Published online Jan 27, 2026. doi: 10.4240/wjgs.v18.i1.114477
Revised: October 18, 2025
Accepted: November 21, 2025
Published online: January 27, 2026
Processing time: 123 Days and 15.8 Hours
Chronic calculous cholecystitis (CCC), caused by persistent gallstone-induced inflammation, can lead to development of small cell neuroendocrine carcinoma (SCNEC) of the gallbladder through chronic irritation, cellular damage, and re
A 64-year-old woman with a history of CCC presented with persistent right upper quadrant pain. Imaging revealed a mass in the gallbladder and hepatic hilum. The patient underwent radical cholecystectomy and hepatic resection. Histopathological examination confirmed SCNEC with synaptophysin and CD56 positivity and Ki-67 of 80%. Due to severe hepatic dysfunction (total bilirubin ≥ 300 μmol/L, albumin ≤ 25 g/L), chemotherapy was contraindicated. The patient’s condition deteriorated rapidly, and she died 2 months postoperatively.
Early detection and surgical intervention are critical for improving outcomes in gallbladder SCNEC associated with chronic inflammation.
Core Tip: Gastrointestinal neuroendocrine tumors are relatively common, but gallbladder small cell carcinoma (SCC) is an extremely rare subtype, with limited clinical reports and no established treatment guidelines. Most clinicians manage SCC as gallbladder cancer, yet the 5-year survival rate for early- and mid-stage SCC exceeds 94%, significantly higher than that of typical gallbladder adenocarcinoma. Misdiagnosis and delayed treatment can significantly increase mortality. Due to the rarity of cases, clinicians often lack familiarity with the clinical features and imaging characteristics of gallbladder SCC, leading to diagnostic challenges. This report highlights the importance of timely recognition and radical surgical management to improve outcomes.
- Citation: Yu JQ, He XS, Xue YZ, Hu LY, Wen X, Yang XD, Zhou X. Gallbladder small cell carcinoma from chronic cholecystitis: A case report and review of literature. World J Gastrointest Surg 2026; 18(1): 114477
- URL: https://www.wjgnet.com/1948-9366/full/v18/i1/114477.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v18.i1.114477
Gallbladder cancer (GBC) is a rare malignancy originating in the extrahepatic biliary tract[1]. It accounts for 80%-95% of all biliary tract tumors, and the prognosis is poor, with a 5-year survival rate of only 5%[2]. Adenocarcinoma is the most common histological subtype, accounting for approximately 85% of GBC cases, while rare subtypes include squamous cell carcinoma, adenosquamous carcinoma, neuroendocrine carcinoma, undifferentiated tumor, and sarcoma[3]. Most GBC cases are associated with chronic calculous cholecystitis (CCC), with gallstones identified in approximately 90% of cases[4]. GBC is not sensitive to radiotherapy or chemotherapy[5]. Therefore, radical resection is still the main treatment method for gallbladder malignancy. The key to determine the scope of surgical resection is to accurately determine the depth of tumor infiltration before surgery. National Comprehensive Cancer Network guidelines recommend stage 4b and stage 5 resection for stage T2 GBC, along with extended hepatectomy, hilar lymph node dissection, or bile duct resection[6].
Small cell neuroendocrine carcinoma (SCNEC) of the gallbladder is a rare and highly aggressive neuroendocrine tumor characterized by early lymphatic and distant metastases, with a 5-year survival rate of 0%[7]. Since the first case was reported by Albores et al[8] in 1981, < 100 cases have been documented in the literature, mostly as individual case reports. Consequently, the clinical understanding of this rare entity remains limited.
In this paper, we present a case of SCNEC of the gallbladder confirmed by pathology following surgical resection at our hospital. Through this case report and literature review, we aimed to enhance the understanding of this rare and challenging malignancy.
On August 21, 2018, a 48-year-old woman was admitted to the Department of Hepatobiliary Surgery at The Second Affiliated Hospital of Jiaxing University with complaints of intermittent upper abdominal pain for 2 days.
The patient initially presented to the emergency department on August 21, 2018, with intermittent upper abdominal pain. She had a 20-year history of hypertension, but her endocrine function and blood glucose levels were normal upon admission. The preoperative diagnosis was: Acute attack of CCC, gallbladder mass, hypertension, bilateral adnexal cyst, and posterior uterine mass. Laparoscopic cholecystectomy was performed in our hospital. Postoperatively, the patient survived for 2 months after being diagnosed with SCNEC of the gallbladder.
The patient underwent left thyroidectomy 20 years ago and recovered well. She also had a 5-year history of hypertension, for which she regularly took one 20 mg tablet of nifedipine daily. Her blood pressure was usually controlled at 130/85 mmHg. There were no other significant medical conditions.
The patient denied any family history of cancer.
The patient experienced right upper abdominal pain with a positive Murphy’s sign. There were no abdominal distension, nausea, vomiting, or signs of jaundice (skin yellowing or dark urine). She reported no chest tightness, shortness of breath, diarrhea, black stool, or intermittent cessation of bowel movements. The patient did not complain of urinary symptoms, including hematuria, frequent urination, urgency, or dysuria.
Laboratory tests on admission showed no significant abnormalities. During surgery, a mass was identified in the right upper abdomen, which was adherent to the surrounding omentum and small intestine, although it did not involve the spleen, stomach, or colon. The hilar and duodenal ligament lymph nodes were swollen and merged into a mass approximately 7 cm × 7 cm in size.
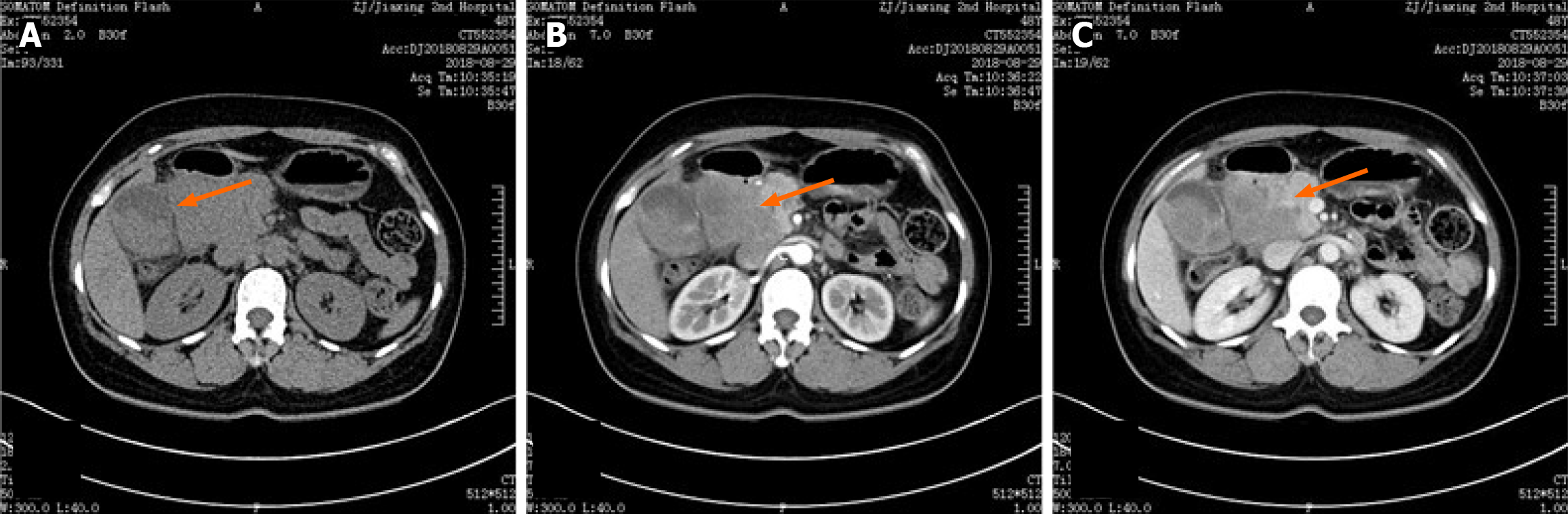
Abdominal computed tomography (CT; plain and enhanced scan; Figure 1). Plain CT revealed gallbladder enlargement with thickening of the cystic wall, and multiple nodular dense shadows within the gallbladder and cystic duct. On contrast enhancement, the gallbladder wall exhibited mild enhancement with adjacent oozing shadows, and a mixed-density mass in the hepatic portal region showed mild enhancement (Figure 1A). The arterial enhancement pattern (Figure 1B) correlated with mild enhancement of the hepatic portal mass. CT also revealed a nodule in the left posterior portion of the uterus.
The patient was diagnosed with primary SCNEC of the gallbladder, stage T3N2M1. Based on hematoxylin and eosin staining and immunohistochemical analysis, the gallbladder tumor was diagnosed as poorly differentiated neuroendocrine carcinoma, with features consistent with SCNEC.
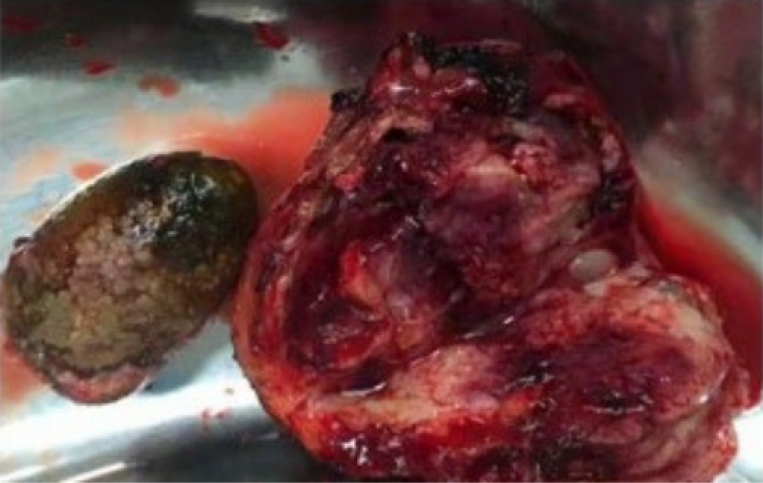
Given the patient's young age, the nature of the gallbladder tumor was initially unclear. After careful discussion with her husband, a decision was made to proceed with surgery. During the operation, the gallbladder was found to be significantly enlarged, measuring approximately 12 cm × 4 cm × 3 cm. The gallbladder wall was thickened, and the tumor was prominently located at the base of the gallbladder bed. Multiple gallstones were also present within the gallbladder (Figure 2).
During surgery, the hilar and duodenal ligament lymph nodes were found to be swollen and merged into a mass approximately 7 cm × 7 cm in size. The mass adhered to the omentum and small intestine. After separating the adhesions, the borders of the tumor were not clearly defined by the surrounding blood vessels. Additionally, a 1.5-cm diameter tumor was discovered in the rectouterine pouch. This mass had a tough texture and poor mobility, suggesting possible metastatic involvement. The triangular structure of the gallbladder was obscured, and only a partial resection of the visible gallbladder could be performed.
The tumor was immediately sent for frozen section examination. After 30 minutes, the results indicated that the gallbladder malignancy had invaded through all layers, and conventional pathological sections with immunohistochemistry were required for further analysis. We informed the patient's family that the tumor was at an advanced stage, with extensive invasion and metastasis within the abdominal cavity. Radical surgery requires removal of most of the liver, including the caudate lobe, left extrahepatic lobe, left intrahepatic lobe, and right posterior lobe, but this is extremely likely to cause postoperative liver failure. Therefore, it was not feasible, and we decided to terminate the operation at that point.
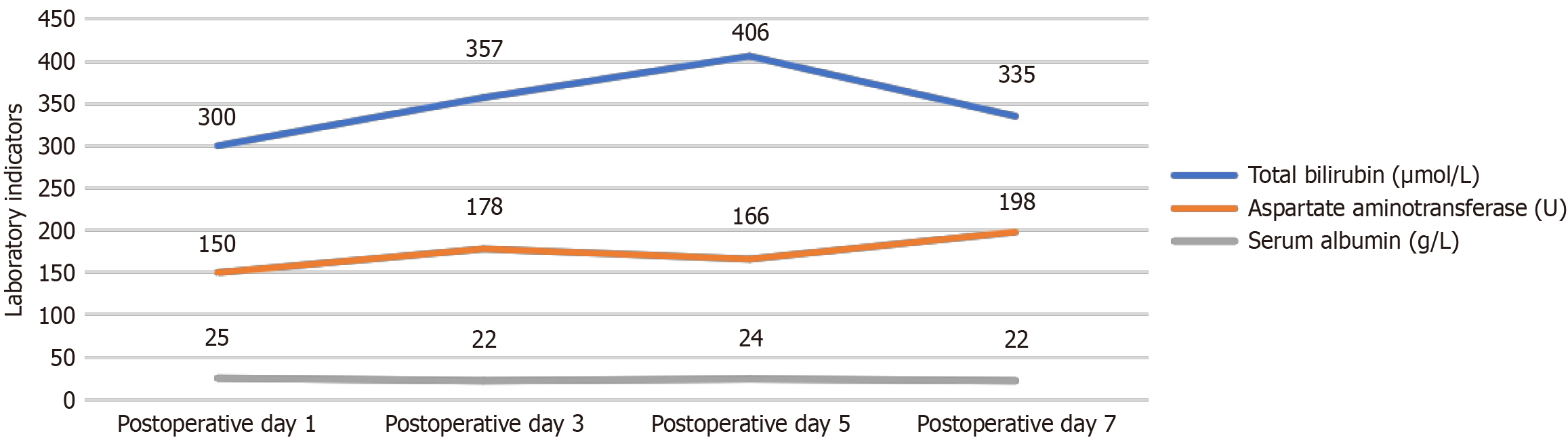
After laparoscopic surgery, the patient survived for 2 months following initial diagnosis of SCNEC. SCNEC of the gallbladder is an aggressive and challenging disease. Following surgery, the patient’s bilirubin levels increased within 1 month, and liver function became abnormal. She was extremely weak. The patient’s postoperative laboratory tests revealed markedly abnormal results: Total bilirubin ≥ 300 μmol/L, aspartate aminotransferase ≥ 150 U/L, and serum albumin ≤ 25 g/L (Figure 3). Given her severely impaired liver function and poor general condition, chemotherapy could not be administered. After discussing her case through a multidisciplinary team (MDT) approach, we decided to proceed with percutaneous transhepatic cholangiography drainage as a symptomatic treatment, hoping to improve her liver function. However, by early February 2018, the patient developed refractory anemia (with gastrointestinal bleeding ruled out). Despite our best efforts, chemotherapy was not an option. The patient died 2 months after surgery.
Neuroendocrine tumors are rare malignancies originating from diffuse neuroendocrine cells, representing < 1% of all malignant tumors. They are most commonly found in the gastrointestinal (66%) and respiratory (31%) tracts[9]. Gastrointestinal neuroendocrine tumors are more frequently located in the rectum, jejunum, and pancreas[10]. Recently, studies have found that the occurrence of epithelial malignant tumors, such as colorectal cancer, is associated with chronic inflammation[11]. The patient also had a history of CCC. The presence of gallstones leads to periodic cell death and regeneration of the epithelial layer cells, which maintains the gallbladder in a constant state of inflammatory stimulation[12]. Since the 19th century, researchers have been concerned about the correlation between chronic cholecystitis (especially with mixed gallstones) and GBC. Hsing et al[13] reported that chronic gallstone disease contributes to a 21-57-fold increase in the risk of developing GBC. Mhatre et al[14] also found that gallstones were associated with a high incidence of GBC. Therefore, we also believe that chronic inflammatory stimulation is closely related to the development of gallbladder neuroendocrine cancer. Gallbladder neuroendocrine cancers, however, are extremely rare[15]. According to the American Cancer Institute’s Surveillance, Epidemiology, and End Results database, gallbladder neuroendocrine tumors account for only 0.5% of all neuroendocrine tumors and 2.1% of all gallbladder tumors[16]. Duffy et al[17] reported that neuroendocrine cancer constituted 3% of the 435 cases of GBC at Memorial Sloan Kettering Cancer Center, NY, United States, from 1995 to 2005.
SCNEC of the gallbladder is a rare and aggressive subtype of gallbladder neuroendocrine carcinoma[18]. Currently, limited research has been conducted on its therapy, and its origin remains unclear. Some researchers speculate that SCNEC arises from metaplasia of the gallbladder wall epithelium, with CCC potentially contributing to the development of metaplastic tissue in the gallbladder wall[19-21].
The clinical presentation of SCNEC is often delayed due to vague or nonspecific symptoms. Many patients present with symptoms similar to CCC, including right upper quadrant pain and discomfort. Additionally, symptoms of acute cholecystitis or cholangitis, such as bloating, jaundice, and fever, may complicate the diagnosis. In rare cases, patients may present with carcinoid syndrome, manifesting as skin flushing, abdominal pain, diarrhea, asthma, and other symptoms. Some patients may also exhibit cancer-associated syndromes, including hypercalcemia, Cushing's syndrome, hyponatremia, and neurological symptoms[22,23].
Histologically, gallbladder SCNEC can be classified into oat cell, small round cell, and mixed types. About 25% of cases of small cell carcinoma (SCC) also have an associated adenocarcinoma component[24]. Additionally, mixed forms of gallbladder SCNEC with squamous cell carcinoma, clear cell carcinoma, or sarcoma have been reported[25,26]. SCNEC of the gallbladder is easily missed or misdiagnosed, and postoperative histopathological examination remains the gold standard for diagnosis. The cytoplasm of the tumor cells typically expresses neuroendocrine markers such as Syn and chromogranin A[27], and more recently, CD56 has been identified as a key marker for diagnosis[28]. In this case, both Syn and CD56 were positive.
Gallbladder SCNEC is highly malignant, with rapid disease progression, frequent recurrence, and metastasis occurring shortly after surgery. Liver invasion or lymph node metastasis is common, and there are currently no established surgical guidelines for its management[29]. Chemotherapy remains the cornerstone of treatment, and protocols commonly used for small cell lung cancer (SCLC) are applied, including the etoposide plus cisplatin (EP) regimen, which has demonstrated an effective rate of 69%-73% in SCLC[30,31]. However, the effects are usually short-lived, with a median survival < 1 year. In the present case, the EP regimen was considered but ultimately not administered following an MDT discussion. The patient’s postoperative total bilirubin was ≥ 300 μmol/L, aspartate aminotransferase ≥ 150 U/L, and serum albumin ≤ 25 g/L, indicating severe hepatic dysfunction. Her overall performance status (Eastern Cooperative Oncology Group 4) also suggested poor tolerance to cytotoxic therapy. Therefore, chemotherapy was contraindicated due to the high risk of toxicity and limited potential benefit. Treatment of metastatic disease with chemotherapy has shown a significant survival benefit compared to no chemotherapy, and the use of platinum doublet with etoposide demonstrated a nonsignificant 4-month improvement in survival compared to other regimens[32]. In contrast, Imai et al[30] reported a case of gallbladder SCC treated with surgery followed by adjuvant chemotherapy using etoposide and cisplatin, in which the patient achieved markedly prolonged survival. This contrast highlights the potential benefit of systemic chemotherapy in improving outcomes when patients are in a suitable physiological condition to tolerate treatment.
Due to the limited efficacy of current chemotherapy options, researchers are exploring new drugs and therapeutic approaches to improve treatment outcomes. Targeted therapies have shown promise in other neuroendocrine cancers. For example, sunitinib has demonstrated significant results in pancreatic neuroendocrine tumors, with a phase 3 clinical study in France showing a progression-free survival of 11.4 months in the treatment group, compared to 5.5 months in the placebo group[33]. However, no clinical studies have been conducted specifically on gallbladder SCNEC.
In recent years, immunotherapy has made significant advances in the treatment of cancers. Immune checkpoint inhibitors, including anti-PD-1 agents (nivolumab and pembrolizumab) and anti-PD-L1 agents (atezolizumab and durvalumab), have demonstrated efficacy in hepatocellular carcinoma and biliary tract cancers[34,35]. In particular, the TOPAZ-1 trial reported improved overall survival with durvalumab plus gemcitabine-cisplatin, and nivolumab achieved durable responses in refractory cases[35,36]. Approximately 20%-30% of biliary tract tumors express PD-L1, supporting the rationale for immunotherapy in this tumor type[37-39]. Although no clinical studies have addressed gallbladder SCC, these findings suggest that checkpoint inhibitors may offer potential benefit. It is hoped that ongoing advances in basic and clinical research will lead to the development of targeted immunotherapy regimens, which could become a milestone in the treatment of this rare and aggressive disease.
Although early-stage SCNEC of the gallbladder can achieve a 5-year survival rate > 94% with timely surgical intervention, most cases are diagnosed at an advanced stage, when curative treatment is no longer feasible. This case underscores the importance of early detection in high-risk populations, particularly patients with long-standing CCC. Routine imaging surveillance and comprehensive clinical assessment should be considered to facilitate early diagnosis and improve prognosis. Establishing clear screening and management protocols for high-risk patients may help detect SCNEC at a curable stage and significantly enhance long-term survival.
The following conclusions can be drawn from this case. The 5-year survival rate of patients with GBC diagnosed at an early stage is significantly higher than that of patients with intermediate or advanced-stage GBC. Therefore, we recommend active surgical intervention for patients with gallbladder stones and other conditions that meet the indications for cholecystectomy. For patients unwilling to undergo surgical treatment, regular follow-up examinations are mandatory. Currently available radiomics-based robotic deep learning techniques can improve the detection rate of early-stage GBC. The author is currently drafting a meta-analysis focusing on the diagnostic accuracy of GBC after deep learning applications, aiming to enrich the diagnostic methods for early-stage GBC.
SCNEC of the gallbladder is an extremely rare and highly malignant tumor with an insidious onset and poor prognosis. Patients with chronic cholecystitis should be particularly vigilant when there is irregular thickening of the gallbladder wall or unclear ultrasound findings. Early treatment, including surgical resection followed by adjuvant chemotherapy, is recommended to improve outcomes. In advanced stages, a chemotherapy-based MDT approach is advised. Due to the rarity of this disease, most reports on treatment and prognosis are based on case reports or retrospective studies, with no prospective studies available to date. Therefore, there is an urgent need for international multicenter randomized controlled trials to determine the most effective treatment strategies for this aggressive disease.
| 1. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 514] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 2. | Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. 2022;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 3. | Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol. 2017;23:3978-3998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 212] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (4)] |
| 4. | Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, Fraumeni JF Jr. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - A systematic review. Eur J Surg Oncol. 2019;45:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 6. | Benson AB 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Schmidt C, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Zhu AX, Hoffmann KG, Darlow S. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 7. | Eltawil KM, Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol. 2010;44:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Albores-Saavedra J, Cruz-Ortiz H, Alcantara-Vazques A, Henson DE. Unusual types of gallbladder carcinoma. A report of 16 cases. Arch Pathol Lab Med. 1981;105:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, Brierley J, Siu LL. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. 2008;31:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Oberg K. Neuroendocrine tumors: recent progress in diagnosis and treatment. Endocr Relat Cancer. 2011;18 Suppl 1:E3-E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, Zhang D, Xia H, Xu X, Jie Z, Su L, Li X, Li X, Li J, Xiao L, Huber-Schönauer U, Niederseer D, Xu X, Al-Aama JY, Yang H, Wang J, Kristiansen K, Arumugam M, Tilg H, Datz C, Wang J. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 1036] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 12. | Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4:695-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Hsing AW, Bai Y, Andreotti G, Rashid A, Deng J, Chen J, Goldstein AM, Han TQ, Shen MC, Fraumeni JF Jr, Gao YT. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2007;121:832-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Mhatre S, Richmond RC, Chatterjee N, Rajaraman P, Wang Z, Zhang H, Badwe R, Goel M, Patkar S, Shrikhande SV, Patil PS, Davey Smith G, Relton CL, Dikshit RP. The Role of Gallstones in Gallbladder Cancer in India: A Mendelian Randomization Study. Cancer Epidemiol Biomarkers Prev. 2021;30:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1875] [Article Influence: 81.5] [Reference Citation Analysis (1)] |
| 16. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3314] [Article Influence: 184.1] [Reference Citation Analysis (0)] |
| 17. | Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH, O'Reilly EM. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 18. | Yao X, Wu K, Lu B, Lin F. Neuroendocrine carcinoma of the gallbladder: A case report and literature review. Medicine (Baltimore). 2024;103:e39147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Matsuo S, Shinozaki T, Yamaguchi S, Matsuzaki S, Takami Y, Hayashi T, Kanematsu T. Small-cell carcinoma of the gallbladder: report of a case. Surg Today. 2000;30:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Chuang SS, Lin CN, Chu CH, Chen FF. Small cell carcinoma of the gallbladder: report of two cases. Pathol Oncol Res. 1999;5:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Kuwabara H, Uda H. Small cell carcinoma of the gall-bladder with intestinal metaplastic epithelium. Pathol Int. 1998;48:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Uribe-Uribe NO, Jimenez-Garduño AM, Henson DE, Albores-Saavedra J. Paraneoplastic sensory neuropathy associated with small cell carcinoma of the gallbladder. Ann Diagn Pathol. 2009;13:124-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Ng ES, Venkateswaran K, Ganpathi SI, Chuah BY. Small cell gallbladder carcinoma complicated by paraneoplastic hyponatremia: a case report and literature review. J Gastrointest Cancer. 2010;41:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Shimizu T, Tajiri T, Akimaru K, Arima Y, Yoshida H, Yokomuro S, Mamada Y, Taniai N, Mizuguchi Y, Kawahigashi Y, Naito Z. Combined neuroendocrine cell carcinoma and adenocarcinoma of the gallbladder: report of a case. J Nippon Med Sch. 2006;73:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Cavazza A, Abrate M, Corrado S, Ciamprone G, Putrino I, Scotti R, De Marco L, Piana S. [Localized lymph node lymphangiomyoma. Description of 2 cases]. Pathologica. 2000;92:530-533. [PubMed] |
| 26. | Takahashi Y, Fukushima J, Fukusato T, Shiga J. Sarcomatoid carcinoma with components of small cell carcinoma and undifferentiated carcinoma of the gallbladder. Pathol Int. 2004;54:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Maitra A, Tascilar M, Hruban RH, Offerhaus GJ, Albores-Saavedra J. Small cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and molecular pathology study of 12 cases. Am J Surg Pathol. 2001;25:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Beach DF, Klump WJ, Haddad G, Reid LM, Schwarting R, Hageboutros A. Extrapulmonary small cell: a novel case of small cell carcinoma of the thyroid gland. Med Oncol. 2012;29:1405-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Maitra A, Krueger JE, Tascilar M, Offerhaus GJ, Angeles-Angeles A, Klimstra DS, Hruban RH, Albores-Saavedra J. Carcinoid tumors of the extrahepatic bile ducts: a study of seven cases. Am J Surg Pathol. 2000;24:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Imai H, Matsui S, Tokuyama Y, Osada S, Tomita H. Small cell carcinoma of the gallbladder successfully treated by surgery and adjuvant chemotherapy. Am Surg. 2008;74:272-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Usmani S, Pazooki M, Bilgrami SF. Small cell carcinoma of the gall bladder: role of adjuvant chemotherapy. J Gastrointest Cancer. 2010;41:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Carrera C, Kunk P, Rahma O. Small Cell Carcinoma of the Gallbladder: Case Report and Comprehensive Analysis of Published Cases. J Oncol. 2015;2015:304909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Vinik A, Bottomley A, Korytowsky B, Bang YJ, Raoul JL, Valle JW, Metrakos P, Hörsch D, Mundayat R, Reisman A, Wang Z, Chao RC, Raymond E. Patient-Reported Outcomes and Quality of Life with Sunitinib Versus Placebo for Pancreatic Neuroendocrine Tumors: Results From an International Phase III Trial. Target Oncol. 2016;11:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3442] [Article Influence: 382.4] [Reference Citation Analysis (1)] |
| 35. | Burris HA 3rd, Okusaka T, Vogel A, Lee MA, Takahashi H, Breder V, Blanc JF, Li J, Bachini M, Żotkiewicz M, Abraham J, Patel N, Wang J, Ali M, Rokutanda N, Cohen G, Oh DY. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer (TOPAZ-1): patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024;25:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 36. | Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, Schell MJ, Zhou JM, Mahipal A, Kim BH, Kim DW. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020;6:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 37. | Ahn S, Lee JC, Shin DW, Kim J, Hwang JH. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. 2020;10:12348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Frega G, Cossio FP, Banales JM, Cardinale V, Macias RIR, Braconi C, Lamarca A. Lacking Immunotherapy Biomarkers for Biliary Tract Cancer: A Comprehensive Systematic Literature Review and Meta-Analysis. Cells. 2023;12:2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Mody K, Starr J, Saul M, Poorman K, Weinberg BA, Salem ME, VanderWalde A, Shields AF. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J Gastrointest Oncol. 2019;10:1099-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/