Published online Mar 27, 2014. doi: 10.4240/wjgs.v6.i3.42
Revised: January 15, 2014
Accepted: February 16, 2014
Published online: March 27, 2014
Processing time: 108 Days and 0.1 Hours
Mucinous cystic adenoma (MCA) of the pancreas is a rare benign cystic tumor with ovarian-like stroma and lack of communication with the pancreatic ductal system. The ovarian tissue is incorporated from the left gonad within the dorsal pancreas during embryogenesis. Consequently, congenital dorsal agenesis of the pancreas (DAP) cannot be associated with MCA. We report the case of a giant MCA associated with atrophy of the dorsal pancreas mimicking complete DAP. Pancreato-magnetic resonance imaging failed to identify the dorsal pancreas but the absence of diabetes mellitus and compression of the splenic vein with major tributaries rectified the diagnosis of secondary atrophy of the distal pancreas. Unusual proximal location of the cyst in the pancreas may have induced chronic obstruction of both the dorsal pancreatic duct and the splenic vein, with secondary atrophy of the distal pancreas.
Core tip: Mucinous cystic adenoma (MCA) of the pancreas is a benign tumor with ovarian-like tissue located in the body or the tail of the pancreas. We report the first case of atrophy of the distal pancreas secondary to compression by a giant MCA. We raise the question of underlying dorsal agenesis of the pancreas (DAP) but as ovarian-like tissue of MCA comes from the close migration of the left gonad and the dorsal pancreas during embryogenesis MCA can not be associated with true DAP. Finally, the absence of diabetes mellitus, and thrombosis of the splenic vein confirmed the secondary atrophy caused by a mechanism of compression.
- Citation: Gagnière J, Dupré A, Ines DD, Tixier L, Pezet D, Buc E. Giant mucinous cystic adenoma with pancreatic atrophy mimicking dorsal agenesis of the pancreas. World J Gastrointest Surg 2014; 6(3): 42-46
- URL: https://www.wjgnet.com/1948-9366/full/v6/i3/42.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i3.42
Dorsal agenesis of the pancreas (DAP) is a rare disease that is frequently asymptomatic except when associated with polysplenia syndrome. Etiology remains unclear, but dysgenesis of the dorsal bud during embryogenesis seems to be the most plausible explanation. Confounding diagnoses include pseudo-agenesis of the dorsal pancreas following acute pancreatitis or compression by a tumor[1,2]. In such cases, the mechanism involves pancreatic duct obstruction with atrophy of pancreatic acini replaced by fat. However, endocrine cells generally still persist and prevent the occurrence of diabetes mellitus. Benign cystic or non-cystic tumors cannot usually induce pancreatic atrophy since invasive contingents are missing. We herein present the first documented case of a giant mucinous cystic adenoma (MCA) of the pancreas responsible for secondary atrophy of the dorsal pancreas and mimicking a complete DAP.
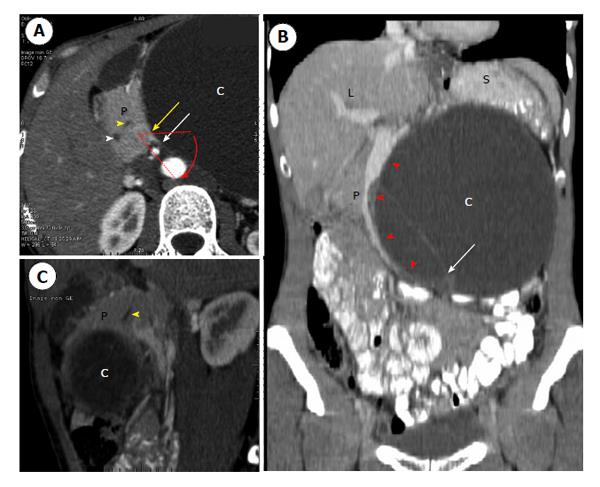
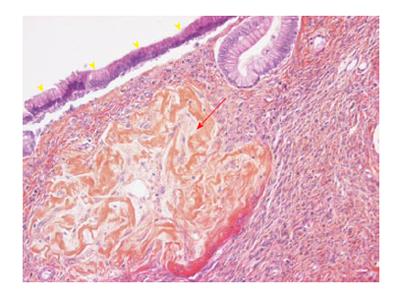
A 36-year-old female was referred to a first institution for exploration of an asymptomatic abdominal mass. She had no previous medical or surgical history. Physical examination showed a large painless epigastric mass. Ultrasound (US) showed a well-limited cyst in the epigastric area 15 cm × 10 cm in size with a thick wall, heterogeneous content and peripheral calcifications. Laboratory test results including amylase, lipase and serum glucose levels were within the normal range. The serum tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were normal. Contrast-enhanced computed tomography (CT) scan confirmed a well-defined, low-density, 17 cm × 11 cm, unilocular cystic tumor (Figure 1). It seemed to originate from the proximal part of the distal pancreas but the rotation to the left of both the head of the pancreas and the superior mesenteric vessels rendered the exact location of the cyst inconclusive (Figure 1). Thin septa, contrast enhancement and calcifications were also observed. Magnetic resonance imaging (MRI) and endoscopic US-guided fine needle aspiration (EUS-FNA) of the cyst were not performed preoperatively as giant benign MCA was suspected. The patient underwent surgical enucleation of an exophytic 14 cm × 10 cm cystic tumor of the pancreas. In his operative report, the surgeon noted a difficult procedure with accidental intraoperative rupture of the cyst. The postoperative period was uneventful and the patient was discharged on postoperative day 7. The pathological report confirmed a multilocular, thick-walled, 14 cm × 10 cm cyst with intracystic hemorrhage and disruption. Microscopically, the cyst was lined by tall columnar, mucin-containing epithelial cells, surrounded by an ovarian-like stroma (Figure 2). The epithelium was benign and positive for cytokeratins 7 and 19, which is consistent with the diagnosis of pancreatic MCA. Enucleation was complete and conservative as pancreatic parenchyma was absent on the specimen.
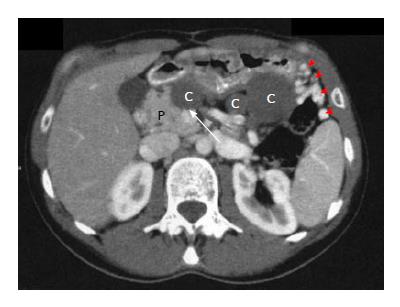
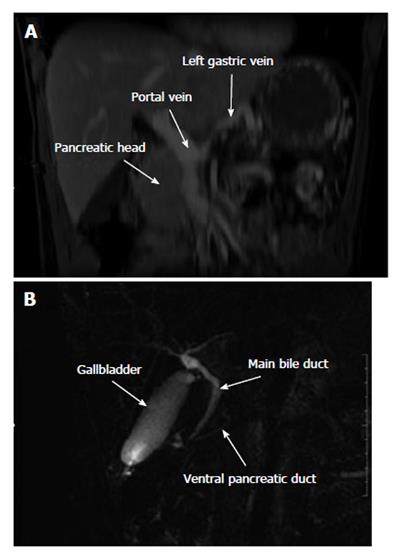
An abdominal CT-scan was performed 6 mo after surgery for exploration of abdominal tenderness and showed four low-density homogenous cystic lesions with contrast-enhanced wall in the previous pancreatic enucleation area (Figure 3). The body and tail of the pancreas were not visible on CT nor on the upper part of the head of the pancreas, suggesting complete DAP. Magnetic resonance cholangiopancreatography (MR-CP) showed absence of the body and tail of the pancreas with no accessory pancreatic duct that would confirm the diagnosis of DAP (Figure 4). There was no other pancreatic anomaly and no polysplenia. The patient was then referred to our institution. A second interpretation of the scan pictures showed splenic vein obstruction with major tributaries around the stomach, suggesting segmental portal hypertension (Figure 3). These features were also present on the initial CT. The serum levels of glucose, amylase and lipase were still normal. The suspected diagnoses were either recurrence of the MCA following difficult and incomplete primary resection, as suggested by intraoperative rupture, or multiple pseudocysts due to a latent post-operative pancreatic leak. A second-look laparoscopy was advocated because of abdominal tenderness, risk of recurrence of the MCA and because EUS-FNA failed to distinguish MCA from pancreatic pseudocysts. Laparoscopic exploration showed extra-pancreatic multiple cysts close to the first duodenum at the anterior part of the head of the pancreas, without pancreatic leak. The body and tail of the pancreas were also absent. Intraoperative pathology examination of the cysts confirmed pseudocysts with fat necrosis. Postoperative course was uneventful and the patient was discharged on postoperative day 3. One year after initial resection the patient had no diabetes mellitus, and routine blood parameters, in particular serum glucose level, were normal.
To the best of our knowledge, there are no documented reports of pancreatic cyst-including benign MCA-associated with congenital or secondary atrophy of the distal pancreas. MCA is a rare benign cystic tumor characterized by an ovarian stroma underlying the epithelium of the cyst. Differential diagnosis includes other benign cystic lesions such as serous cystic neoplasm, intraductal papillary mucinous neoplasm and post-pancreatitis pseudocysts[3]. Clinical presentation (female sex, location in the distal pancreas and no history of pancreatitis) and paraclinical investigations (MRI and EUS-FNA showing no pancreatic duct communication, wall calcifications and high level of intra-cystic CA19-9 and CEA) are suggestive of MCA[4]. Prophylactic resection is warranted as malignant transformation can occur in 6%-27% of cases[5,6]. The origin of ovarian stroma remains unclear. It has been suggested to derive from ectopic tissue within the pancreas incorporated throughout close migration of the left primordial gonad and dorsal pancreatic bud during embryogenesis[7-9], which would explain the predilection of MCA for the body-tail region of the pancreas. Consequently, the association of complete DAP with MCA is theoretically not possible.
DAP is a congenital agenesis of the pancreas that can be partial or complete. It is a rare event since only 54 cases have been reported in the literature[10]. The pancreas develops from ventral and dorsal endodermal buds during embryogenesis. The ventral bud gives rise to the major part of the head and uncinate process, which drains through the duct of Wirsung (i.e., the main pancreatic duct). The dorsal bud forms the upper part of the head, body and tail of the pancreas and drains through the duct of Santorini (i.e., the accessory pancreatic duct). Each bud develops a tree-like ductal system and, during growth and rotation of the gut in the seventh week of gestation, the two buds fuse and form the main pancreatic gland. Exocrine secretion is consistent in both dorsal and ventral pancreas, whereas insulin-secreting cells of the islets of Langerhans are located predominantly in the dorsal pancreas[11]. Rarely, DAP is complete with lack of structures originating from the dorsal pancreas - such as minor papilla, accessory pancreatic duct, body and tail[12]. When DAP is partial, which is most frequently the case, the minor papilla with a remnant accessory pancreatic duct and the body of the pancreas usually persist[13]. Confounding diagnosis is secondary atrophy of the distal pancreas due to chronic obstruction of the pancreatic duct. In this case, atrophy involves predominantly the exocrine tissue while endocrine cells are still present and prevent the occurrence of diabetes mellitus[1,2].
In our patient, despite arguments for congenital DAP, compression of the main pancreatic duct by the giant cyst with secondary atrophy of the distal pancreas was the most probable hypothesis. As discussed above, the association of complete DAP with MCA is not possible. Although there was no accessory pancreatic duct on MR-CP, the unusual proximal location within the dorsal pancreas of the MCA could have induced atrophy of the distal pancreas with no or undetectable remnant accessory pancreatic duct. This is consistent with atrophy of the splenic vein and collateral vascularization developed from the gastric veins, usually absent in congenital DAP[10,13]. Atrophy may have been worsened by intraoperative injury in what was described as a difficult procedure, as shown by the presence of a postoperative pseudocyst close to the head of the pancreas (Figure 3). Another argument for secondary atrophy is the absence of diabetes mellitus, since congenital DAP involves both endocrine and exocrine secretions with diabetes mellitus in around 40% of cases[2].
To the best of our knowledge, there is no published report of the effects of MCA on the distal pancreas. There are at least two reasons for this[14,15]. First, observations usually focus on the size, symptoms and management of the MCA, with little or no information about the distal pancreas. Second, MCAs are located in the distal position and usually spare the proximal pancreatic parenchyma. The pathological report usually insists on the features of the MCA, but not in the distal pancreas, and whether it is atrophied or absent. There have been reports of DAP associated with non-invasive tumors[16,17]. In these cases, the diagnosis of DAP was based on atrophy of the distal pancreas but in no instance it was possible to differentiate congenital agenesis from secondary atrophy. The unusual occurrence of congenital DAP makes the association with tumor very unlikely and we suggest that, as in our case, most DAPs associated with huge tumors are the result of secondary atrophy. Furthermore, tumors located within the dorsal pancreas cannot be associated with DAP given that complete agenesis of an organ cannot lead to the development of a tumor because neoplastic transformation can not occur from cells that do not exist.
Our observation is a reminder that the management of huge benign tumors is problematic. Preoperative imaging must be rigorous to detect congenital or acquired anomalies of the pancreas, and to describe pancreatic ductal anatomy. Resection must be conservative as often as possible, to avoid injury of the ductal system and secondary occurrence of pancreatic fistula or pseudocysts. Non-visualization of distal pancreas can be the consequence of long term compression of the main pancreatic duct. However, islets cells may still be present and accidental resection of the atrophic pancreas can lead to secondary diabetes mellitus. Thus, we recommend addressing these patients to tertiary centers for adequate preoperative evaluation and surgical management.
A 36-year-old female presented with asymptomatic abdominal mass.
Painless huge epigastric mass with no digestive repercussion.
Gastric tumor, liver tumor, liver cyst, pancreatic tumor.
WBC 9.80 k/μL, HGB 14.0 mg/dL, glucose 7 mmol/L, lipase 80 U/L, CRP 2.9 mg/L. Carbohydrate antigen 19-9 and carcinoembryonic antigen were within normal limits.
Computed tomography showed a huge mucinous cystic adenoma (MCA) that originated from the head or the body of the pancreas, with thrombosis of the splenic vein and complete atrophy of the pancreas distal to the cyst mimicking dorsal agenesis of the pancreas (DAP).
Specimen showed a cyst lined by tall columnar epithelial cells surrounded by an ovarian-like stroma, positive for cytokeratins 7 and 19, consistent with the diagnosis of MCA.
The patient was treated by enucleation of the cyst, but recurrence of multiple cysts six months later led to second-look laparoscopy, which showed pseudocysts resulting from the initial surgery.
Atrophy of the dorsal pancreas is usually observed in invasive tumors or in chronic pancreatitis but not in non-invasive benign tumors.
DAP is defined as embryological agency of the dorsal pancreatic bud resulting in lack of development of the superior part of the head, body and tail of the pancreas.
Right-sided pancreatic MCA can lead to atrophy of the distal pancreas but can not be associated with DAP as MCAs usually originate from the dorsal pancreas.
Splenic vein thrombosis and absence of diabetes mellitus are good markers of secondary atrophy of the pancreas.
| 1. | Gold RP. Agenesis and pseudo-agenesis of the dorsal pancreas. Abdom Imaging. 1993;18:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Sakpal SV, Sexcius L, Babel N, Chamberlain RS. Agenesis of the dorsal pancreas and its association with pancreatic tumors. Pancreas. 2009;38:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Scoazec JY, Vullierme MP, Barthet M, Gonzalez JM, Sauvanet A. Cystic and ductal tumors of the pancreas: diagnosis and management. J Visc Surg. 2013;150:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Goh BK, Tan YM, Chung YF, Chow PK, Cheow PC, Wong WK, Ooi LL. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30:2236-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 318] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1458] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 7. | Nishimura H. Atlas of human prenatal histology. Tokyo-New York: Igaku-Shoin 1983; . |
| 8. | Tuchmann-Duplessis H. Embryologie travaux pratiques et enseignement dirigé. Paris: Masson 1968; . |
| 9. | Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 402] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Schnedl WJ, Piswanger-Soelkner C, Wallner SJ, Reittner P, Krause R, Lipp RW, Hohmeier HE. Agenesis of the dorsal pancreas and associated diseases. Dig Dis Sci. 2009;54:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg. 1974;179:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Mohapatra M, Mishra S, Dalai PC, Acharya SD, Nahak B, Ibrarullah M, Panda K, Mishra SS. Imaging findings in agenesis of the dorsal pancreas. Report of three cases. JOP. 2012;13:108-114. [PubMed] |
| 13. | Schnedl WJ, Reisinger EC, Schreiber F, Pieber TR, Lipp RW, Krejs GJ. Complete and partial agenesis of the dorsal pancreas within one family. Gastrointest Endosc. 1995;42:485-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Teixeira F, Moutinho V, Ushinohama A, Akaishi E, Utiyama E, Rasslan S. Giant mucinous cystic neoplasm of the pancreas. J Gastrointest Surg. 2010;14:1197-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Mizutani S, Nakamura Y, Ogata M, Watanabe M, Tokunaga A, Tajiri T. A case of giant mucinous cystic neoplasm of the pancreas resected with laparoscopic surgery. J Nippon Med Sch. 2009;76:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Nakamura Y, Egami K, Maeda S, Hosone M, Onda M. Solid and papillary tumor of the pancreas complicating agenesis of the dorsal pancreas. J Hepatobiliary Pancreat Surg. 2001;8:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Ulusan S, Bal N, Kizilkilic O, Bolat F, Yildirim S, Yildirim T, Niron EA. Case report: solid-pseudopapillary tumour of the pancreas associated with dorsal agenesis. Br J Radiol. 2005;78:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
P- Reviewers: Azhar R, Guan YS, Klinge U, Pavlidis TE S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ