Published online May 27, 2012. doi: 10.4240/wjgs.v4.i5.126
Revised: October 15, 2011
Accepted: October 25, 2011
Published online: May 27, 2012
Presacral tumors are rare, but can comprise a great variety of histological types. Congenital tumors are the most common. Once the diagnosis is established, surgical resection is essential because of the potential for malignancy or infection. Previous biopsy is not necessary or may be even harmful. To decide the best surgical approach (abdominal, sacral or combined) an individual and multidisciplinary analysis must be carried out. We report three cases of cystic presacral masses in which a posterior approach (Kraske procedure) enabled complete resection, the only way to decrease local recurrence. All patients had a satisfactory recovery. A brief overview of retrorectal tumors is presented, focusing on classification, clinical presentation, diagnosis and surgical management.
- Citation: Aranda-Narváez JM, González-Sánchez AJ, Montiel-Casado C, Sánchez-Pérez B, Jiménez-Mazure C, Valle-Carbajo M, Santoyo-Santoyo J. Posterior approach (Kraske procedure) for surgical treatment of presacral tumors. World J Gastrointest Surg 2012; 4(5): 126-130
- URL: https://www.wjgnet.com/1948-9366/full/v4/i5/126.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i5.126
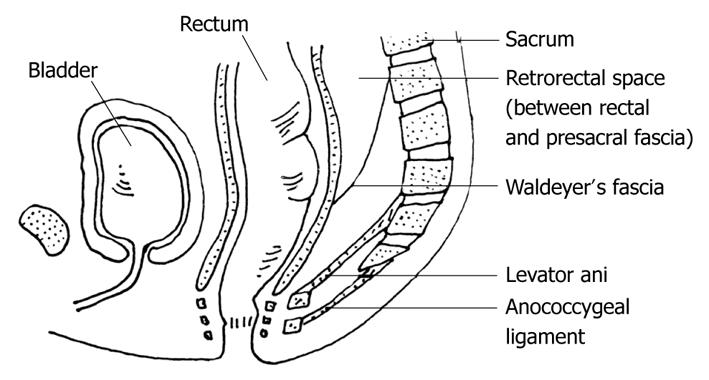
The presacral space is a peculiar anatomic area between the posterior wall of the rectum and the anterior surface of the sacrum, extending upwards to the peritoneal reflection and downwards to the rectosacral fascia (Waldeyer). The lateral boundaries are the endopelvic fascia (lateral ligaments), the ureters and the iliac vessels (Figure 1). A great variety of lesions can occur, due to the presence of multiple embryologic remnants and miscellany of tissue types within this area. Several classifications have been suggested by different authors, but none are universally accepted. Two of the most important concepts for categorization of a presacral mass are the benign or malignant character, and the subdivision into five categories according to the origin of the retrorectal mass [congenital (the most common), inflammatory, neurogenic, osseous, and miscellaneous (Table 1)]. It is also important to consider the cystic or solid character of these lesions, as solid masses are more likely to be malignant. These lesions may be silent over prolonged periods of time and often are discovered incidentally. Surgical resection is the best therapeutic option once the diagnosis is established, even in asymptomatic patients as there is potential for growth or malignancy[1-6]. However, surgical management may be challenging, not only technically but in determining the most appropriate approach: anterior, posterior or combined. In our experience, limited and conditioned by the low incidence of these lesions (as low as 1 in 40 000 hospital admissions), a trans-sacral approach (Kraske) provides an excellent surgical access and allows complete excision without morbidity.
| Benign | Malignant |
| Congenital | |
| Developmental cyst | Chordoma |
| Epidermoid cyst | Teratocarcinoma |
| Dermoid cyst | |
| Tailgut cyst | |
| Teratoma | |
| Anterior meningocele | |
| Rectal duplication | |
| Adrenal rest tumors | |
| Inflammatory | |
| Granuloma | - |
| Pelvirectal abscess | |
| Neurogenic | |
| Neurofibroma | Neurofibrosarcoma |
| Ependymoma | Malignant Schwanoma |
| Neuroblastoma | |
| Schwannoma | |
| Neurolemmoma | |
| Ganglioneuroma | |
| Osseus | |
| Osteoma | Chondrosarcoma |
| Simple bone cyst | Osteosarcoma |
| Ewing’s sarcoma | |
| Others | |
| Myelolipoma | Metastatic disease |
| Fibroma | Liposarcoma |
| Lymphangioma | Carcinoid |
| Desmoid tumor | Fibrosarcoma |
| Leiomyoma |
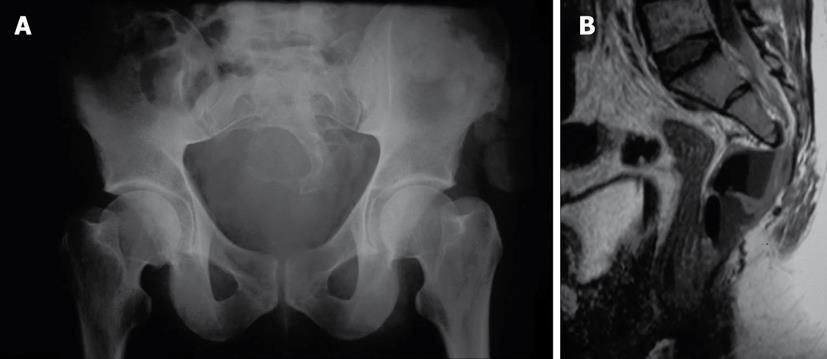
A 24-year-old male developed subacute headache, fever and vomiting. Neurological examination and lumbar punction suggested the diagnosis of acute meningitis. Culture of cerebrospinal fluid revealed the presence of faecal flora (Anaerobic enterococci, Bacteroides fragilis, Eschericia coli). These clinical findings and an unsatisfactory clinical evolution (with the appearance of significant weakness of both legs and repeated episodes of urinary retention) led to an exhaustive lumbar radiological study looking for a possible extraneurological origin. Simple X-ray images of the pelvis showed a sacral bony defect with a rounded concave border but without bony destruction (“scimitar sign”). Computed tomography (CT) and magnetic resonance imaging (MRI) diagnosed an anterior meningocele (Figure 2) in contact with the posterior wall of the rectum, with an air-liquid level that suggested the presence of a neuroenteric fistula that was diagnosed by rectoscopy. These findings explained the development of meningitis with faecal flora. The patient underwent a diverting colostomy, indicated to avoid continuous faecal contamination. Excision of the meningocele by a posterior approach was performed after the complete resolution of the meningeal disease, with appropriate closure of the rectum and of the dural space.
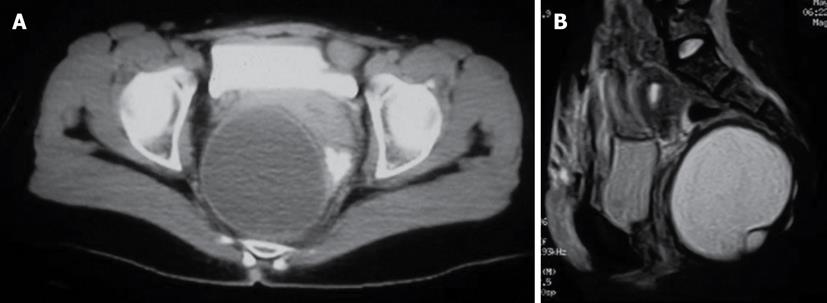
A 44-year-old woman underwent a gynecologic examination because of chronic pelvic pain. This exploration revealed an occupation of the Douglas space. The diagnosis of a cystic, hypogastric mass of 10 cm diameter was established by ultrasonography (US) and CT, but its relationship with the genital system was not clear. Exploration through a low transverse incision (Pfannenstiel) performed by the gynecologist revealed a presacral mass under the peritoneal reflection. A colorectal surgeon was informed and an elective and definitive treatment using a trans-sacral approach was performed after obtaining imaging of the sacral area with MRI to rule out potential neural, rectal or vesicle involvement (Figure 3).
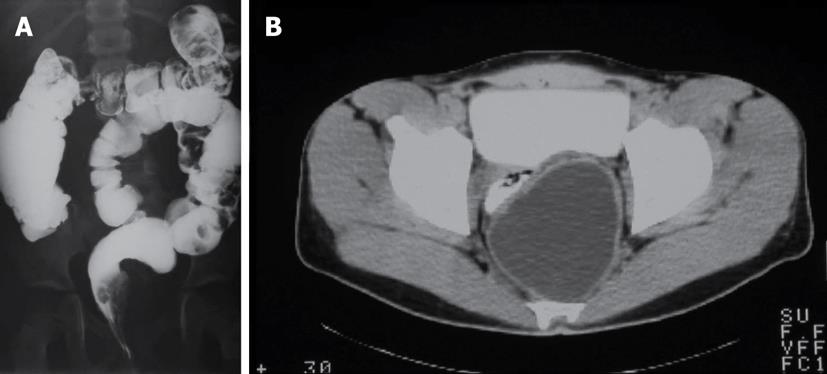
A 20-year-old male had severe constipation since infancy. A tentative diagnosis of ultrashort segment Hirschprung's disease was established, and an internal anal sphincterotomy was performed. At the age of 8 years he developed an episode of perianal pain with local inflammatory signs, and the patient underwent drainage of the left ischiorectal space, releasing purulent material with no microbiological findings. Chronic constipation continued and a complete radiologic examination was conducted, showing an effect in the lateral wall of the rectum (double-contrast barium enema) created by a 9 cm diameter mass in the presacral space (CT) (Figure 4). This was excised by an elective posterior approach.
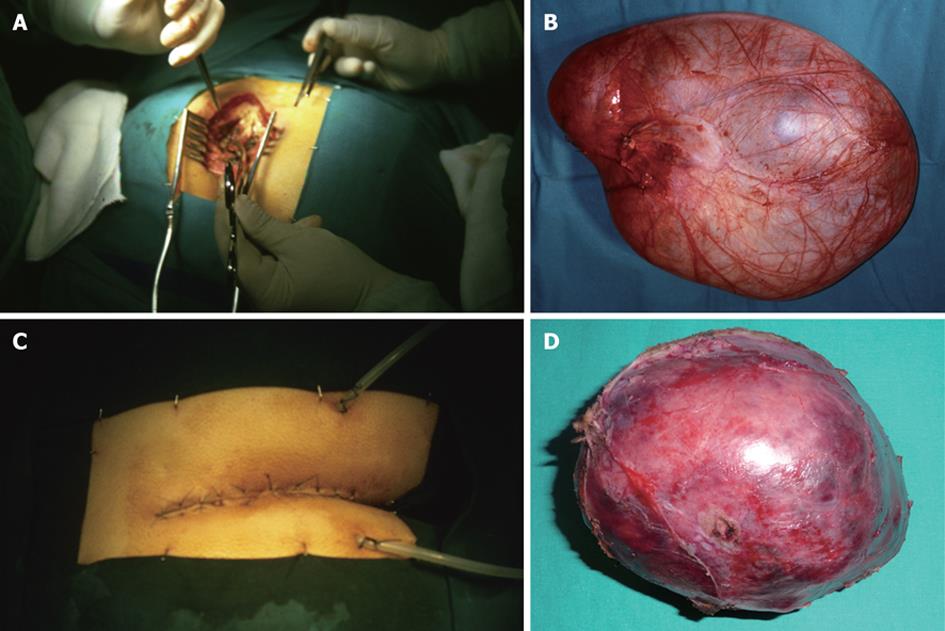
The three lesions were removed transperineally using a posterior approach (Kraske), with the patient placed in the prone-jackknife position with the buttocks spread. After an incision over the lower portion of the sacrum and coccyx down to the anus (which may be performed transversally or parasacral), the anococcygeal ligament was transected and the levator ani muscles were retracted laterally to allow the right approach to the presacral space. To provide a good exposure, excision of the coccyx was necessary in all three cases. Careful dissection was performed to separate the tumor from the rectum. Adequate reconstruction of the perineum and wound closure using suction drains completed the surgical technique[3]. Tumors were excised successfully, with complete resection in all three cases (Figure 5). The patients had an uncomplicated postoperative stay and a satisfactory recovery without morbidity. The histopathology revealed, respectively: meningocele, retrorectal cystic hamartoma and mature cystic teratoma (dermoid cyst). Follow-up with CT and MRI has not revealed any radiological evidence of local recurrence and the patients remain asymptomatic.
Although rare, presacral lesions can include various types of tumors. Two-thirds of them are congenital. The most common tumors encountered during infancy are teratomas, but the most common malignancy is sacrococcygeal chordoma. Almost 50% of presacral tumors are malignant or have areas of malignant change within them, with solid tumors having a higher probability of malignancy than cystic lesions (60% vs 10%)[1,2,6]. Retrorectal masses are typically slow growing, so vague pain in the perineal area is the most common symptom reported by patients, during malignant or inflammatory processes. Other clinical presentations are chronic constipation, rectal or urinary incontinence or sexual dysfunction. The presence of a midline sinus or fistulous tract in the sacrococcygeal area may indicate an underlying retrorectal mass, and this possibility must be taken into account in patients with refractory perianal suppuration or fistula. Complications related with pregnancy have also been described. Recurrent headaches associated with bowel movements should raise the suspicion of an anterior meningocele. However, retrorectal tumors may be silent over prolonged periods and are often only discovered incidentally[3-5].
A comprehensive clinical assessment including digital rectal examination is essential to establish a diagnosis. A simple X-ray of the sacrum may reveal indirect signs such as the presence of a presacral occupation with bony destruction (chordoma), the “scimitar sign” (anterior meningocele) or retrorectal calcifications (teratoma). CT and/or MRI of the sacral area have become the best diagnostic modalities for these lesions. Imaging is important not only for defining the radiological appearance and extension of the tumor, but also for diagnosing involvement with sacral or adjacent structures. These parameters are essential for establishing a preliminary diagnosis and deciding the best surgical approach. MRI has become particularly useful in delineating soft-tissue planes and defining bony and nerve invasion. Other diagnostic modalities like proctoscopy, barium enema or transrectal US may be helpful in selected cases. Needle biopsy should be avoided because complications including meningitis after punction of a meningocele, infection of a cystic tumor, malignant extension through the biopsy tract or bleeding can occur. Only in cases of unresectable lesions, or in patients with significant comorbidity that precludes pelvic surgery, may biopsies be performed for histological diagnosis if required to indicate adjuvant or palliative therapy.
Excision of a retrorectal tumor is essential, even in asymptomatic patients. The possibility of malignancy, potential dystocia in women of childbearing age, future malignant transformation (teratomas) or infection (meningoceles, cystic tumors) dictate that an aggressive attitude to the treatment of these lesions must be adopted. With complete excision of retrorectal tumors, survival for benign lesions is excellent and most local recurrences can be resected. Prognosis of malignant masses depends on the biological behaviour of the tumor. For chordomas, 5-year survival rate ranges from 67% to 84%, although local recurrence is common. Some data suggest that postoperative radiation therapy may increase the recurrence-free interval after resection of these tumors. A poorer prognosis has been reported for other malignancies[1-6].
A multidisciplinary team involving colorectal surgeons, neurosurgeons, orthopedic and plastic surgeons must decide between the various operative approaches recommended from large series of presacral tumors[7-10] (Table 2): the abdominal or anterior, the transsacral or posterior, and the combined abdominosacral. Other approaches such as transvaginal or transrectal routes have been less commonly reported. A wide en bloc resection must be performed to improve prognosis and survival, decreasing risk of recurrence. If sacrectomy is necessary, at least one side of S2 must be preserved in order to avoid urinary and bowel disturbance[6].
| Author (yr) | Patients | Period (yr) | Male/female | Age (yr) | Benign/malignant | Surgical approach (A/P/C) |
| Uhlig et al[7] 1975 | 63 | 30 | 17:46 | - | 37/26 | - |
| Jao et al[8] 1985 | 120 | 20 | 46:74 | 43 (0-81) | 69/51 | 21/79/2 (85% resectibility rate) |
| Wang et al[9] 1995 | 45 | 15 | 20:25 | 41 (15-76) | 23/22 | 24/13/6 (95% resectibility rate) |
| Lev-Chelouche et al[10] 2003 | 42 | 10 | 14:28 | 40 (21-84) | 21/21 | 18/21/3 |
An abdominal approach is especially indicated for lesions with the lowest extent above the S4 in the absence of nerve involvement. An anterior approach can achieve an excellent exposure of pelvic structures, iliac vessels and ureters. Usually a large midline incision is needed, but recent papers suggest that, adequate exposure and resection can be achieved with laparoscopic access, especially when malignancy has been excluded[11].
For larger lesions that extend above and below S4, the procedure of choice must be the combined approach, beginning with the patient in modified lithotomy position and entering the retroperitoneal space through the areolar plane between the mesorectum and the presacral fascia, to gain access for dissection of the upper part of the lesion. As progression in the deep pelvis and identification of planes between the tumor and surrounding tissues become more difficult, the patient may be repositioned in the jack-knife position or left in the modified lithotomy position for the perineal phase of the procedure, according to the preferences of the surgeon and the necessity of proctectomy with possible creation of a low-colorectal or coloanal anastomosis.
For benign lesions that do not extend above S4, or when there is sacral involvement, a posterior approach is preferred. If the proximal extent of the retrorectal mass is palpable on digital rectal exploration, it must be assumed that a complete excision by the posterior approach is feasible. Details of this surgical technique have been previously described. Coccygectomy can be indicated not only for exposure but for allowing the complete removal of a potential communication route and consequent recurrence of cystic lesions or teratomas. The major disadvantages that have been argued for the posterior approach are the absence of control over pelvic vessels, and the potential for injury to the lateral pelvic nerves[2,3,6]. These drawbacks may be minimized by the careful selection of cases amenable to this approach: low-lying tumors, tumors that are benign or cystic or those originating from or penetrating into the sacral bone. Our experience suggests that even larger cystic lesions may be excised safely by the posterior approach, though they are normally opened and drained to provide a complete resection.
In summary, the posterior approach can be helpful in the surgical management and outcome of low-lying presacral masses. This approach must be included in the therapeutic arsenal of colorectal surgeons and performed in specialized units.
| 1. | Glasgow SC, Birnbaum EH, Lowney JK, Fleshman JW, Kodner IJ, Mutch DG, Lewin S, Mutch MG, Dietz DW. Retrorectal tumors: a diagnostic and therapeutic challenge. Dis Colon Rectum. 2005;48:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Hobson KG, Ghaemmaghami V, Roe JP, Goodnight JE, Khatri VP. Tumors of the retrorectal space. Dis Colon Rectum. 2005;48:1964-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Buchs N, Taylor S, Roche B. The posterior approach for low retrorectal tumors in adults. Int J Colorectal Dis. 2007;22:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Glasgow SC, Dietz DW. Retrorectal tumors. Clin Colon Rectal Surg. 2006;19:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Bullard Dunn K. Retrorectal tumors. Surg Clin North Am. 2010;90:163-71, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Ghosh J, Eglinton T, Frizelle FA, Watson AJ. Presacral tumours in adults. Surgeon. 2007;5:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Uhlig BE, Johnson RL. Presacral tumors and cysts in adults. Dis Colon Rectum. 1975;18:581-589. [PubMed] |
| 8. | Jao SW, Beart RW, Spencer RJ, Reiman HM, Ilstrup DM. Retrorectal tumors. Mayo Clinic experience, 1960-1979. Dis Colon Rectum. 1985;28:644-652. [PubMed] |
| 9. | Wang JY, Hsu CH, Changchien CR, Chen JS, Hsu KC, You YT, Tang R, Chiang JM. Presacral tumor: a review of forty-five cases. Am Surg. 1995;61:310-315. [PubMed] |
| 10. | Lev-Chelouche D, Gutman M, Goldman G, Even-Sapir E, Meller I, Issakov J, Klausner JM, Rabau M. Presacral tumors: a practical classification and treatment of a unique and heterogeneous group of diseases. Surgery. 2003;133:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Chen Y, Xu H, Li Y, Li J, Wang D, Yuan J, Liang Z. Laparoscopic resection of presacral teratomas. J Minim Invasive Gynecol. 2008;15:649-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
Peer reviewer: Tsukasa Hotta, MD, PhD, Department of Surgery, Wakayama Medical University, School of Medicine, 811-1, Kimiidera, Wakayama 641-8510, Japan
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM