Published online Dec 27, 2013. doi: 10.4240/wjgs.v5.i12.321
Revised: November 19, 2013
Accepted: December 12, 2013
Published online: December 27, 2013
Processing time: 157 Days and 1 Hours
AIM: To evaluate the timing of chemotherapy in gastric cancer by comparing survival outcomes in treatment groups.
METHODS: Patients with surgically resected gastric adenocarcinoma from 1988 to 2006 were identified from the Los Angeles County Cancer Surveillance Program. To evaluate the population most likely to receive and/or benefit from adjunct chemotherapy, inclusion criteria consisted of Stage II or III gastric cancer patients > 18 years of age who underwent curative-intent surgical resection. Patients were categorized into three groups according to the receipt of chemotherapy: (1) no chemotherapy; (2) preoperative chemotherapy; or (3) postoperative chemotherapy. Clinical and pathologic characteristics were compared across the different treatment arms.
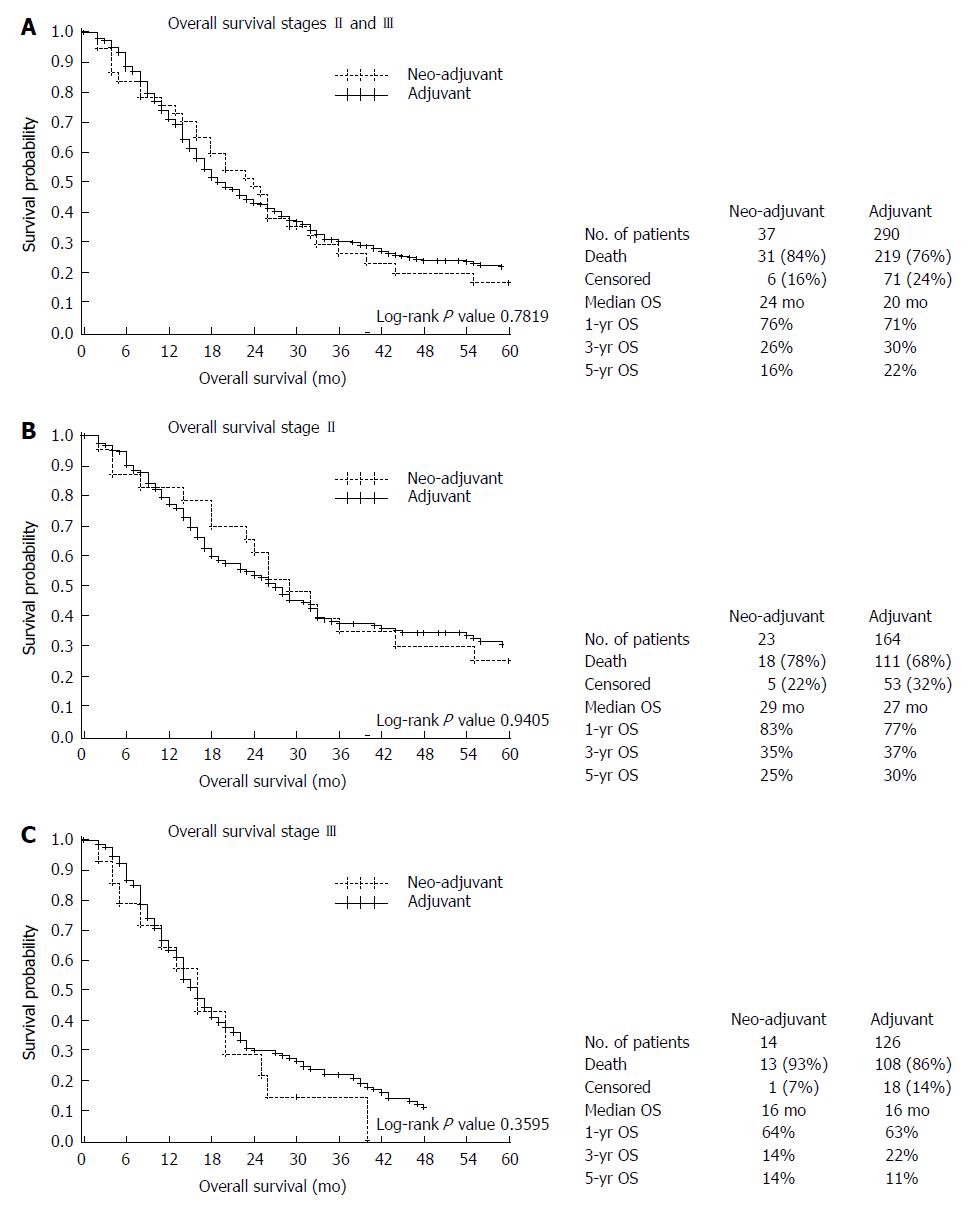
RESULTS: Of 1518 patients with surgically resected gastric cancer, 327 (21.5%) received perioperative chemotherapy. The majority of these 327 patients were male (68%) with a mean age of 61.5 years; and they were significantly younger than non-chemotherapy patients (mean age, 70.7; P < 0.001). Most patients had tumors frequently located in the distal stomach (34.5%). Preoperative chemotherapy was administered to 11.3% of patients (n = 37) and postoperative therapy to 88.7% of patients (n = 290). An overall survival benefit according to timing of chemotherapy was not observed on univariate or multivariate analysis. Similar results were observed with stage-specific survival analyses (5-year overall survival: Stage II, 25% vs 30%, respectively; Stage III, 14% vs 11%, respectively). Therefore, our results do not identify a survival advantage for specific timing of chemotherapy in locally advanced gastric cancer.
CONCLUSION: This study supports the implementation of a randomized trial comparing the timing of perioperative therapy in patients with locally advanced gastric cancer.
Core tip: Curative intent surgical resection offers the best survival potential in conjunction with chemotherapy for patients with gastric cancer. Few studies have evaluated the optimal timing of chemotherapy. This study shows that in the setting of resectable gastric cancer, there is no survival advantage based on the timing of chemotherapy.
- Citation: Arrington AK, Nelson R, Patel SS, Luu C, Ko M, Garcia-Aguilar J, Kim J. Timing of chemotherapy and survival in patients with resectable gastric adenocarcinoma. World J Gastrointest Surg 2013; 5(12): 321-328
- URL: https://www.wjgnet.com/1948-9366/full/v5/i12/321.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i12.321
Despite an overall decrease in the incidence and mortality in gastric cancer patients in the United States, nearly 22000 patients will be diagnosed with gastric cancer in the United States each year[1]. More alarmingly, gastric cancer remains the 2nd leading cause of cancer-related deaths worldwide accounting for an estimated 436930 deaths in 2013[2,3]. In Western societies, where screening is not routine (compared to Asian countries[4,5]), the majority of patients present with regional or distant disease[6,7], and the 5-year overall survival is 30%-40%[8-10]. In these cases, the only hope for long term survival remains surgical resection with curative intent[11]. Despite aggressive surgical measures, rates of disease recurrence remain high following surgery[12,13]. In fact, approximately 50% to 90% of patients die as a result of disease relapse[14]. As a result, much attention has been placed on the identification of optimal adjunct therapies for gastric cancer.
Adjunct therapies for gastric cancer may be administered in the pre-operative (i.e., neoadjuvant) or post-operative (i.e., adjuvant) settings. Multiple trials have assessed these treatment strategies; and potential benefits have been drawn from both options[14-19]. In a landmark study, the investigators of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial reported an overall survival benefit with a regimen that incorporated neoadjuvant and adjuvant (i.e., perioperative) timing of chemotherapy consisting of epirubicin, cisplatin, and fluorouracil (ECF) when compared to surgery alone[18]. From the Intergroup 0116 trial, investigators reported a 9 mo improvement in survival (27 mo vs 36 mo) when adjuvant chemoradiation was administered compared to surgery alone[17]. More recently, the CLASSIC trial investigators reported an overall survival benefit from adjuvant chemotherapy (capecitabine and oxaliplatin) compared to surgery alone[20]. However, there has been no trial that has directly compared neoadjuvant versus adjuvant chemotherapy administration. Using a large, population-based cohort, the objective of this study was to assess whether the timing of chemotherapy affects the survival of patients following surgical resection for gastric cancer.
The Los Angeles County (LAC) Cancer Surveillance Program (CSP) is the cancer registry that collects information for all cancer diagnoses in LAC since 1972. As part of the National Cancer Institute’s Surveillance, Epidemiology, and End Results program, CSP routinely collects data on patient demographics, primary tumor site, tumor morphology, disease stage at diagnosis, treatment received, and follow-up. CSP monitors the quality of data by performing annual reviews and training of staff. Approval to conduct this study was obtained from the Institutional Review Boards of the City of Hope and the State of California.
Using the CSP registry, we identified patients diagnosed with gastric adenocarcinoma from 1988 to 2006. Inclusion criteria consisted of Stage II or III gastric cancer patients above 18 years of age who underwent curative-intent surgical resection. As Stage I disease is more likely to be treated by surgery alone and patients with Stage IV disease are more likely not to undergo surgery given metastatic/unresectable disease, these patients were excluded from the current study. Therefore, this study was designed to evaluate the population most likely to receive and/or benefit from adjunct chemotherapy (Stage II or III).
Specifically, we included only gastric cancer patients with International Classification of Diseases for Oncology histology codes for adenocarcinoma: 8140-8145, 8210-8211, 8480-8481, and 8490. In CSP, the location of tumor was categorized as proximal, distal, middle, or whole stomach. For each patient, stage was categorized according to the American Joint Commission on Cancer (AJCC) 7th Edition classification system. Furthermore, size and depth of tumor invasion were categorized by AJCC T-stage as: T1A, T1B, T2, T3, or T4. The presence or absence of nodal involvement was designated by AJCC N-stage as: N0, N1, N2, or N3. Our survival analysis included only patients with AJCC Stage II and III gastric cancer. Finally, we obtained data regarding the timing of chemotherapy administration (none, preoperative, or postoperative). As the CSP database only codes the date of the first chemotherapy treatment, patients who did receive preoperative (neoadjuvant) chemotherapy may or may not have received subsequent postoperative chemotherapy. Therefore, we could not distinguish between neoadjuvant and perioperative chemotherapy in this database. Thus, this study compares neoadjuvant chemotherapy, adjuvant chemotherapy, and no chemotherapy.
Patients were categorized into three groups according to the receipt of chemotherapy: (1) no chemotherapy; (2) neoadjuvant chemotherapy (± postoperative chemotherapy); or (3) adjuvant chemotherapy.
Clinical and pathologic characteristics were compared across the different treatment arms by χ2 analyses for categorical variables and student’s t test for continuous variables. Cox-proportional hazards modeling was used to evaluate the role of chemotherapy and other variables on overall survival as represented by hazard ratios (HR) with 95%CI. Variables included in the univariate analyses were age, sex, race/ethnicity, tumor location, AJCC stage, T-stage, N-stage, tumor grade, tumor size, lymph node number, and timing of chemotherapy regimen (neoadjuvant ± adjuvant vs adjuvant alone). Variables included in the multivariate analyses were age, AJCC stage, and timing of chemotherapy regimen (preoperative vs postoperative). Because tumor size, T stage and lymph node status are multi-collinear with AJCC stage, the multivariate model included AJCC stage alone to represent the staging variable.
Survival was defined as survival throughout the study period (1988-2006). Mortality was defined through the database used as all-cause mortality since date of diagnosis of gastric cancer. Overall survival (OS) for the treatment arms was calculated by the Kaplan-Meier method, and differences in survival were compared by the log-rank test. Proportional hazard assumptions for the Cox models were tested by calculating scaled Schoenfeld residuals with results indicating model fit. Two-sided P values < 0.05 were considered to be statistically significant. All statistical analyses were completed using SAS software, (SAS institute Inc. Cary, NC).
Of 1518 patients with AJCC Stage II or III surgically resected gastric cancer between 1988 and 2006, 22% of patients (n = 327) received chemotherapy as part of their cancer treatment. The demographics of the study cohort are presented in Table 1. Most tumors were observed in the proximal (26%) or distal stomach (35%) and were poorly differentiated (68%) by histology. The majority (76%) of the study cohort had lymph node positive disease; and most patients had Stage II disease (63%) rather than Stage III disease (37%). Patients who did not receive chemotherapy were more likely to have lymph node negative disease than those who did receive chemotherapy (28% vs 12%, P < 0.001).
| Characteristic | No chemon = 1191 | Receivedchemotherapyn = 327 | P value | |
| Age, yr | Mean ± SD | 71 ± 12 | 61 ± 14 | < 0.0001 |
| Sex | Men | 741 (62) | 221 (68) | 0.0743 |
| Women | 450 (38) | 106 (32) | ||
| Race/ ethnicity | Non-hispanic white | 438 (37) | 118 (36) | 0.9833 |
| Black | 112 (9) | 33 (10) | ||
| Hispanic white | 322 (27) | 88 (27) | ||
| Asian/pacific islanders | 319 (27) | 88 (27) | ||
| Tumor location | Proximal | 296 (25) | 98 (30) | 0.0290 |
| Distal | 423 (36) | 100 (31) | ||
| Whole | 167 (14) | 59 (18) | ||
| Middle | 305 (26) | 70 (21) | ||
| Grade | Well differentiated | 26 (2) | 1 (0) | 0.0178 |
| Moderately differentiated | 312 (26) | 70 (21) | ||
| Poorly differentiated | 792 (66) | 234 (72) | ||
| Undifferentiated | 40 (3) | 18 (6) | ||
| Unknown | 21 (2) | 4 (1) | ||
| Tumor size | ≤ 5 cm | 462 (39) | 117 (36) | 0.6048 |
| >5 cm | 507 (43) | 145 (44) | ||
| Unknown | 222 (19) | 65 (20) | ||
| T stage1 | T2 | 108 (9) | 24 (7) | 0.1556 |
| T3 | 620 (52) | 163 (50) | ||
| T4a | 319 (27) | 96 (29) | ||
| T4b | 144 (12) | 44 (13) | ||
| N stage1 | N0 | 334 (28) | 38 (12) | < 0.0001 |
| N1 | 786 (66) | 261 (80) | ||
| N2 | 31 (3) | 15 (5) | ||
| N3 | 40 (3) | 13 (4) | ||
| Node status | N- | 334 (28) | 38 (12) | < 0.0001 |
| N+ | 857 (72) | 289 (88) | ||
| AJCC7 group | II | 767 (64) | 187 (57) | 0.0168 |
| III | 424 (36) | 140 (43) | ||
As shown in Table 1, patients who did not receive chemotherapy were older than those who did (71 vs 61, respectively, P < 0.001). Within the chemotherapy groups (Table 2), more patients received adjuvant chemotherapy (n = 290, 89%) than neoadjuvant chemotherapy (n = 37, 11%, P < 0.001). The mean ages of patients receiving chemotherapy were similar (65 and 61 years, neoadjuvant and adjuvant, respectively). The majority of patients were male in both chemotherapy groups. There was no difference in race/ethnicity, tumor location, tumor grade, T stage, N stage, node status or AJCC stage group.
| Characteristic | Neoadjuvantn = 37 | Adjuvantn = 290 | P value | |
| Age, yr | Mean (± SD) | 65 (± 10) | 61 (± 14) | 0.0583 |
| Sex | Men | 23 (62) | 198 (68) | 0.4543 |
| Women | 14 (38) | 92 (32) | ||
| Race/ ethnicity | Non-hispanic white | 18 (49) | 100 (34) | 0.2885 |
| Black | 3 (8) | 30 (10) | ||
| Hispanic white | 6 (16) | 82 (28) | ||
| Asian/pacific islanders | 10 (27) | 78 (27) | ||
| Tumor location | Proximal | 12 (32) | 86 (30) | 0.9189 |
| Distal | 10 (27) | 90 (31) | ||
| Whole | 6 (16) | 53 (18) | ||
| Middle | 9 (24) | 61 (21) | ||
| Grade | Well differentiated | 0 (0) | 1 (0) | 0.9087 |
| Moderately differentiated | 7 (19) | 63 (22) | ||
| Poorly differentiated | 27 (73) | 207 (71) | ||
| Undifferentiated | 2 (5) | 16 (6) | ||
| Unknown | 1 (3) | 3 (1) | ||
| Tumor size | ≤ 5 cm | 10 (27) | 107 (37) | 0.2297 |
| > 5 cm | 16 (43) | 129 (44) | ||
| Unknown | 11 (30) | 54 (19) | ||
| T stage1 | T2 | 0 (0) | 24 (8) | 0.4283 |
| T3 | 19 (51) | 144 (50) | ||
| T4a | 15 (41) | 81 (28) | ||
| T4b | 3 (8) | 41 (14) | ||
| N stage1 | N0 | 7 (19) | 31 (11) | 0.2684 |
| N1 | 27 (73) | 234 (81) | ||
| N2 | 1 (3) | 14 (5) | ||
| N3 | 2 (5) | 11 (4) | ||
| Node status | N- | 7 (19) | 31 (11) | 0.1413 |
| N+ | 30 (81) | 259 (89) | ||
| AJCC7 group | II | 23 (62) | 164 (57) | 0.5160 |
| III | 14 (38) | 126 (43) | ||
Patients who received chemotherapy were compared according to the timing of administration of chemotherapy (neoadjuvant vs adjuvant). By Kaplan-Meier method, a difference in overall survival was not observed between the neoadjuvant vs adjuvant chemotherapy groups (Figure 1A). This was consistent when the groups were evaluated by stage of disease as well (Figure 1B and C). Next, univariate and multivariate analyses were performed to identify factors associated with improved survival (Table 3). In univariate analysis, younger age, lower T stage, node negative status and Stage II disease were associated with improved survival. On multivariate analysis, older age and Stage III disease were independently associated with shorter survival. All other factors fell out of multivariate analysis and were not significant. When grouped by stage, age continued to be an independent predictor of survival in both the Stage II and III gastric cancer patients (data not shown).
| Factor | n (%) | Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |||
| Chemo status1 | Neo-Adjuvant | 37 (11) | - | - | - | - |
| Adjuvant | 290 (89) | 0.95 (0.65-1.38) | 0.7854 | 0.97 (0.67-1.43) | 0.8961 | |
| Age1, yr | Mean ± SD | 61.5 ± 14 | 1.02 (1.01-1.03) | 0.0008 | 1.02 (1.01-1.03) | 0.0006 |
| Sex | Men | 221 (68) | - | - | ||
| Women | 106 (32) | 1.00 (0.77-1.31) | 0.9859 | |||
| Race/ethnicity | Non-hispanic white | 118 (36) | - | - | ||
| Black | 33 (10) | 1.48 (0.96-2.27) | 0.0765 | |||
| Hispanic white | 88 (27) | 1.16 (0.84-1.59) | 0.3576 | |||
| Asian/pacific islanders | 88 (27) | 1.00 (0.73-1.37) | 1.0000 | |||
| Tumor location | Proximal | 98 (30) | - | - | ||
| Distal | 100 (31) | 0.90 (0.65-1.24) | 0.5173 | |||
| Whole | 59 (18) | 0.99 (0.69-1.43) | 0.9621 | |||
| Middle | 70 (21) | 0.94 (0.67-1.33) | 0.7395 | |||
| Grade | Well differentiated | 1 (0) | - | - | ||
| Moderately differentiated | 70 (21) | 0.38 (0.05-2.73) | 0.3338 | |||
| Poorly differentiated | 234 (72) | 0.48 (0.07-3.43) | 0.4642 | |||
| Undifferentiated | 18 (6) | 0.57 (0.07-4.31) | 0.5843 | |||
| Unknown | 4 (1) | 0.41 (0.04-4.55) | 0.4687 | |||
| Tumor size | ≤ 5cm | 117 (36) | - | - | ||
| > 5cm | 145 (44) | 1.33 (1.02-1.75) | 0.0379 | |||
| Unknown | 65 (20) | 0.98 (0.66-1.46) | 0.9322 | |||
| T stage | T2 | 24 (7) | - | - | ||
| T3 | 163 (50) | 2.18 (1.17-4.05) | 0.0135 | |||
| T4a | 96 (29) | 2.90 (1.54-5.45) | 0.0009 | |||
| T4b | 44 (13) | 4.86 (2.48-9.53) | < 0.0001 | |||
| N stage | N0 | 38 (12) | - | - | ||
| N1 | 261 (80) | 1.47 (0.99-2.20) | 0.0588 | |||
| N2 | 15 (5) | 1.98 (0.95-4.11) | 0.0666 | |||
| N3 | 13 (4) | 1.90 (0.94-3.84) | 0.0731 | |||
| Node status | N- | 38 (12) | - | - | ||
| N+ | 289 (88) | 1.51 (1.01-2.25) | 0.0449 | |||
| AJCC7 group1 | II | 187 (57) | - | - | - | - |
| III | 140 (43) | 1.79 (1.39-2.30) | < 0.0001 | 1.81 (1.41-2.33) | < 0.0001 | |
Despite advances in medical and surgical therapies, the prognosis of advanced gastric cancers remains poor, with a dismal 5-year relative survival rate of 28%[1]. With locoregional and distant recurrence rates approaching > 70%, an emphasis has been placed on identifying effective adjunct therapeutic regimens[18,17,20-23]. Systemic chemotherapy has been a logical choice, but the optimal timing of its administration remains unclear. In this study we compared the outcomes of Stage II-III surgically resected gastric cancer patients who received neoadjuvant (± postoperative chemotherapy) versus adjuvant chemotherapy and observed no difference in overall survival between the two treatment groups.
Several trials have examined the use of chemotherapy in the management of gastric cancer. One landmark study, the MAGIC trial by Cunningham et al[18], showed that perioperative chemotherapy with ECF decreased tumor size and stage while improving both progression-free and overall survival when compared to surgery alone. In this randomized controlled prospective study, 503 patients were randomly assigned to either perioperative ECF chemotherapy (three cycles preoperative and three cycles postoperative) or surgery alone. Of the perioperative group, only 41.6% completed all six cycles of chemotherapy due to disease progression, toxic effects, or complications[18]. The use of neoadjuvant chemotherapy decreased tumor size (3 cm vs 5 cm, P < 0.001) and stage of the pathologic surgical specimens. Therefore, by administering chemotherapy neoadjuvantly, the chance of curative resection by downstaging the tumor is increased. Other benefits of neoadjuvant chemotherapy include the elimination of micrometastasis, the improvement of tumor-related symptoms, and the determination of whether the tumor is chemotherapy-sensitive[18]. The MAGIC trial concluded perioperative chemotherapy should be considered in patients with resectable gastric cancers. Although we observed no difference between neoadjuvant and adjuvant timing of chemotherapy, nevertheless our findings suggest that patients with Stage II or Stage III gastric cancer indeed benefit from chemotherapy in conjunction with surgery. The 3-year OS was 16.6% in the MAGIC trial compared to 35% and 37% in the neoadjuvant and adjuvant arms, respectively, in our study. Given the inherent limitations of our database, we could not assess the effect of neoadjuvant chemotherapy on downstaging the gastric cancer, an outcome also not reported in the MAGIC trial.
The use of adjuvant chemotherapy has also been a treatment choice in the setting of chemotherapy. Adjuvant chemotherapy provides the benefit of removing disease burden upfront with a surgical resection, followed by chemotherapy. The potential downfall of adjuvant chemotherapy is the delay in beginning systemic treatment in the postoperative period for recovery from surgery. Further, downstaging tumor is not possible with adjuvant chemotherapy. Bang et al[20] evaluated the use of adjuvant chemotherapy after gastrectomy with D2 lymph node dissection in patients with Stage II-IIIB gastric cancer in the recent CLASSIC (Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer) trial. In this phase III randomized controlled, multi-institutional study, 1035 patients were randomized to surgery alone or surgery followed by chemotherapy (capecitabine plus oxaliplatin). The CLASSIC trial found a 34% reduction in the risk of death in the chemotherapy arm (HR = 0.66, 95%CI: 0.51-0.85; P = 0.0015) and a 5-year overall survival that was significantly increased in the chemotherapy arm (78% chemotherapy arm vs 69% surgery alone arm, P < 0.0029)[20,24]. However, the CLASSIC trial was reported in an eastern hemisphere patient population and has not been readily accepted in the United States[20,24]. Further, the CLASSIC trial had a 56% rate of grade 3 or 4 adverse effects (neutropenia, nausea, and vomiting) requiring dose modifications in a significant portion of patients, thereby limiting completion of adjuvant therapy[20].
The MAGIC and CLASSIC trials, however, did not examine the role and timing of radiation for gastric cancer. Adjuvant chemoradiation has been shown to improve overall survival in patients with locally advanced gastric cancer. Macdonald et al[16,17,25-27] reported in the INT0116 Phase III randomized multi-institutional trial of adjuvant chemoradiation compared to surgery alone that adjuvant chemoradiation improved overall survival and disease-free survival. Though Macdonald et al[16,17,25-27] evaluated adjuvant chemoradiation to surgery alone, there are no phase III trials that directly compare neoadjuvant with adjuvant chemoradiation. With the database used for this study, only receipt of radiation could be determined. Therefore, we could not assess the timing of radiation in relation to surgery and to exclude bias of radiation in our analysis, patients who received radiation were excluded from this current study.
Our study is not without its limitations. Due to the retrospective nature of this study, there may be patient selection bias. Surgical techniques and chemotherapy options have drastically changed over time. Therefore, resectability criteria have changed in that time period as well. Given the 18-year time period in our study, selection bias could play a role in neoadjuvant chemotherapy determination, surgical resectability criteria used, and adjuvant chemotherapy recommendations. However, we cannot determine in this retrospective study whether these biases would skew the results in any direction. To account for this, we limited our study to patients with Stage II and Stage III disease given that, in general, they are likely to have resectable disease. Stage I disease was omitted as Stage I patients routinely did not receive chemotherapy. The staging information in the CSP database is based on pathologic staging at the time of surgery. Therefore, we acknowledge that downstaging and decreased tumor burden could have occurred in the neoadjuvant chemotherapy group. However, to limit the potential bias of downstaging disease stage in the cohort that received neoadjuvant chemotherapy, Stage I and IV disease was omitted from our analysis. In particular, Stage I disease should be treated with surgery first, potentially followed with adjuvant chemotherapy. As such, patients who were documented to have Stage I disease and received neoadjuvant chemotherapy were more likely to have had downstaging of disease.
Another limitation is that although the CSP database provides coding for the receipt of chemotherapy and the first date of chemotherapy, we do not have data on the exact chemotherapy regimen (the type of chemotherapy, number of cycles, dose reductions, etc.) or on the successful completion of chemotherapy. Therefore, patients who did have preoperative chemotherapy determined by the first date of surgery could have also received postoperative chemotherapy.
Although the neoadjuvant cohort is smaller than the adjuvant group, our data does not appear underpowered. Prior to the study, a power calculation was performed. Assuming 80% power with a 2-sided log-rank alpha of 0.05 and based on the parameter estimates for neoadjuvant and adjuvant survival in stage II patients at 1 year (83% vs 77%, respectively), it would take a sample size of 699 patients in each group to find these differences statistically significant. This however, assumes that the 1 year survival curves (neoadjuvant vs adjuvant) are parallel and do not cross. Given that the survivals do cross (Figure 1), and due to the fact that the 3 year and 5 year results showing adjuvant survival is longer than neoadjuvant survival, a larger sample size would not change our overall conclusion.
As the management of gastric cancer continues to evolve, many questions remain unanswered. The extent of surgical resection, choice of adjunct therapy, and timing of therapy remain under debate. In this study, we compared survival following postoperative and preoperative chemotherapy, with similar outcomes observed between the preoperative and postoperative chemotherapy regimens. On the basis of these observations, we propose that a randomized, controlled trial be conducted to define the optimal timing of chemotherapy administration in the management of surgically resectable gastric cancer.
This study was presented at the 7th Annual Academic Surgical Congress held February 14-16, 2012.
Gastric adenocarcinoma is cancer of the stomach. The outcome of patients diagnosed with gastric cancer is determined by stage of disease. Though surgery remains the best curative-intent treatment, recurrence rates are unfortunately high. Multiple studies have evaluated the benefit of surgery alone or combined with chemotherapy, but there are few studies evaluating the timing of chemotherapy around surgery.
Multimodality therapies are now being investigated for the treatment of gastric cancer, such as chemoradiation and intraperitoneal chemotherapy; yet these therapies are not standard of care.
The mainstay of treatment is surgical resection either with perioperative chemotherapy or postoperative chemotherapy. Most chemotherapy regimens are either 5-fluorouracil (5-FU) based or cisplatin/oxaliplatin based. Asian studies have also shown benefit with adjuvant S-1, an oral dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine based on a biochemical modulation of 5-FU.
This study indicates that chemotherapy and surgery provides the best survival benefits for patients with gastric cancer. There was no difference in survival when comparing neoadjuvant chemotherapy to adjuvant chemotherapy alone.
Gastric adenocarcinoma: a cancer of the stomach; Neoadjuvant chemotherapy: chemotherapy given prior to surgical resection; Adjuvant chemotherapy: chemotherapy given after surgical resection.
The authors investigated outcomes for patients with gastric cancer treated with surgery and chemotherapy using a population-based cancer registry (n = 327). They demonstrated that both chemotherapy and surgical resection are critically important treatment modalities, while reporting no difference in overall survival between patients given neoadjuvant chemotherapy or adjuvant chemotherapy.
| 1. | National Cancer Institute. SEER Stat Fact Sheets: Stomach Cancer 2013. Available from: http://seer.cancer.gov/statfacts/html/stomach.html. |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10., in Cancer IAfRo (ed). Lyon, France: IARC 2013; . |
| 3. | Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14 Suppl 2:ii31-ii36. [PubMed] |
| 4. | Choi KS, Jun JK, Lee HY, Park S, Jung KW, Han MA, Choi IJ, Park EC. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci. 2011;102:1559-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921-932. [PubMed] |
| 7. | Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 454] [Article Influence: 13.8] [Reference Citation Analysis (7)] |
| 8. | Zhang H, Liu C, Wu D, Meng Y, Song R, Lu P, Wang S. Does D3 surgery offer a better survival outcome compared to D1 surgery for gastric cancer A result based on a hospital population of two decades as taking D2 surgery for reference. BMC Cancer. 2010;10:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Tian S. Does D2 plus Para-Aortic Nodal Dissection surgery offer a better survival outcome compared to D2 surgery only for gastric cancer consistently A definite result based on a hospital population of nearly two decades. Scand J Surg. 2013;102:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Thompson AM, Rapson T, Gilbert FJ, Park KG. Hospital volume does not influence long-term survival of patients undergoing surgery for oesophageal or gastric cancer. Br J Surg. 2007;94:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Shridhar R, Almhanna K, Hoffe SE, Fulp W, Weber J, Chuong MD, Meredith KL. Increased survival associated with surgery and radiation therapy in metastatic gastric cancer: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2013;119:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Choi JY, Ha TK, Kwon SJ. Clinicopathologic Characteristics of Gastric Cancer Patients according to the Timing of the Recurrence after Curative Surgery. J Gastric Cancer. 2011;11:46-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, Boot H, Putter H, Peeters KC, van de Velde CJ. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 15. | Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, Hirabayashi N, Mikata S, Iwahashi M, Fukushima R. Induction of a Pathological Complete Response by Four Courses of Neoadjuvant Chemotherapy for Gastric Cancer: Early Results of the Randomized Phase II COMPASS Trial. Ann Surg Oncol. 2013;Epub ahead of print. [PubMed] |
| 16. | Macdonald JS, Benedetti J, Smalley S, Haller D, Hundahl S, Jessup J, Ajani J, Gunderson L, Goldman B, Martenson J. Chemoradiation of resected gastric cancer: A 10-year follow-up of the phase III trial INT0116 (SWOG 9008), ASCO Annual Meeting, 2009, pp 4515. Available from: http: //meeting.ascopubs.org/cgi/content/abstract/27/15S/4515. |
| 17. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2460] [Article Influence: 98.4] [Reference Citation Analysis (1)] |
| 18. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4735] [Article Influence: 236.8] [Reference Citation Analysis (7)] |
| 19. | Yang L, Song Y, Zhou AP, Qin Q, Chi Y, Huang J, Wang JW. A phase II trial of oxaliplatin plus S-1 as a first-line chemotherapy for patients with advanced gastric cancer. Chin Med J (Engl). 2013;126:3470-3474. [PubMed] |
| 20. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1331] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 21. | Gunderson LL. Gastric cancer--patterns of relapse after surgical resection. Semin Radiat Oncol. 2002;12:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Takashima A, Boku N, Kato K, Nakamura K, Mizusawa J, Fukuda H, Shirao K, Shimada Y, Ohtsu A. Survival prolongation after treatment failure of first-line chemotherapy in patients with advanced gastric cancer: combined analysis of the Japan Clinical Oncology Group Trials JCOG9205 and JCOG9912. Gastric Cancer. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, Konishi M, Yasui H, Terashima M, Goto M. Survival analysis of adjuvant chemotherapy with S-1 plus cisplatin for stage III gastric cancer. Gastric Cancer. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Lee KH, Kim HH, Ji J, Chen JS, Lim Y. Adjuvant Capecitabine and Oxaliplatin (Xelox) for Gastric Cancer After D2 GastrectomyL: Final Results from the CLASSIC Trial. Ann Oncol. 2013;24 (suppl 4): iv14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Macdonald JS. Adopting postoperative chemoradiotherapy in resected gastric cancer. Gastrointest Cancer Res. 2009;3:245-246. [PubMed] |
| 26. | Macdonald JS. Gastric cancer--new therapeutic options. N Engl J Med. 2006;355:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Macdonald JS. Role of post-operative chemoradiation in resected gastric cancer. J Surg Oncol. 2005;90:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
P- Reviewers: Hajifathalian K, He ST, Lesquereux LM S- Editor: Wen LL L- Editor: A E- Editor: Wang CH