Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.107544
Revised: April 15, 2025
Accepted: May 21, 2025
Published online: July 27, 2025
Processing time: 117 Days and 3.4 Hours
Pyogenic liver abscess (PLA) is a prevalent liver infection with gradual onset and severe symptoms, including fever, abdominal pain, jaundice, and vomiting. Complications like sepsis or toxic shock can also occur.
To investigate the clinical value of early ultrasound-guided percutaneous drai
This retrospective analysis included 143 patients with PLA who were admitted to the Department of General Surgery between January 2018 and March 2023. All patients underwent ultrasound-guided PCD. Based on the liquefaction status of the abscess, patients were divided into two groups: Liquefied group and non-liquefied group. Clinical outcomes, including puncture success rate, puncture duration, length of hospital stay, time to fever resolution, abscess shrinkage rate, and complication rates, were compared between the two groups.
The puncture success rate for all patients was 99.3%, with a postoperative com
Early ultrasound-guided PCD can be safely and effectively performed in PLA, even when the abscess is not fully liquefied or is non-liquefied, supporting the clinical feasibility of early intervention.
Core Tip: This study demonstrated that early ultrasound-guided percutaneous drainage (PCD) is safe and effective for pyogenic liver abscess (PLA), even in non-liquefied abscesses. By analyzing 143 PLA patients, we found that non-liquefied cases treated with early PCD had shorter hospital stays (3.9 vs 5.1 days), faster fever resolution (2.4 vs 4.9 days), and quicker abscess shrinkage compared to liquefied abscesses, with no increase in complications. These results challenge the traditional requirement for abscess liquefaction prior to intervention, advocating for early PCD to accelerate recovery and reduce healthcare burdens. This approach redefines clinical strategies for PLA management.
- Citation: Qiu F, Yang TC, Han W. Clinical application of ultrasound-guided surgical puncture and drainage in early treatment of pyogenic liver abscess. World J Gastrointest Surg 2025; 17(7): 107544
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/107544.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.107544
Pyogenic liver abscess (PLA) is a common hepatic infectious disease that typically presents with an insidious onset and severe clinical symptoms[1]. Patients may experience fever, abdominal pain, jaundice, and vomiting, and in some cases, complications such as sepsis or toxic shock may develop[2]. If not treated promptly, PLA can lead to multiple organ failure and even death, with a mortality rate as high as 8.6%[3]. Depending on the etiology, PLA can be classified into bacterial and parasitic types, with bacterial infections being the most common[4]. These infections usually originate from the liver’s portal vein or biliary system. Furthermore, due to the rich blood supply to the liver, liver abscesses can also spread hematogenously or from adjacent organ infections to the liver[5].
Currently, the main treatment methods for PLA include antibiotic therapy and interventional treatment. For smaller abscesses (diameter < 3 cm), systemic antibiotic therapy is usually sufficient. For patients with single hepatic abscesses larger than 3 cm and no severe complications, a combination of antibiotics and ultrasound-guided percutaneous catheter drainage (PCD) is widely used[6,7]. PCD, as an interventional treatment, can effectively drain the abscess, alleviate the infection, and shorten the course of the disease. Although PCD is widely applied in the treatment of PLA, there is no unified standard regarding the optimal timing for its use[8]. The generally accepted view is that PCD should be performed after the abscess has reached a certain level of liquefaction, meaning the formation of sufficient fluid areas within the abscess to facilitate drainage[9].
This study aimed to explore the clinical value of early ultrasound-guided PCD in the treatment of PLA, particularly in cases where the abscess has not yet fully liquefied, in order to provide a reference for the timing and strategy of PCD-based treatment in liver abscess management.
This retrospective analysis included 143 consecutive patients diagnosed with PLA who met the inclusion criteria and were admitted to the General Surgery Department of our hospital between January 2018 and March 2023. A complete enumeration sampling method was adopted, systematically enrolling all eligible cases during this period to minimize selection bias. Patients were categorized into non-liquefied and liquefied groups based on abscess liquefaction status. Collected clinical data included age, sex, medical history (e.g., diabetes, biliary tract disease), abscess diameter, and location. This study was approved by the Beijing Luhe Hospital Ethics Committee.
The inclusion criteria were: (1) Ultrasound or abdominal computed tomography (CT) suggesting liver abscess; and (2) Safe percutaneous access confirmed by ultrasound. The exclusion criteria included: (1) Severe coagulopathy; (2) No safe ultrasound-guided access; (3) Abscess cavity < 2 cm; (4) Massive ascites; and (5) Altered mental status, agitation, or uncooperative behavior preventing stable positioning.
This study utilized the Sonosen EDGE for ultrasound examination, with a C60X abdominal convex array probe operating at a frequency of 2-5 MHz. The drainage catheter used was an 8.5F pigtail catheter with a guidewire (COOK, Jiangsu Yubang, Jiangsu Jianzhiyuan). All patients underwent ultrasound-guided PCD at the bedside.
Procedure: Preoperative routine assessments were performed to exclude severe coagulation disorders and other contraindications for puncture. Based on ultrasound scanning results, the patient’s position (supine or left lateral decubitus) and puncture site were selected, with the lowest intercostal or subcostal puncture point typically chosen to ensure a clear puncture path. During the puncture process, ultrasound scanning was first performed to determine the puncture path and patient position, followed by disinfection of the puncture area and draping with sterile surgical towels. After applying the coupling agent to the ultrasound probe, the patient was covered with sterile gloves or a sterile ultrasound cover. Local anesthesia was administered under ultrasound guidance (in-plane needle injection) to the liver capsule. The skin and subcutaneous tissue were incised about 3 mm, and the puncture drainage kit was assembled. A small amount of drainage fluid was collected for bacterial culture, the drainage bag was connected, and the drainage tube was fixed. Finally, a sterile dressing was applied.
The evaluated outcomes included postoperative hospital stay, time to fever resolution (the days from the onset of fever until the body temperature remains below 37.3 °C for at least 24 hours), time for neutrophil count to return to normal, puncture duration (from disinfection to the completion of drainage tube fixation), time for abscess reduction by more than 50%, bacterial culture results, and perioperative complications (e.g., septic shock, hemorrhage).
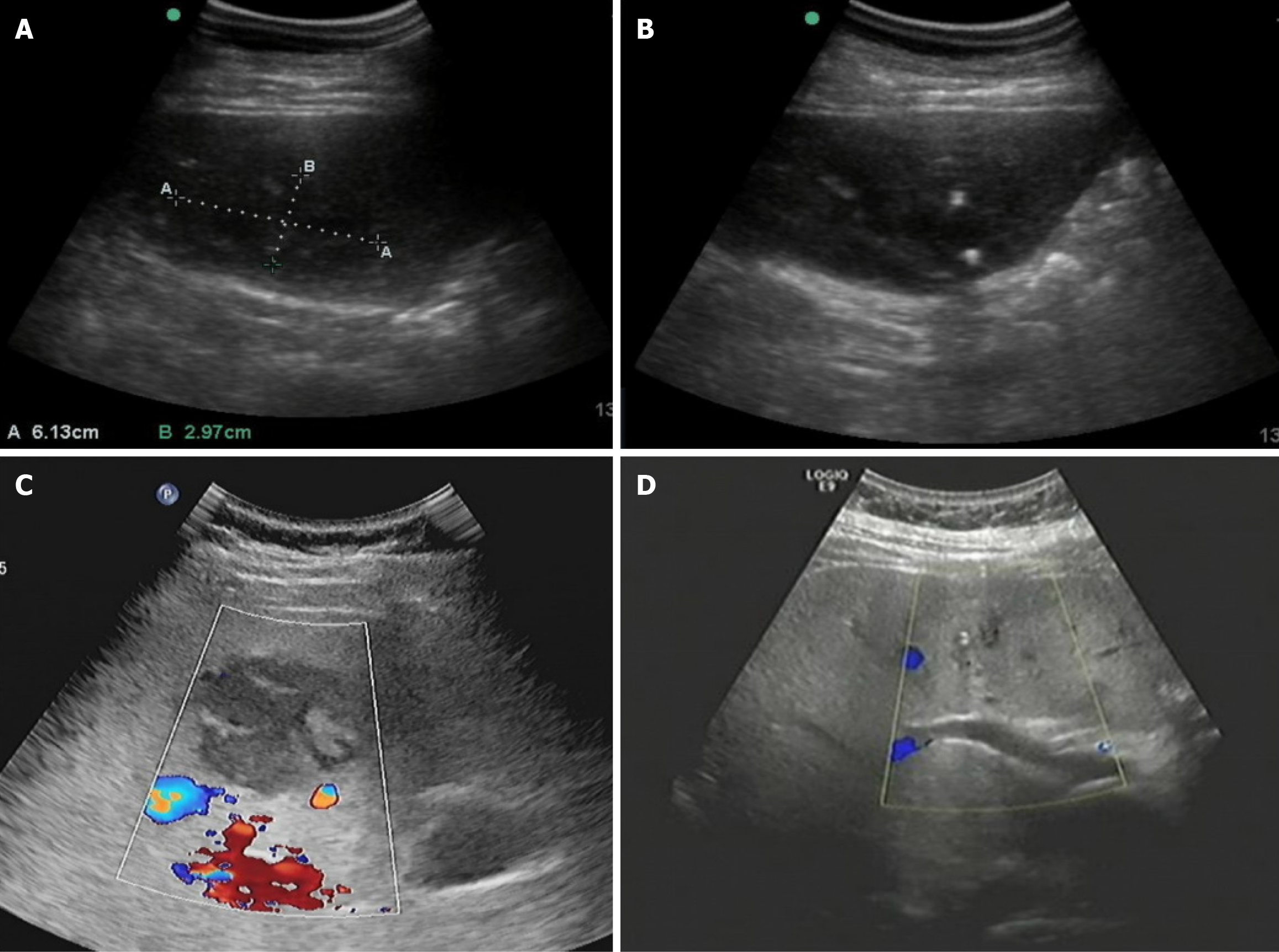
“Non-liquefied” was defined as the absence of a liquid hypoechoic area within the abscess on ultrasound examination; “liquefied” referred to the presence of a liquid hypoechoic area greater than 1 cm in diameter within the abscess, as determined by the experienced radiologist.
Statistical analyses were performed using SPSS version 26.0. Continuous data are expressed as the mean ± SD, and group comparisons were made using independent samples t-test. Categorical data are presented as frequencies (%), and group comparisons were performed using the χ2 test. A P value of < 0.05 was considered statistically significant.
Overall, this study included 143 patients who underwent PCD treatment, with ages ranging from 18 to 87 years, and an average age of 58.1 ± 14.2 years. Among the patients, 53.1% were male. Additionally, 55.9% of patients had diabetes, 16.8% (24 cases) had a history of biliary stones, and 4.9% (7 cases) had biliary malignancies (Table 1).
| Patient | n = 143 |
| Age, (year) | 58.1 ± 14.2 (18-87) |
| Sex | |
| Male | 76 (53.1) |
| Female | 67 (46.9) |
| Biliary calculus | 24 (16.8) |
| Biliary malignancies | 7 (4.9) |
| Abscess diameter (cm) | 7.2 ± 2.5 |
| Abscess location | |
| Left liver | 42 (29.4) |
| Right liver | 99 (69.2) |
| Caudal lobe | 2 (1.4) |
| Abscess liquefaction status | |
| Non-liquefied | 59 (41.3) |
| Liquefied | 84 (58.7) |
The puncture success rate for all patients was 99.3%. In one sclerosing abscess case, the drainage tube could not be inserted, but was gradually withdrawn without bleeding or bile leakage. The average puncture duration was 11.3 ± 5.4 minutes. The average postoperative hospital stay was (4.9 ± 2.8) days, and the average time to postoperative fever resolution was (1.9 ± 1.2) days. A total of eight postoperative complications occurred, including three cases of drainage tube protrusion requiring secondary puncture and drainage, and five cases of transient septic shock, which improved after fluid resuscitation and dilation treatment. Postoperative reexamination in five cases showed that the drainage tube was inserted through the costal diaphragm angle. No pulmonary complications, such as pneumothorax, occurred during puncture or after extubation, and no damage to the gastrointestinal tract, gallbladder, or other surrounding organs was observed in any of the punctures. The positive rate of drainage culture was 72%, with Klebsiella pneumoniae accounting for 75.7%, Escherichia coli for 15.3%, and other bacteria (such as Citrobacter freundiensis and Streptococcus.) for 2.1%. No perioperative deaths occurred, and all patients were discharged with improvement after treatment (Table 2).
| Result | n = 143 |
| Puncture success rate (%) | 99.3 |
| Puncture duration (minute) | 11.3 ± 5.4 |
| Time to fever resolution (days) | 1.9 ± 1.2 |
| Time for neutrophil count to return to normal (days) | 2.4 ± 0.8 |
| Postoperative hospital stay (days) | 2.9 ± 2.8 |
| Time for abscess reduction by more than 50% (days) | 7.1 ± 3.3 |
| Bacterial culture | |
| Positive | 103 (72) |
| Klebsiella pneumoniae | 78 (75.7) |
| Escherichia coli | 22 (15.3) |
| Other | 3 (2.1) |
| Negative | 40 (28) |
| Intraoperative complications | |
| Catheterization failure | 1 (0.7) |
| Postoperative complication | |
| Catheter dislodgement | 3 (2.1) |
| Septic shock | 5 (3.5) |
Patients were categorized into the non-liquefied and liquefied groups based on abscess liquefaction observed in imaging examinations. The results showed that, compared to the liquefied group, the non-liquefied group had significantly shorter hospitalization duration (3.9 ± 1.8 days vs 5.1 ± 2.7 days), time to fever resolution (2.4 ± 1.1 days vs 4.9 ± 2.2 days), and time required for abscess reduction by more than 50% (4.7 ± 1.5 days vs 8.6 ± 3.3 days) (P < 0.05). No significant differences were observed between the two groups in terms of puncture success rate, complication rate, and time for neutrophil count to return to normal. Regarding complications, two cases of postoperative catheter dislodgement occurred in the non-liquefied group, whereas the liquefied group had six cases of complications, including one case of catheter dislodgement and five cases of transient septic shock (Table 3 and Figure 1).
| Patient | Non-liquefied group (n = 59) | Liquefied group (n = 84) | t/χ2 value | P value |
| Puncture success rate | 58 (98.3) | 84 (100) | 1.434 | 0.924 |
| Postoperative hospital stay (days) | 3.9 ± 1.8 | 5.1 ± 2.7 | -2.903 | 0.004 |
| Time to fever resolution (days) | 2.4 ± 1.1 | 4.9 ± 2.2 | -7.935 | 0.000 |
| Time for abscess reduction by more than 50% (days) | 4.7 ± 1.5 | 8.6 ± 3.3 | -8.445 | 0.000 |
| Time for neutrophil count to return to normal (days) | 1.5 ± 1.26 | 1.9 ± 1.85 | 0.478 | 0.633 |
| Perioperative complications | 2 (3.4) | 6 (7.1) | 0.924 | 0.470 |
PLA is common in patients with diabetes and biliary tract diseases, and is primarily caused by Klebsiella pneumoniae and Escherichia coli[10]. If not treated promptly, septic shock can occur, putting the patient’s life at risk. Compared to open and laparoscopic surgery, PCD is more aligned with the trend of rapid rehabilitation surgery, with a success rate of over 90%[11]. Its therapeutic effect is essentially equivalent to that of surgical treatment, offering advantages such as less trauma, shorter hospital stays, and lower treatment costs[12,13]. Moreover, compared to percutaneous needle aspiration, PCD performs better in terms of success rate, reduction of abscess by more than 50% after treatment, and improvement of clinical symptoms[8,14]. However, current guidelines do not provide clear criteria for selecting the timing of PCD treatment. It is generally believed that PCD should be implemented when abscess liquefaction is favorable[9,15], but this view lacks solid clinical evidence.
The timing of abscess liquefaction is uncertain, and there is the possibility of a solid abscess that is difficult to liquefy. When patients undergo repeated examinations without symptom improvement, potential conflicts arise between doctors and patients, resulting in wasted medical resources. Clinical imaging examinations, including ultrasound, CT, and magnetic resonance imaging, are used to assess the degree of abscess liquefaction. However, clinical experience has shown that when imaging indicates that the abscess is well liquefied, the pus extracted during drainage is often very thin, suggesting that the imaging examination is delayed in determining the optimal puncture time. In other words, by the time the lesion is clearly displayed on imaging, it may have already begun liquefying[16]. This delay in treatment can put patients at risk for complications such as abscess rupture, infection, and toxic shock, leading to potential death or disability. Early and effective treatment is therefore crucial.
The results of our study indicate that early percutaneous drainage of PLA, without waiting for imaging evidence of good liquefaction, can be successfully performed once the liver abscess is diagnosed and the location of the lesion is clearly identified. Even if only a small amount of pus is extracted, it effectively reduces the pressure within the abscess cavity, improving antibiotic efficacy and relieving clinical symptoms. This approach helps reduce symptoms earlier, enhances the effectiveness of antibiotics, and decreases the occurrence of complications.
A study by Ahmed et al[17] analyzed 272 cases of bacterial liver abscess treated with PCD, with results showing a success rate of 96.2%, an average time to clinical symptom improvement of 6.6 days, and an abscess reduction time of 5.5 days for more than 50% shrinkage. Our study results are comparable to those of Ahmed et al[17] in terms of key clinical indicators. Additionally, the retrospective comparative analysis in this study showed that, with no significant difference in puncture success rate and complication rate, the postoperative hospitalization time, time to fever resolution, and time to more than 50% abscess reduction in the non-liquefied group were all significantly shorter than those of the liquefied group.
Although this study provides insights into PCD for early-stage PLA, several limitations should be acknowledged. First, the limited sample size may reduce the statistical power of the findings and affect their generalizability. Additionally, the reliance on historical medical records could introduce information bias, such as incomplete documentation of clinical variables (e.g., antibiotic regimens, comorbidities) or variability in imaging interpretations among radiologists. Heterogeneity in operator expertise (e.g., differences in procedural techniques among physicians) might also influence clinical outcomes, despite adherence to standardized protocols. Furthermore, unmeasured confounders, including subtle variations in abscess characteristics or undocumented comorbidities, could bias the results. Second, the absence of long-term follow-up data, such as recurrence rates or longitudinal liver function assessments, limits the evaluation of treatment sustainability. Future studies should incorporate larger sample sizes and adopt prospective, multicenter designs to validate these findings and further assess the long-term efficacy of early PCD intervention.
This study suggests that early percutaneous drainage of bacterial liver abscesses does not necessarily need to wait for the abscess to liquefy, offering a high success rate and low complication rate, and effectively reducing the risk of worsened infection. It also saves medical resources and costs. This simple procedure has the potential for wider clinical application.
| 1. | Tian LT, Yao K, Zhang XY, Zhang ZD, Liang YJ, Yin DL, Lee L, Jiang HC, Liu LX. Liver abscesses in adult patients with and without diabetes mellitus: an analysis of the clinical characteristics, features of the causative pathogens, outcomes and predictors of fatality: a report based on a large population, retrospective study in China. Clin Microbiol Infect. 2012;18:E314-E330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Bakirova GH, Alharthy A, Corcione S, Aletreby WT, Mady AF, De Rosa FG, Karakitsos D. Fulminant septic shock due to Edwardsiella tarda infection associated with multiple liver abscesses: a case report and review of the literature. J Med Case Rep. 2020;14:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Chen YC, Lin CH, Chang SN, Shi ZY. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000-2011. J Microbiol Immunol Infect. 2016;49:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Khim G, Em S, Mo S, Townell N. Liver abscess: diagnostic and management issues found in the low resource setting. Br Med Bull. 2019;132:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Shi SH, Zhai ZL, Zheng SS. Pyogenic Liver Abscess of Biliary Origin: The Existing Problems and Their Strategies. Semin Liver Dis. 2018;38:270-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Mobasher-Jannat A, Akhavan-Moghadam J. Percutaneous drainage for giant pyogenic liver abscess-does size matter? Am J Surg. 2017;214:770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Liu CH, Gervais DA, Hahn PF, Arellano RS, Uppot RN, Mueller PR. Percutaneous hepatic abscess drainage: do multiple abscesses or multiloculated abscesses preclude drainage or affect outcome? J Vasc Interv Radiol. 2009;20:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Lin JW, Chen CT, Hsieh MS, Lee IH, Yen DH, Cheng HM, Hsu TF. Percutaneous catheter drainage versus percutaneous needle aspiration for liver abscess: a systematic review, meta-analysis and trial sequential analysis. BMJ Open. 2023;13:e072736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Li Z, Liu A, Xu J, Li Y, Liu J, Liu Y, Zhu H. Early percutaneous catheter drainage in protecting against prolonged fever among patients with pyogenic liver abscess: a retrospective cohort study. Ann Med. 2022;54:2269-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Huang Y, Guo H, Li Y. A case of necrotic pneumonia caused by Streptococcus pneumoniae was diagnosed using a pneumonia antigen test in BALF: A case report. Medicine (Baltimore). 2024;103:e39571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Meddings L, Myers RP, Hubbard J, Shaheen AA, Laupland KB, Dixon E, Coffin C, Kaplan GG. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Ferraioli G, Garlaschelli A, Zanaboni D, Gulizia R, Brunetti E, Tinozzi FP, Cammà C, Filice C. Percutaneous and surgical treatment of pyogenic liver abscesses: observation over a 21-year period in 148 patients. Dig Liver Dis. 2008;40:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Luo M, Yang XX, Tan B, Zhou XP, Xia HM, Xue J, Xu X, Qing Y, Li CR, Qiu JF, Li YL. Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis. Eur J Clin Microbiol Infect Dis. 2016;35:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Al-Sayaghi KM, Alhujaily M, Zaky MK, Alhasan AS, Babikir TB, Alnehmi FS, Abdalrahman HH, Abdelmalik MAA, Ali AM, Fadlalmola HA, Swamy DSV. Percutaneous needle aspiration versus catheter drainage in the management of liver abscess: an updated systematic review and meta-analysis. ANZ J Surg. 2023;93:840-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Anand M, Sahi PK, Mandal A. Pediatric Liver Abscess: Outcomes of Protocol-based Management and Predictors of Poor Outcome. Pediatr Infect Dis J. 2023;42:549-556. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Gyorffy EJ, Frey CF, Silva J Jr, McGahan J. Pyogenic liver abscess. Diagnostic and therapeutic strategies. Ann Surg. 1987;206:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Ahmed M, Alam J, Hussain S, Aslam M. Prospective randomized comparative study of percutaneous catheter drainage and percutaneous needle aspiration in the treatment of liver abscess. ANZ J Surg. 2021;91:E86-E90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/