, Li X, Wang Z. Germline mutations of the VHL gene in seven Chinese families with von Hippel-Lindau disease. Int J Mol Med. 2012;29:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Anlauf M, Schlenger R, Perren A, Bauersfeld J, Koch CA, Dralle H, Raffel A, Knoefel WT, Weihe E, Ruszniewski P. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Balas D, Senegas-Balas F, Delvaux M, Bertrand C, Fagot-Revurat P, Rumeau JL, Ribet A. Silent human pancreatic glucagonoma and “A” nesidioblastosis. Pancreas. 1988;3:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Klöppel G, Anlauf M, Perren A, Sipos B. Hyperplasia to neoplasia sequence of duodenal and pancreatic neuroendocrine diseases and pseudohyperplasia of the PP-cells in the pancreas. Endocr Pathol. 2014;25:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Koth CM, Murray JM, Mukund S, Madjidi A, Minn A, Clarke HJ, Wong T, Chiang V, Luis E, Estevez A. Molecular basis for negative regulation of the glucagon receptor. Proc Natl Acad Sci USA. 2012;109:14393-14398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA. 2003;100:1438-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 474] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 18. | Yu R, Dhall D, Nissen NN, Zhou C, Ren SG. Pancreatic neuroendocrine tumors in glucagon receptor-deficient mice. PLoS One. 2011;6:e23397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Runge S, Gram C, Brauner-Osborne H, Madsen K, Knudsen LB, Wulff BS. Three distinct epitopes on the extracellular face of the glucagon receptor determine specificity for the glucagon amino terminus. J Biol Chem. 2003;278:28005-28010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Authier F, Desbuquois B. Glucagon receptors. Cell Mol Life Sci. 2008;65:1880-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Unson CG, Cypess AM, Kim HN, Goldsmith PK, Carruthers CJ, Merrifield RB, Sakmar TP. Characterization of deletion and truncation mutants of the rat glucagon receptor. Seven transmembrane segments are necessary for receptor transport to the plasma membrane and glucagon binding. J Biol Chem. 1995;270:27720-27727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Klammer M, Messina DN, Schmitt T, Sonnhammer EL. MetaTM - a consensus method for transmembrane protein topology prediction. BMC Bioinformatics. 2009;10:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Yu R. Pancreatic α-cell hyperplasia: facts and myths. J Clin Endocrinol Metab. 2014;99:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595-2604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 25. | Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947-2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21085] [Cited by in RCA: 21205] [Article Influence: 1116.1] [Reference Citation Analysis (0)] |
P- Reviewer: Guan YS S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/