Published online Feb 27, 2013. doi: 10.4240/wjgs.v5.i2.16
Revised: October 21, 2012
Accepted: December 1, 2012
Published online: February 27, 2013
Processing time: 218 Days and 21.2 Hours
AIM: To analyze our experience in patients with duodenal gastrointestinal stromal tumors (GIST) and review the appropriate surgical approach.
METHODS: We retrospectively reviewed the medical records of all patients with duodenal GIST surgically treated at our medical institution between 2002 and 2011. Patient files, operative reports, radiological charts and pathology were analyzed. For surgical therapy open and laparoscopic wedge resections and segmental resections were performed for limited resection (LR). For extended resection pancreatoduodenectomy was performed. Age, gender, clinical symptoms of the tumor, anatomical localization, tumor size, mitotic count, type of resection resectional status, neoadjuvant therapy, adjuvant therapy, risk classification and follow-up details were investigated in this retrospective study.
RESULTS: Nine patients (5 males/4 females) with a median age of 58 years were surgically treated. The median follow-up period was 45 mo (range 6-111 mo). The initial symptom in 6 of 9 patients was gastrointestinal bleeding (67%). Tumors were found in all four parts of the duodenum, but were predominantly located in the first and second part of the duodenum with each 3 of 9 patients (33%). Two patients received neoadjuvant medical treatment with 400 mg imatinib per day for 12 wk before resection. In one patient, the GIST resection was done by pancreatoduodenectomy. The 8 LRs included a segmental resection of pars 4 of the duodenum, 5 wedge resections with primary closure and a wedge resection with luminal closure by Roux-Y duodeno-jejunostomy. One of these LRs was done minimally invasive; seven were done in open fashion. The median diameter of the tumors was 54 mm (14-110 mm). Using the Fletcher classification scheme, 3/9 (33%) tumors had high risk, 1/9 (11%) had intermediate risk, 4/9 (44%) had low risk, and 1/9 (11%) had very low risk for aggressive behaviour. Seven resections showed microscopically negative transsection margins (R0), two showed positive margins (R1). No patient developed local recurrence during follow-up. The one patient who underwent pancreatoduodenectomy died due to progressive disease with hepatic metastasis but without evidence of local recurrence. Another patient died in complete remission due to cardiac disease. Seven of the nine patients are alive disease-free.
CONCLUSION: In patients with duodenal GIST, limited surgical resection with microscopically negative margins, but also with microscopically positive margins, lead to very good local and systemic disease-free survival.
- Citation: Hoeppner J, Kulemann B, Marjanovic G, Bronsert P, Hopt UT. Limited resection for duodenal gastrointestinal stromal tumors: Surgical management and clinical outcome. World J Gastrointest Surg 2013; 5(2): 16-21
- URL: https://www.wjgnet.com/1948-9366/full/v5/i2/16.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i2.16
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of gastrointestinal tract. GIST arise from interstitial cells of Cajal or Cajal-like precursor cells which are located in the muscular layer of gastrointestinal organs[1]. GIST can be distinguished from other mesenchymal tumors by immunohistochemistry: in contrast to sarcomas, leiomyomas, and myoblastomas, they show a KIT expression (CD117)[2,3]. The most common site where GIST are found is the stomach with 50%-60%. The next frequent locations are the small bowel (25%) and the colo-rectum (10%). GIST with duodenal origin are very rare and represent only about 5% of all GIST.
In the absence of metastatic disease, surgical resection is the primary curative approach to treat GIST. Since longitudinal submucosal spread is very limited and lymph node involvement is rare, margin-negative resection without lymphadenectomy is the commonly accepted surgical treatment[4]. In the stomach, limited resections are technically simple in the majority of cases due to anatomic circumstances. By contrast, local resections in the duodenum are more challenging. The direct proximity to the pancreatic head, the papilla of Vater and the mesenteric root make limited resections technically demanding and thus, clear resection margins are often barely obtainable.
To evaluate the results and outcome of surgical therapy of duodenal GIST, we retrospectively analysed all patients who underwent resection extended by pancreatoduodenectomy or limited to local resections at our institution within the last 10 years.
Between 01/2002 and 12/2011, 9 patients underwent surgical treatment for duodenal GIST at the Department of Surgery at University of Freiburg in Germany. This review was carried out retrospectively by analyzing the patient files, operative reports, radiological charts and pathology reports. CD117, CD34, Desmin, Smooth Muscle Actin and S100 were stained immunohistochemically. According to our protocols for GIST diagnosis, tumors were classified as GIST either if CD117 was positive, or if typical morphology was present along with CD34 positivity in immunohistochemistry.
Open and laparoscopic wedge resections and segmental resections were performed for limited resection (LR). For extended resection (ER), pancreatoduodenectomy was performed.
Age, gender, clinical symptoms of the tumor, anatomical localization, tumor size, mitotic count, type of resection (LR vs ER), resectional status, neoadjuvant therapy, adjuvant therapy, risk classification and follow-up details were analysed in this retrospective study. Risk stratification of the GIST was carried out according to the criteria proposed by Fletcher et al[2] (Table 1).
| Risk | Tumor size (cm) | Mitotic count (/50 HPF) |
| Very low-risk | > 2 | < 5 |
| Low-risk | 2-5 | < 5 |
| Intermediate risk | < 5 | 6-10 |
| 5 - 10 | < 5 | |
| High-risk | > 5 | > 5 |
| > 10 | any mitotic rate | |
| any size | > 10 |
Between 01/2002 and 12/2011, nine patients underwent surgery for duodenal GIST at the Department of Surgery at University of Freiburg. The medium age was 58 years (range 43-75 years) and there were 5 men and 4 women. The tumors were located in all 4 parts of the duodenum. Three tumors were found each in part 1 and 2 of the duodenum. Two tumors were located in the third portion of the duodenum and one in the fourth portion of the duodenum. In one patient, the duodenal GIST was an incidental finding and the other 8 were symptomatic. The most frequent initial symptom was upper gastrointestinal bleeding which was present in 6 patients (Table 2).
| Patient no. | Age (yr) | Sex | Initial symptom | Location | Surgical procedure | Resectional status |
| 1 | 51 | M | Bleeding | 2 | Open wedge resection + duodenojejunostomy | R0 |
| 2 | 63 | M | Bleeding | 3 | Open wedge resection | R0 |
| 3 | 52 | M | Bleeding | 1 | Laparoscopic wedge resection | R0 |
| 4 | 62 | F | Jaundice | 1 | Pancreatoduodenectomy | R0 |
| 5 | 58 | M | Abdominal pain | 1 | Open wedge resection | R0 |
| 6 | 69 | F | Incidental finding | 2 | Open wedge resecction | R1 |
| 7 | 43 | F | Bleeding | 4 | Segmental resection | R0 |
| 8 | 75 | M | Bleeding | 3 | Open wedge resection | R0 |
| 9 | 49 | F | Bleeding | 2 | Tumor resection without duodenal resection | R1 |
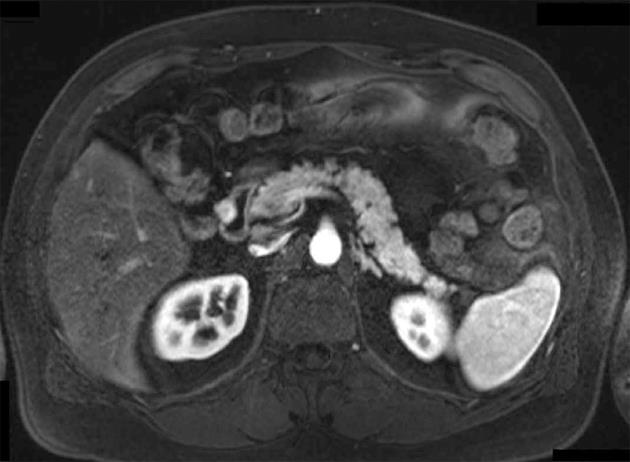
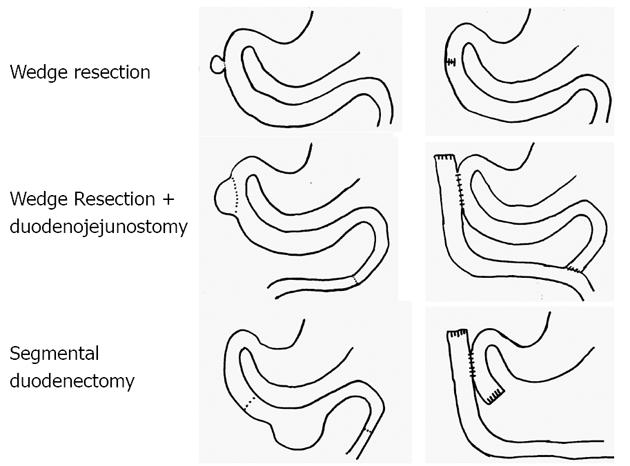
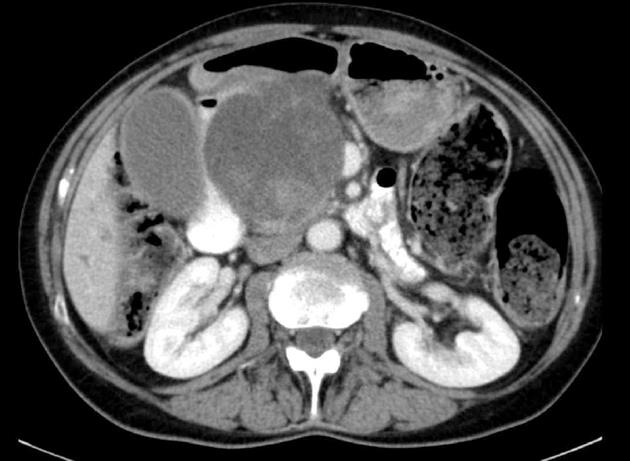
All 9 patients underwent curative resection including one ER with pancreatoduodenectomy and 8 LR (Table 3). The 8 LR included 1 segmental resection of pars 4 of the duodenum, 1 laparoscopic wedge resection with primary closure (Figure 1), 4 open wedge resections with primary closure and 1 open wedge resection with luminal closure by Roux-Y Duodeno-Jejunostomy. Figure 2 illustrates the different surgical procedures applied for LR of duodenal GIST in our study. In one patient, intraoperative exploration and intraoperative examination in frozen section revealed the residual tumor after neoadjuvant imatinib therapy to be strictly periduodenal with no involvement of the duodenal wall. In this patient an extraduodenal tumorectomy was performed. The patient who underwent pancreatoduodenectomy with portal vein resection (Figure 3) had a concomitant small liver metastasis in Segment 2 of the liver which was detected intraoperatively and resected in the same session by atypical liver resection. All patients had macroscopically clear margins after resection of the tumor. However, 2 patients showed microscopically positive transsection margins (R1) (Table 2). Postoperative course after LR and ER in all patients was uneventful. After LR, the patients were discharged medium on the 8th postoperative day (range 5-16 d). The patient after ER left the hospital on the 22nd postoperative day.
| Patient no. | c-KIT | Tumor size (mm) | Mitotic count (/50 HPF) | Risk | Neoadj. therapy | Adjuvant therapy | Disease relapse | Status |
| 1 | pos. | 32 | 5 | Low risk | No | No | No | NED at 6 mo |
| 2 | pos. | 35 | 14 | High risk | No | Imatinib | No | NED at 7 mo |
| 3 | pos. | 14 | 1 | Very low risk | No | No | No | NED at 13 mo |
| 4 | pos. | 110 | 35 | High risk | No | No | Liver | Died of PD at 3 mo |
| 5 | pos. | 30 | 0 | Low risk | No | No | No | NED at 90 mo |
| 6 | pos. | 25 | 0 | Low risk | No | No | No | NED at 111 mo |
| 7 | pos. | 110 | 4 | High risk | No | No | No | NED at 92 mo |
| 8 | pos. | 50 | 0 | Low risk | Imatinib | No | No | Died with NED at 37 mo |
| 9 | pos. | 80 | 1 | Intermediate | Imatinib | Imatinib | No | NED at 39 mo |
In our series, 2 patients received neoadjuvant medical treatment with 400 mg imatinib per day for 12 wk before resection. In both patients, neoadjuvant therapy was started due to large duodenal GIST in which cross-sectional imaging showed infiltration of other organs and complete resectability did not appear possible. In one patient neoadjuvant imatinib therapy was started because of suspected tumor infiltration of the Vena cava in computed tomography (CT). The other patient underwent initial duodenotomy with operative haemostasis of the bleeding GIST in an outside hospital, the resection of the remaining tumor was carried out in our institute after 12-wk neoadjuvant imatinib therapy by tumorectomy of the remaining tumor. Neoadjuvant treatment was initiated because of a large duodenal GIST infiltrating the liver hilum and the retroperitoneum and a complete resection initially appeared impossible. Due to microscopically margin-positive resectional status in the second procedure, adjuvant chemotherapy with imatinib was continued for 12 mo postoperative. Besides this patient only one other patient-with a high risk tumor-received postoperative adjuvant imatinib therapy for one year (Table 3).
The medium tumor size was 54 mm; diameters ranged from 14 mm to 110 mm. Immunohistochemically, all duodenal GIST were positive for KIT (CD117). Six of nine GIST were also positive for CD34. Using the Fletcher classification scheme[2], 3/9 tumors had high risk, 1/9 had intermediate risk, 4/9 had low risk, and 1/9 had very low risk for aggressive behaviour (Table 3). Microscopically margin-negative resectional status (R0) was achieved in 6 of 8 patients undergoing LR. The final resectional status after LR was margin positive in two patients. The patient with the large, high-risk GIST treated by pancreatoduodenectomy showed negative transsection margins and lymph node status.
One patient with a large, high-risk GIST, which was completely resected by pancreatoduodenectomy, died 3 months after surgery due to progressive disease with hepatic metastasis. Another 75-year-old patient died of cardiopulmonary disease 37 mo after surgery without evidence of local or distant tumor recurrence. Both patients with R1 resection are alive 39 and 111 mo after surgery without evidence of local or distant manifestation of GIST disease. All other patients who were surgically treated by LRs are alive without evidence of GIST disease (Table 3).
GIST are a distinct group of mesenchymal tumors with predominantly gastrointestinal distribution. They are molecularly characterized by mutations in the KIT proto-oncogene which results in over-expression of the transmembrane tyrosinkinase receptor KIT (CD117). GIST have been described nearly everywhere in the gastrointestinal tract but also rarely affect extragastrointestinal sites such as omentum and mesentery[5].
It is known that GIST of different anatomical sites not only vary in morphology and gene expression but also in clinical presentation and clinical outcome[2,6,7]. For this reason, it is necessary to characterize the different subsets of GIST as precisely as possible and to identify and define tailored diagnostic approaches and surgical strategies for management of GIST. In this retrospective analysis, we have focused on the relatively small subset of duodenal GIST and present our experience in their management over the last decade. In contrast to the relatively common gastric GIST, GIST of the duodenum are rare and represent only 3%-5% of all GIST[3]. We identified nine patients who were surgically treated for duodenal GIST in our institution over the last ten years. Duodenal GIST can be located in all four parts of the duodenum, although our study shows a descending distribution beginning with three GIST each in both the first and the second part of the duodenum, followed by two in the third and one in the fourth part.
In this series, 8 of 9 patients presented with symptoms. According to the literature[3,8] the most common clinical symptom is gastrointestinal bleeding, which was the initial symptom in 6 of our 9 patients. Three of these 6 patients presented with massive gastrointestinal bleeding and endoscopic attempts to stop the bleeding were unsuccessful. In these patients, the GIST resection was performed as an emergency operation. Only one small 25 mm tumor presented as asymptomatic and was incidentally found in endoscopic retrograde cholangiopancreatography for cholecystolithiasis. Since gastrointestinal bleeding was the most frequent initial symptom, gastrointestinal endoscopy was routinely the method for detection and verification of the duodenal GIST, which usually appear as submucosal swelling with or without mucosal ulceration. In our population, tumors located not only in the upper duodenum but also in the third and fourth part of the duodenum could be macroscopically detected in endoscopy and verified as GIST by pathology workup of the biopsy samples from endoscopy. Besides gastrointestinal endoscopy, cross-sectional imaging by CT or magnetic resonance imaging are useful. They can be used not only for examination of the local extension of the primary tumor, but also for the detection of metastasis[9]. However, it is often impossible to obtain histological samples for the verification of diagnosis through cross-sectional imaging-guided biopsy from duodenal GIST. Finally F-fluoro-2-deoxy-D-glucose positron emission tomography-CT seems to be very promising, especially for early response evaluation within one to two weeks after starting neoadjuvant medical treatment of GIST[9].
Surgical resection with the achievement of microscopically clear resection margins is the therapeutical goal of treatment in cases of primary non-mestastatic GIST-not only of the duodenum[4]. As lymph node involvement is very rare in adult GIST[10-14] and even the largest clinicopathologic series of duodenal GIST[3] did not detect lymphatic spread in 167 patients, routine lymph node dissection is not required in duodenal GIST. Supporting these findings, we did not detect any lymph node recurrence in either LRs or in ER in this study.
We performed LRs of duodenal GIST if the primary tumor showed no infiltration of other organs and lymph node involvement was excluded in cross-sectional imaging and in intraoperative macroscopic examination. This is the same strategy we apply in surgical therapy of GIST of the stomach and the small bowel. In our series, only one patient required pancreatoduodenectomy and atypical liver resection of a small metastasis. This was necessary due to a large, high-risk tumor infiltrating the pancreatic head. Although it was possible to achieve a microscopically margin-negative resection and no lymphatic involvement was seen in the specimen, the patient died due to tumor progression with liver metastasis three months after resection. LRs were carried out in all other patients, independent of tumor location within the duodenum (parts 1-4).
Wedge resections with primary closure, wedge resections with duodenal closure by Roux-Y duodeno-jejunostomy and segmental duodenectomy and extraduodenal tumorectomy for residual GIST after neoadjuvant therapy were performed in this study as LRs. Longitudinal wedge resection usually resulted in only limited defects of the duodenal wall. These limited defects were closed directly by interrupted sutures in transverse direction. This technique was utilized for tumors located in all first three parts of the duodenum. In one patient with larger GIST located in the second part of the duodenum and without involvement of the papilla of Vater, the reconstruction was carried out with a side-to-side duodeno-jejunostomy at the site of the duodenal wall defect and a Roux-Y limb as described by Goh et al[13]. Another option for LR of small and large GIST located in the third and fourth part of the duodenum is segmental duodenectomy. In this study, resection using a segmental duodenectomy of the fourth part of the duodenum and reconstruction using a side-to-side Roux-Y duodeno-jejunostomy was performed in one patient with an 11 cm high-risk GIST of the fourth portion of the duodenum.
Intraoperatively, all patients of our study showed macroscopically clear resection margins. However, patho-histological examination of the specimens revealed positive margins in two patients after LR. This fact could be a drawback in LRs of duodenal GIST. Due to the narrow and complex anatomy of the pancreaticoduodenal area with the proximity to the pancreas, the bile duct, the mesenteric root, the portal vein and the papilla of Vater, clear resection margins of LRs of duodenal GIST often measure only a few millimetres. Although the findings above represent a rate of 25% of margin-positive resections in the limited surgical approach of our series, both margin-positive patients are well and without evidence of local or systemic tumor recurrence 111 and 39 mo after surgery.
Although there is no official market authorization for neoadjuvant treatment of GIST with imatinib, it was used for downstaging of the tumors in two of our patients because large duodenal GIST infiltrating adjacent organs or large vessels made the possibility of a complete resection questionable. A pathologic response rate of 86% and a rate of complete resections after treatment with imatinib of 89% have been reported for neoadjuvant therapy of GIST[14]. Different studies were able to demonstrate that adjuvant imatinib treatment was able to significantly increase recurrence-free survival[15,16]. In case of high-risk duodenal GIST with a > 30% risk of recurrence or microscopically margin-positive resection, adjuvant imatinib treatment was also carried out in our patients after 2007. In our series, none of the patients treated with imatinib before or after surgery had recurrent disease. Adjuvant imatinib was planned for the only patient with recurrent tumor after resection PD, but therapy was not started due to rapid worsening of the general state of health in progressive metastatic tumor disease.
In conclusion, LR offers an excellent option for the surgical treatment of duodenal GIST. LR shows good results in terms of tumour-free survival and missing evidence of tumor recurrence in all of our patients treated by LR. Although microscopically positive margins were evident in 2 cases, no local recurrence was detected. Nevertheless, negative resection margins should be achieved in LR. If it is not possible by LR, tumor resection should be performed by ER.
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the gastrointestinal tract. GIST arise from interstitial cells of Cajal which are located in the muscular layer of gastrointestinal organs. GIST of the duodenum are rare tumors and represent only a small subgroup of all GIST.
Up to now, various surgical procedures have been described for their treatment. Both extended resections (ER) by pancreatoduodenectomy and limited local resections (LR) are performed. The presented study was to evaluate the results of LR and ER in patients with duodenal GIST and to find the appropriate surgical approach in future cases.
This study shows that LR of duodenal GIST promise very good results in terms of local and systemic disease-free survival (DFS). Both margin negative, but also margin positive LR showed good results concerning DFS.
As LR offers good results concerning DFS it can be advocated as therapy of duodenal GIST. Different techniques of LR for duodenal GIST can be applied for surgical therapy.
GIST: Gastrointestinal stromal tumors are mesenchymal tumors of gastrointestinal tract. LR: Limited resection means a surgical resection of the tumor without larger tumor-negative resection margins and without regional lymphadenectomy. ER: Extended resection means a surgical resection of the tumor with larger tumor-negative resection margins and regional lymphadenectomy. DFS: Disease-free survival describes the chances of staying free of disease after a particular treatment for cancer.
Duodenal GIST is very rare. Surgical treatment is another problem for duodenal GIST because of localization. Therefore, these cases are important.
| 1. | Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 522] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2170] [Article Influence: 90.4] [Reference Citation Analysis (1)] |
| 3. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 280] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg. 2003;90:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Llenas-García J, Guerra-Vales JM, Moreno A, Ibarrola C, Castelbon FJ, Fernández-Ruiz M, Meneu JC, Ballestin C, Moreno E. Primary extragastrointestinal stromal tumors in the omentum and mesentery: a clinicopathological and immunohistochemical study. Hepatogastroenterology. 2008;55:1002-1005. [PubMed] |
| 6. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1340] [Article Influence: 70.5] [Reference Citation Analysis (33)] |
| 7. | Antonescu CR, Viale A, Sarran L, Tschernyavsky SJ, Gonen M, Segal NH, Maki RG, Socci ND, DeMatteo RP, Besmer P. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10:3282-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Winfield RD, Hochwald SN, Vogel SB, Hemming AW, Liu C, Cance WG, Grobmyer SR. Presentation and management of gastrointestinal stromal tumors of the duodenum. Am Surg. 2006;72:719-722; discussion 722-7273. [PubMed] |
| 9. | Antoch G, Herrmann K, Heusner TA, Buck AK. [Imaging procedures for gastrointestinal stromal tumors]. Radiologe. 2009;49:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1698] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 11. | Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Aparicio T, Boige V, Sabourin JC, Crenn P, Ducreux M, Le Cesne A, Bonvalot S. Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol. 2004;30:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 13. | Goh BK, Chow PK, Ong HS, Wong WK. Gastrointestinal stromal tumor involving the second and third portion of the duodenum: treatment by partial duodenectomy and Roux-en-Y duodenojejunostomy. J Surg Oncol. 2005;91:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Goh BK, Chow PK, Chuah KL, Yap WM, Wong WK. Pathologic, radiologic and PET scan response of gastrointestinal stromal tumors after neoadjuvant treatment with imatinib mesylate. Eur J Surg Oncol. 2006;32:961-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Eisenberg BL, Smith KD. Adjuvant and neoadjuvant therapy for primary GIST. Cancer Chemother Pharmacol. 2011;67 Suppl 1:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Hohenberger P, Eisenberg B. Role of surgery combined with kinase inhibition in the management of gastrointestinal stromal tumor (GIST). Ann Surg Oncol. 2010;17:2585-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
P- Reviewer Akyuz U S- Editor Huang XZ L- Editor A E- Editor Xiong L