Published online Jul 27, 2014. doi: 10.4240/wjgs.v6.i7.122
Revised: May 26, 2014
Accepted: June 20, 2014
Published online: July 27, 2014
Processing time: 201 Days and 19.9 Hours
Hepatic ischemia-reperfusion injury (IRI) is a pathophysiological event post liver surgery or transplantation and significantly influences the prognosis of liver function. The mechanisms of IRI remain unclear, and effective methods are lacking for the prevention and therapy of IRI. Several factors/pathways have been implicated in the hepatic IRI process, including anaerobic metabolism, mitochondria, oxidative stress, intracellular calcium overload, liver Kupffer cells and neutrophils, and cytokines and chemokines. The role of nitric oxide (NO) in protecting against liver IRI has recently been reported. NO has been found to attenuate liver IRI through various mechanisms including reducing hepatocellular apoptosis, decreasing oxidative stress and leukocyte adhesion, increasing microcirculatory flow, and enhancing mitochondrial function. The purpose of this review is to provide insights into the mechanisms of liver IRI, indicating the potential protective factors/pathways that may help to improve therapeutic regimens for controlling hepatic IRI during liver surgery, and the potential therapeutic role of NO in liver IRI.
Core tip: This review provides insights into several key mechanisms of liver ischemia-reperfusion injury, including the effects of anaerobic metabolism and the role of mitochondria, oxidative stress, intracellular calcium overload, liver Kupffer cells and neutrophils, and cytokines and chemokines; and summarizes the protective effects of nitric oxide.
- Citation: Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin MG, Li W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg 2014; 6(7): 122-128
- URL: https://www.wjgnet.com/1948-9366/full/v6/i7/122.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i7.122
In recent years, liver resection and liver transplantation have been widely adopted in clinical practice for the treatment of liver diseases. Hepatic ischemia-reperfusion injury (IRI) occurs substantially during liver resection or transplantation and remains a major cause of liver nonfunction or functional failure following liver surgery. This non-negligible injury has become a bottleneck which has restricted the use of marginal liver donors and the development of extensive liver resection. Hepatic IRI includes both warm and cold IRI - two types that share similar pathophysiological processes. The mechanisms of liver IRI have been widely investigated, but nevertheless remain largely unclear. The factors/pathways have been implicated in the hepatic IRI process include anaerobic metabolism, mitochondria, oxidative stress, intracellular calcium overload, liver Kupffer cells (KCs) and neutrophils, and cytokines and chemokines. More importantly, an effective prevention or treatment method is still lacking. Therefore, an effective method for preventing or minimizing hepatic IRI during liver surgery is urgently needed. A better understanding of the mechanisms in the development of IRI will provide insights into improving the treatment regimen for IRI. In this review, the authors comprehensively discuss the mechanisms of liver IRI and describe the role of nitric oxide (NO) in protecting the liver from IRI.
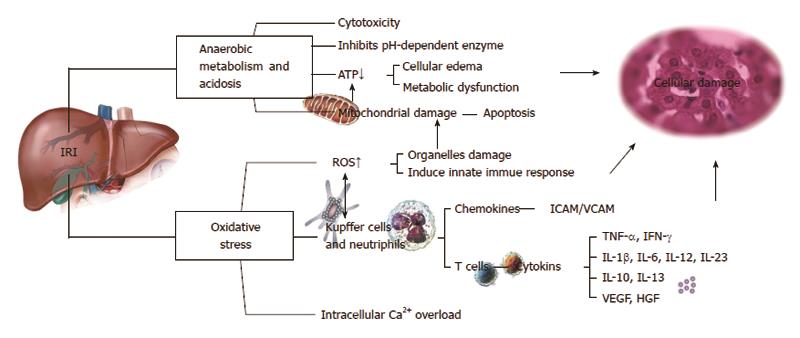
IRI exerts wide-ranging metabolic effects on the body. During the state of hepatic ischemia, the metabolic pattern is shifted from aerobic to anaerobic, the redox process of the hepatocytes is blocked, adenosine triphosphate (ATP)-dependent cellular metabolic activities are gradually stopped, and intracellular ATP is rapidly depleted. Conversely, there is accumulation of acidic metabolites, such as lactic acid and ketone bodies, which is caused by enhanced anaerobic glycolysis. This is accompanied by hypofunction of mitochondrial oxidative phosphorylation, resulting in the decrease of pH values between tissues and cells, known as metabolic acidosis. Studies have shown that this change plays a role in protecting the liver cells[1,2]. However, the pH values restore to normal after reperfusion, and further enhance pH-dependent enzyme activation, such as activation of proteases and phospholipases, further worsening the damage of tissues and organs. This is called the pH paradox[3]. The toxicity of acidic metabolites caused by a lower ATP supply mainly impairs the cellular functions of homeostasis, signaling interactions, and sodium/potassium ATPase (Na+/K+-ATPase), causing mitochondrial damage and resulting in microcirculation failure and cellular destruction[4].
IRI exerts effects not only on the body as a whole, but also at the cellular level. The mitochondria are the location where oxidative phosphorylation mainly takes place, and the mitochondria participate in multiple pathophysiological processes of IRI. A large number of reactive oxygen species (ROS) and reactive nitrogen species are generated in the mitochondria during the state of ischemia. Hypoxia undermines the process of oxidative phosphorylation in cells and obstructs the production of ATP, causing disorders of the cytoplasmic ions such as Ca2+, Na+, and H+ in the mitochondria, and finally leads to mitochondrial membrane permeability transition (MMPT)[5]. MMPT is manifested primarily by mitochondrial swelling and the decline of membrane potential[6], which allows soluble molecules of a molecular weight less than 1500 kDa to freely pass through the inner mitochondrial membrane, the so-called “mitochondrial megachannel”[7]. Many studies have indicated that MMPT is related to the process of hepatocyte damage after IRI[5,8].
IRI has many biochemical ramifications. It has been shown that oxidative stress plays a key role in reperfusion injury. Many highly reactive molecules, such as ROS, are induced during the period of hepatic IRI. ROS include superoxide anions, hydroxyl radicals, and peroxide hydrogen, and mainly act on proteins, enzymes, nucleic acids, cytoskeleton, and lipid peroxides, leading to mitochondrial dysfunction and lipid peroxidation[9]. ROS can also damage endothelial cells and destroy the integrity of the microvasculature. ROS can be reduced or overcome by reducing the blood flow and applying endogenous antioxidants, such as superoxide dismutase, catalase, glutathione, vitamin E, or beta-carotene[10]. On the other hand, application of recombinant adenovirus superoxide has been shown to effectively reduce hepatic IRI in mice[11].
Among the biochemical factors affected by IRI, calcium has an especially important role. The electrochemical gradient of the calcium ion plays an important role in maintaining homeostasis of physical calcium (Ca2+). If the calcium level is elevated when ischemia or hypoxia, oxidative stress, toxic substance release or other harmful events occur, this is called Ca2+ overload. Intracellular Ca2+ overload can activate Ca2+-dependent enzymes such as calpains, protein kinase C, and phospholipase C, and ultimately leads to cell death or apoptosis. Recent studies have shown that the increased amount of intracellular Ca2+ is not uniform, but is a local phenomenon. Non-specific calcium channel blockers can inhibit the elevation of intracellular Ca2+ and reduce cellular damage, demonstrating that Ca2+ influx may play a major role in the IRI process[12,13].
It has been demonstrated that liver KCs and neutrophils are involved in the hepatic IRI process. The KCs mainly mediate liver ischemic injury in the earlier stage of reperfusion (within 2 h) by synthesizing and releasing ROS and the pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1β to further activate liver sinusoidal endothelial cells, enhance the expression of the adhesion molecules intercellular adhesion molecule 1 (ICAM-1)/vascular cell adhesion molecule 1 (VCAM-1), further promote the adhesion, migration, and chemotaxis of neutrophils and endothelial cells, and accumulate and activate neutrophils, resulting in subsequent liver cell damage[14]. Studies have shown that endotoxins are also involved in the process of liver IRI[10,15]. Blocking KC activation by the use of gadolinium chloride or methyl palmitate can reduce acute liver cell injury significantly. Activation of neutrophils can directly damage liver cells by the release of oxidants and proteases after reperfusion. Ultimately, myeloperoxidase (halide form, such as Cl-) released from neutrophils changes hydrogen peroxide (H2O2) into hypochlorous acid (HOCl), which is a potent oxidant. These oxidants can directly cause liver cell damage and/or induce protease-mediated injury through inactivation of the endogenous anti-protease system[15,16], suggesting that anti-oxidant or anti-protease therapy would be helpful for preventing IRI.
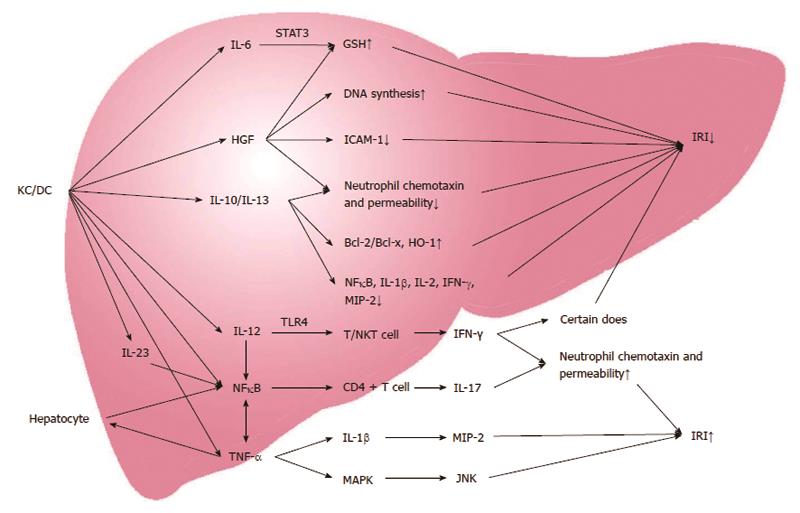
Cytokines play a dual role of anti-inflammatory and pro-inflammatory responses in the process of liver IRI (Figure 1). TNF-α is a key member of the group of endogenous pro-inflammatory and anti-inflammatory molecules, and is a critical factor in triggering the inflammatory cascade. It is secreted by activated KCs and impacts liver tissue and distant organs through paracrine signaling and the endocrine system[17]. TNF-α can bind to the receptors on the surface of liver cells to induce overproduction of the chemokine epithelial neutrophil activating protein-78 (ENA-78) and ROS, activate nuclear factor (NF)-κB, mitogen-activated protein kinase, and c-Jun N-terminal kinase (JNK), and cause liver injury directly[18]. In addition, TNF-α also can upregulate expression of the chemokines ICAM-1, VCAM-1 and P-selectin[19]. Moreover, JNK and ROS can directly act on liver cells to cause liver damage.
In addition to TNF-α, the other important cytokines involved in liver IRI are interferon-gamma (IFN-γ), IL-1β, IL-6, IL-12, IL-23, IL-10, IL-13, vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF). These cytokines promote leukocyte activation in the liver after ischemia through various pathways. IFN-γ is mainly produced by T cells and natural killer T cells, and activated by toll-like receptor-4 and IL-12. IFN-γ can either aggravate liver damage or reduce liver damage through enhancing or downregulating neutrophil accumulation and activation in a dose-dependent manner[20]. IL-1β, IL-6, IL-12, and IL-23 are mainly produced by KCs and hepatocytes. IL-1β can upregulate NO synthesis through the protein kinase B (Akt), NF-κB, and inducible nitric oxide synthase (iNOS) pathways. IL-1β can further upregulate leukocyte aggregation and adhesion by activating NF-κB and macrophage inflammatory protein (MIP)-2, thus damaging the liver cells[21]. IL-12 and IL-23 can also increase TNF-α production by activating NF-κB and signal transducer and activator of transcription (STAT)-4, and further stimulating CD4 T cells to produce IL-17, ensuring the accumulation of neutrophils and aggravating liver damage[22].
On the contrary, IL-6 can activate STAT-3, upregulate glutathione (GSH) expression, and downregulate oxidative stress markers, thus reducing hepatocyte damage and promoting hepatocyte proliferation[23]. IL-10 and IL-13 are mainly produced by KCs and T lymphocytes, and also play a role in alleviating liver damage and promoting liver regeneration. The protective role of IL-10 and IL-13 is mainly mediated by upregulation of heme oxygenase (HO)-1, B-cell lymphoma (Bcl)-2/bcl-x, and downregulation of NF-κB, IL-1β, IL-2, IFN-γ, MIP-2, cytokine-induced neutrophil chemotaxin, E-selectin, and neutrophil aggregation[24,25].
VEGF can be produced by many types of cells including KCs, T cells, sinusoidal endothelial cells and hepatocytes. It plays dual functions in liver IRI. IRI triggers the VEGF receptor and Src tyrosine kinase activation, and upregulates the expression of TNF-α, INF-γ, monocyte chemoattractant protein-1 and E-selectin, all of which result in the accumulation of intrahepatic T lymphocytes, macrophages and neutrophils, producing liver damage. On the other hand, exogenous administration of VEGF can upregulate iNOS production and protect the liver from IRI[26].
HGF is produced by liver non-parenchymal cells, mainly KCs. HGF can increase hepatocyte DNA synthesis, proliferation, and glutathione expression, downregulate the expression of the oxidative stress marker ICAM-1 in sinusoidal endothelial cells, and inhibit cytokine-induced neutrophil chemotaxin and neutrophil permeability, further reducing liver damage and promoting liver cell proliferation[27].
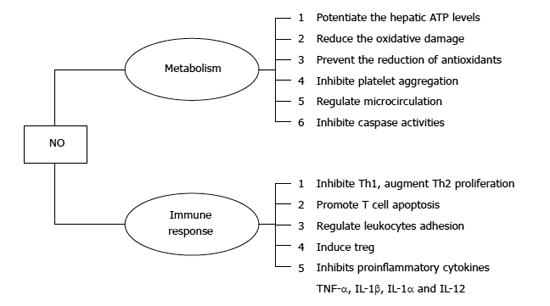
The effects of NO in protecting the liver from IRI have been studied extensively in recent years. NO is a highly reactive free radical produced from L-arginine and oxygen by nitric oxide synthase (NOS) in vivo[28]. Many studies have demonstrated that NO is a versatile signaling mediator involved in a multitude of critical cellular events, such as inhibition of platelet aggregation, regulation of the microcirculation, and inhibition of caspase activities to prevent cell apoptosis[29,30]. It has been shown that both endogenously generated and exogenously administrated NO plays an important role in protecting the liver from IRI[31]. NO has been found to attenuate liver IRI through various mechanisms, including the protection of hepatocytes from apoptosis and the reduction of macrophage infiltration[32]. Complicated mechanisms and numerous molecules are involved in exerting the protective effects of NO against liver IRI, including ATP molecules, endothelin, adhesion molecules, cytokines, free radical species, and antioxidants[33] (Figure 2). NO has been shown to potentiate hepatic ATP levels, reduce oxidative damage, prevent the reduction of antioxidants such as glutathione, and reduce the adverse effects of endothelin during liver IRI[33,34]. Studies have demonstrated that NO affects cellular decisions of life and death by either turning on or shutting off apoptotic pathways, suggesting that NO can function differently depending on the dose and duration of exposure[35,36]. Large amounts of NO may in turn paradoxically damage liver tissue by forming nitrogen peroxide[37], suggesting that the therapeutic safety window of NO is limited.
NO-based therapy has been applied for many years to patients with pulmonary hypertension or cardiopulmonary disorders. The therapeutic application of NO in protecting the liver from IRI has just been emerging. A prospective randomized small group trial with liver transplant patients has demonstrated that NO inhalation in liver recipients during the perioperative period of liver transplantation significantly protects hepatocytes from apoptotic death, accelerates the restoration of liver graft function, and reduces hospital length of stay[38]. Since NO has a very short half-life in vivo, it is not an ideal gas for the treatment of IRI. NO drugs administered to liver donors, such as organic nitrates and sodium nitroprusside, are now being explored as an alternative choice for NO delivery.
Sodium nitrite, a storage form of NO, can release NO during hypoxia and acidosis[39]. Sodium nitrite has now been identified as an important storage reservior of bioavailable NO in the blood and tissues[40]. The reduction of nitrite to NO has been demonstrated to confer cytoprotection against IRI in the heart, liver, brain, and kidney[40]. Interventions that increase NO production by the use of sodium nitrite before the occurrence of ischemia, either through intraperitoneal injection or oral administration, can mediate significant cytoprotection. This strategy has been demonstrated to potently limit acute IRI in both the heart and liver in murine warm IRI models, with the ability to decrease myocardial infarction and hepatocyte apoptosis[40-43].
NO is also an important effector molecule, produced by KCs and dendritic cells (DCs), and is involved in immune regulation and host innate and adaptive immunity[44]. NO inhibits proinflammatory cytokines, including TNF-α, IL-1β, IL-1α and IL-12, which may induce the inflammatory cascade during liver IRI [24-26,33]. It has been reported that NO exerts multiple effects on immune cells, decreasing the number of T helper (Th)1 cells and augmenting Th2 cell proliferation and their cytokine synthesis, regulating leukocyte adhesion and recruitment to the site of infection[45-47], inhibiting Th1 proliferation, and promoting T cell apoptosis[48,49]. Moreover, NO also contributes to the immunosuppressive function of induced T regulatory cells (Treg)[50]. Therefore, NO is involved in the regulation of liver IRI-associated immune responses. The underlying mechanisms are largely unknown and warrant further investigation.
Hepatic IRI is not only a pathophysiological process involving the liver itself, but also a complex systemic process affecting multiple tissues and organs. Hepatic IRI can seriously impair liver function, even producing irreversible damage, which causes a cascade of multiple organ dysfunction. Many factors, including anaerobic metabolism, mitochondrial damage, oxidative stress, intracellular Ca2+ overload, cytokines and chemokines produced by KCs and neutrophils, and NO, are all involved in the regulation of liver IRI processes. The most important pathways of liver IRI are initiated by oxidative stress, anaerobic metabolism and acidosis, further resulting in the cellular damage through induction of apoptosis, immune responses, and cytokine regulations (Figure 3). Inhaled NO or NO-producing drugs have shown positive effects on IRI protection in clinical practice, and may be a good choice for liver IRI therapy in the future. Therefore, further exploration of the mechanisms of IRI on animal models focusing on the regulatory pathway of IRI development, with concomitant development of a more effective method of controlling IRI, will help overcome the challenges in the prevention of IRI and therapeutic strategies.
| 1. | Kanoria S, Glantzounis G, Quaglia A, Dinesh S, Fusai G, Davidson BR, Seifalian AM. Remote preconditioning improves hepatic oxygenation after ischaemia reperfusion injury. Transpl Int. 2012;25:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Guan YF, Pritts TA, Montrose MH. Ischemic post-conditioning to counteract intestinal ischemia/reperfusion injury. World J Gastrointest Pathophysiol. 2010;1:137-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013;19:1683-1698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 123] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Siriussawakul A, Zaky A, Lang JD. Role of nitric oxide in hepatic ischemia-reperfusion injury. World J Gastroenterol. 2010;16:6079-6086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Sastre J, Serviddio G, Pereda J, Minana JB, Arduini A, Vendemiale G, Poli G, Pallardo FV, Vina J. Mitochondrial function in liver disease. Front Biosci. 2007;12:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Aguilar HI, Botla R, Arora AS, Bronk SF, Gores GJ. Induction of the mitochondrial permeability transition by protease activity in rats: a mechanism of hepatocyte necrosis. Gastroenterology. 1996;110:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 422] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl. 2010;16:1016-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Brass CA, Roberts TG. Hepatic free radical production after cold storage: Kupffer cell-dependent and -independent mechanisms in rats. Gastroenterology. 1995;108:1167-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 243] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3970] [Cited by in RCA: 3790] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 12. | Ikeda M, Ariyoshi H, Sakon M, Kambayashi J, Yoshikawa N, Shinoki N, Kawasaki T, Monden M. A role for local calcium gradients upon hypoxic injury in human umbilical vein endothelial cells (HUVEC). Cell Calcium. 1998;24:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 876] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 14. | Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology. 2001;33:902-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Boury NM, Czuprynski CJ. Listeria monocytogenes infection increases neutrophil adhesion and damage to a murine hepatocyte cell line in vitro. Immunol Lett. 1995;46:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Nagendra AR, Mickelson JK, Smith CW. CD18 integrin and CD54-dependent neutrophil adhesion to cytokine-stimulated human hepatocytes. Am J Physiol. 1997;272:G408-G416. [PubMed] |
| 17. | Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 300] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Redaelli CA, Tian YH, Schaffner T, Ledermann M, Baer HU, Dufour JF. Extended preservation of rat liver graft by induction of heme oxygenase-1. Hepatology. 2002;35:1082-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Peralta C, Fernández L, Panés J, Prats N, Sans M, Piqué JM, Gelpí E, Roselló-Catafau J. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583-G589. [PubMed] |
| 21. | Oe S, Hiros T, Fujii H, Yasuchika K, Nishio T, Iimuro Y, Morimoto T, Nagao M, Yamaoka Y. Continuous intravenous infusion of deleted form of hepatocyte growth factor attenuates hepatic ischemia-reperfusion injury in rats. J Hepatol. 2001;34:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Hamada T, Tsuchihashi S, Avanesyan A, Duarte S, Moore C, Busuttil RW, Coito AJ. Cyclooxygenase-2 deficiency enhances Th2 immune responses and impairs neutrophil recruitment in hepatic ischemia/reperfusion injury. J Immunol. 2008;180:1843-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Welborn MB, Moldawer LL, Seeger JM, Minter RM, Huber TS. Role of endogenous interleukin-10 in local and distant organ injury after visceral ischemia-reperfusion. Shock. 2003;20:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Teoh N, Field J, Farrell G. Interleukin-6 is a key mediator of the hepatoprotective and pro-proliferative effects of ischaemic preconditioning in mice. J Hepatol. 2006;45:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Husted TL, Blanchard J, Schuster R, Shen H, Lentsch AB. Potential role for IL-23 in hepatic ischemia/reperfusion injury. Inflamm Res. 2006;55:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Ke B, Shen XD, Lassman CR, Gao F, Busuttil RW, Kupiec-Weglinski JW. Cytoprotective and antiapoptotic effects of IL-13 in hepatic cold ischemia/reperfusion injury are heme oxygenase-1 dependent. Am J Transplant. 2003;3:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: Part I. General considerations and redox biology in hepatitis. J Surg Res. 2010;162:95-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 29. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4983] [Cited by in RCA: 4573] [Article Influence: 240.7] [Reference Citation Analysis (0)] |
| 30. | Mocellin S, Bronte V, Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev. 2007;27:317-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 31. | Abu-Amara M, Yang SY, Seifalian A, Davidson B, Fuller B. The nitric oxide pathway--evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int. 2012;32:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl. 2002;8:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Liu P, Xu B, Spokas E, Lai PS, Wong PY. Role of endogenous nitric oxide in TNF-alpha and IL-1beta generation in hepatic ischemia-repefusion. Shock. 2000;13:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Hsu CM, Wang JS, Liu CH, Chen LW. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock. 2002;17:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Brüne B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003;10:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 432] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 37. | Miyake T, Yokoyama Y, Kokuryo T, Mizutani T, Imamura A, Nagino M. Endothelial nitric oxide synthase plays a main role in producing nitric oxide in the superacute phase of hepatic ischemia prior to the upregulation of inducible nitric oxide synthase. J Surg Res. 2013;183:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 39. | Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308-4313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 40. | Sinha SS, Shiva S, Gladwin MT. Myocardial protection by nitrite: evidence that this reperfusion therapeutic will not be lost in translation. Trends Cardiovasc Med. 2008;18:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 515] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 42. | Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Langston W. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci USA. 2008;105:7540-7545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Li W, Meng Z, Liu Y, Patel RP, Lang JD. The hepatoprotective effect of sodium nitrite on cold ischemia-reperfusion injury. J Transplant. 2012;2012:635179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Chen C, Lee WH, Zhong L, Liu CP. Regulatory T cells can mediate their function through the stimulation of APCs to produce immunosuppressive nitric oxide. J Immunol. 2006;176:3449-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Taylor-Robinson AW, Liew FY, Severn A, Xu D, McSorley SJ, Garside P, Padron J, Phillips RS. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994;24:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 282] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 178] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Roland CR, Walp L, Stack RM, Flye MW. Outcome of Kupffer cell antigen presentation to a cloned murine Th1 lymphocyte depends on the inducibility of nitric oxide synthase by IFN-gamma. J Immunol. 1994;153:5453-5464. [PubMed] |
| 49. | Monsonego A, Imitola J, Zota V, Oida T, Weiner HL. Microglia-mediated nitric oxide cytotoxicity of T cells following amyloid beta-peptide presentation to Th1 cells. J Immunol. 2003;171:2216-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006;27:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
P- Reviewer: Chapel A, Cnossen WR S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Wang CH