Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.107525
Revised: April 17, 2025
Accepted: May 28, 2025
Published online: July 27, 2025
Processing time: 115 Days and 20.2 Hours
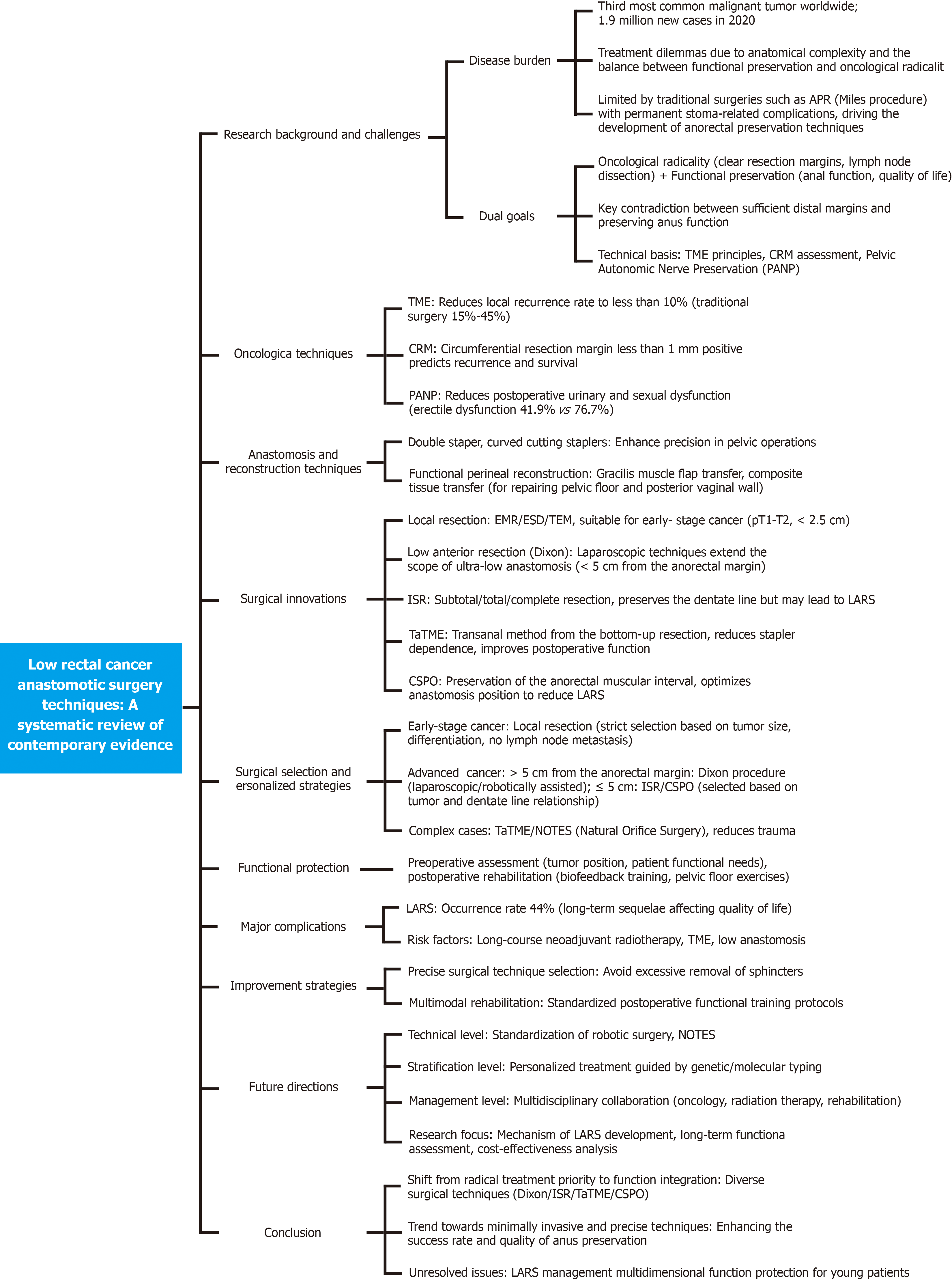
Rectal cancer ranks as the third most prevalent malignancy globally, with an estimated 1.9 million incident cases reported in 2020. The management of low rectal cancer presents significant therapeutic challenges due to its anatomical complexity, and substantially impacts patients' quality of life. While abdominoperineal resection (Miles procedure) ensures oncological radicality, the morbidity associated with permanent colostomy has driven innovations in sphincter-preserving surgical techniques. This review synthesizes current evidence on sphincter-preserving surgical approaches for low rectal cancer. The implementation of total mesorectal excision (TME) principles and enhanced understanding of circumferential resection margin have facilitated the evolution of diverse sphincter-preserving surgical modalities. These include local excision, low anterior resection (Dixon procedure), intersphincteric resection, pull-through procedures, transanal TME, and conventional sphincter-preserving operation. Minimally invasive approaches, particularly laparoscopic and robotic platforms, alongside natural orifice transluminal endoscopic surgery, have demonstrated improved surgical precision and enhanced postoperative recovery outcomes. Novel functional perineal reconstruction techniques offer promising alternatives for patients requiring posterior pelvic exenteration. Nevertheless, the high incidence of low anterior resection syndrome (LARS) and its chronic sequelae remain clinically notable. Evidence indicates that long-course neoadjuvant radiotherapy and TME constitute significant risk factors for LARS development. Contemporary sphincter-preserving surgery for low rectal cancer is advancing toward minimally invasive, personalized, and precision-based approaches. The increasing incidence of early-onset rectal cancer necessitates individualized treatment strategies that balance oncological efficacy with functional preservation. Future directions should focus on standardizing surgical indications, optimizing postoperative rehabilitation protocols, and enhancing treatment outcomes through multidisciplinary integration and technological innovation.
Core Tip: The adoption of total mesorectal excision (TME) principle and better understanding of circumferential resection margin have led to the development of various sphincter-preserving surgeries, such as local excision, Dixon surgery, intersphincteric resection, etc. Minimally invasive methods such as laparoscopic, robotic and natural orifice transluminal endoscopic surgery improve operational precision and postoperative recovery. New perineal reconstruction techniques also benefit some patients. However, the high incidence of low anterior resection syndrome (LARS) and its sequelae are notable. Long-course neoadjuvant radiotherapy and TME are key risk factors for LARS.
- Citation: Wang S, Li AJ, Jiang HH, Lin Y, Ding HB. Sphincter-preserving surgical techniques in low rectal cancer management: A systematic review of contemporary evidence. World J Gastrointest Surg 2025; 17(7): 107525
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/107525.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.107525
Colorectal cancer represents a significant global health burden, ranking as the third most prevalent malignancy and the second leading cause of cancer-related mortality worldwide[1]. According to GLOBOCAN 2020 data, approximately 1.9 million new cases were diagnosed, with a five-year prevalence reaching 5.25 million cases[2]. Among these, low rectal cancer presents unique therapeutic challenges due to its anatomical complexity and profound impact on patients' quality of life (Table 1).
| Cancer type | Number of new cases | Global incidence ranking | Number of deaths | Global mortality ranking |
| Colorectal cancer | 1.9 million | 3rd | 0.9 million | 2nd |
Surgical intervention remains the cornerstone of rectal cancer management, whether for curative intent or palliative relief of obstruction. Historically, abdominoperineal resection (APR, Miles procedure) has been considered the standard surgical approach for low rectal cancer, defined as tumors with inferior margins within 5 cm from the anal verge. However, the necessity of permanent colostomy following APR is associated with significant morbidity, including stoma-related complications[3], genitourinary dysfunction[4], and substantial impairment of patients' psychosocial well-being and quality of life[5].
Recent decades have witnessed significant paradigm shifts in the surgical management of low rectal cancer. The establishment of total mesorectal excision (TME) principles[6] and enhanced understanding of circumferential resection margin (CRM) significance[7], coupled with advances in neoadjuvant chemoradiotherapy and minimally invasive surgical techniques, have markedly improved oncological outcomes in sphincter-preserving procedures[8]. Since the 1990s, technological innovations in surgical instrumentation and minimally invasive platforms have expanded the applications of sphincter-preserving surgery in low rectal cancer management.
Nevertheless, achieving an optimal balance between oncological radicality and functional preservation remains a significant clinical challenge. This comprehensive review synthesizes current evidence on sphincter-preserving surgical approaches for low rectal cancer, focusing on technical innovations, oncological outcomes, and functional results. A conceptual framework diagram is shown in Figure 1.
The fundamental principle of sphincter-preserving surgery in low rectal cancer management encompasses two critical objectives: Achieving oncological radicality while maintaining adequate anal function and quality of life[9,10]. A persistent challenge in this field lies in reconciling the oncological requirement for adequate distal resection margins with the functional demands of sphincter preservation, representing a critical area of ongoing research and clinical debate[11,12].
Oncological radicality in surgical resection mandates complete tumor excision and thorough lymphadenectomy by established oncological principles[11]. Both tumor-specific and technical factors influence the risk of local recurrence. Tumor-specific factors include biological characteristics, depth of invasion, lymph node involvement, and tumor height from the anal verge. Technical and procedural considerations encompass the implementation of TME principles, achievement of adequate circumferential margins, quality of pelvic lymphadenectomy, and appropriate application of the double-stapling technique[12].
In the context of sphincter preservation, surgical intervention inevitably impacts the internal anal sphincter complex and dentate line, potentially affecting postoperative anal function and quality of life[13]. Contemporary sphincter-preserving techniques have evolved to maximize bowel preservation and minimize sphincter and neural damage while adhering to oncological principles. This approach aims to optimize functional outcomes without compromising oncological safety[10].
Recent advances in sphincter-preserving surgical techniques for low rectal cancer have demonstrated promising outcomes. Evidence-based innovations in surgical approaches and technological developments have significantly enhanced both oncological and functional outcomes in sphincter preservation procedures. Implementing these evidence-based strategies has shown potential to optimize surgical outcomes while maintaining adequate oncological safety.
TME has fundamentally transformed the surgical management of rectal cancer. Prior to its implementation, local recurrence rates following rectal cancer surgery were reported to be as high as 34%[14]. The landmark introduction of TME by Hakiman et al[15] marked a turning point in surgical outcomes. While surgery alone was historically associated with local recurrence rates of 15%-45%[16], the integration of TME with chemoradiotherapy has successfully reduced these rates to below 10%.
The preservation of pelvic autonomic nerves has emerged as a crucial consideration in modern surgical approaches, particularly in addressing postoperative urinary and sexual dysfunction. Clinical evidence demonstrates significantly improved functional outcomes with pelvic autonomic nerve preservation (PANP) compared to conventional TME alone. Studies have reported that the incidence of erectile and ejaculatory dysfunction following PANP was 41.9% and 42.45%, respectively, markedly lower than the rates of 76.7% and 67.3% observed with standard TME[9].
The CRM, defined as the minimal distance between the deepest tumor invasion and the mesorectal excision plane, has been established as a critical prognostic indicator. A positive CRM, characterized by a distance less than 1 mm with cancer infiltration, has been consistently identified as an independent risk factor for local recurrence. Research has demonstrated that CRM positivity significantly impacts not only local recurrence rates but also distant metastasis and 5-year survival outcomes[14].
Significant advances in anastomotic techniques have further refined surgical approaches. The double-staple technique has become the standard method for low anterior rectal resection, offering superior precision and reliability compared to manual anastomosis[17]. Recent technological innovations include the development of arc-shaped cutting staplers, which have enhanced access in confined pelvic spaces and improved sphincter preservation rates[18]. Additionally, the introduction of the triple-staple technique has demonstrated particular utility in cases involving narrow pelvic anatomy, effectively reducing operative times[19].
In the realm of reconstructive surgery, Chang et al[20] has pioneered the concept of functional perineal reconstruction, representing a significant advancement beyond traditional wound healing approaches. This innovative methodology emphasizes not only structural repair but also functional restoration, particularly benefiting female patients requiring posterior pelvic exenteration. In these cases, where extensive tissue removal often includes the rectum, anus, and potentially the vagina, this comprehensive approach to reconstruction addresses both anatomical and functional considerations.
Local excision is the complete removal of early-stage tumors with a surrounding 1 cm margin of the standard intestinal wall. Multiple surgical approaches have been developed and validated, including transsexual excision, transanal sphincter excision, transvaginal local excision, and transanal endoscopic minimally invasive surgery. The latter encompasses several advanced techniques: Endoscopic mucosal resection, endoscopic submucosal dissection, and transanal endoscopic microsurgery[21,22]. This surgical approach offers significant advantages, including reduced surgical trauma, lower complication rates, and accelerated postoperative recovery, establishing it as the standard therapeutic approach for precancerous lesions and early-stage cancers.
Evidence suggests that in patients with high-risk pT1 rectal cancer, local resection combined with adjuvant concurrent chemoradiotherapy provides an effective alternative to abdominal radical resection[23]. However, studies demonstrate that disease-free survival rates are significantly lower in the pT2 stage compared to the pT1 stage of disease[24].
Patient selection for local resection requires careful consideration of specific criteria. These include tumor size less than 2.5 cm with circumferential involvement not exceeding one-third of the bowel circumference; T1 or T2 classification with no evidence of per colorectal lymphadenopathy on imaging studies; and well-differentiated adenocarcinoma without lymphovascular invasion, perineural infiltration, or lymph node metastasis.
A primary limitation of local resection is its inability to achieve regional lymph node clearance, with residual metastatic nodes representing a significant source of recurrence. Thus, successful outcomes depend on appropriate patient selection, strict adherence to surgical indications, and complete local resection. For poorly differentiated T2 tumors, adjuvant chemoradiotherapy is indicated, while cases with questionable curative potential should be considered for conversion to radical surgery.
Low anterior resection (LAR) (Dixon procedure) represents the gold standard for sphincter-preserving surgery in low rectal cancer. This surgical approach achieves optimal oncological outcomes while maximizing preservation of anal function[25]. Traditionally, patient selection for the Dixon procedure required a minimum distance of 8 cm between the tumor's inferior margin and the anal verge, along with adequate pelvic space for surgical manipulation. However, advances in laparoscopic techniques and anastomotic technologies have expanded the procedure's applicability, enabling ultra-low anastomosis even in patients with challenging pelvic anatomy, including obesity[26]. Implementation of meticulous surgical techniques and standardized procedural protocols has significantly improved sphincter preservation rates in low rectal cancer management[27].
Since its introduction in 1992, intersphincteric resection (ISR) has emerged as a widely adopted surgical approach for low rectal cancer, with systematic reviews validating its safety and oncological efficacy[28]. Long-term follow-up studies demonstrate favorable outcomes, characterized by low local recurrence rates and promising 5-year survival rates[29]. The procedure encompasses three variants-partial, subtotal, and complete ISR-with the selected technique based on the tumor's relationship to the dentate line[30]. However, the requisite removal of partial or complete internal anal sphincter during the procedure may result in varying degrees of postoperative defecatory dysfunction, with functional outcomes correlating directly with the extent of sphincter resection[31].
Postoperative functional recovery represents a critical clinical consideration in ISR. Research indicates that patients frequently experience low anterior resection syndrome (LARS) in the early postoperative period, manifesting as various degrees of defecatory dysfunction[32]. Functional outcomes are objectively assessed using standardized instruments, primarily the LARS score and Wexner incontinence scoring system[33]. Notably, structured pelvic floor rehabilitation programs have demonstrated significant efficacy in improving functional outcomes for most patients[34]. Multiple factors influence functional recovery, including surgical technique, tumor location, and neoadjuvant chemoradiotherapy[35]. Therefore, comprehensive preoperative assessment and individualized postoperative rehabilitation protocols are essential for optimal outcomes[13].
Pull-through procedures for rectal cancer were developed to address the technical challenges encountered during pelvic surgery[36]. These surgical techniques have evolved into two distinct methodological approaches, each with specific indications and technical considerations.
The first approach, known as the eversion pull-through technique or Welch procedure, involves abdominal mobilization of the rectum followed by transection distal to the tumor. The distinctive feature of this procedure is the eversion of the distal rectum through the anus, accompanied by exteriorization of the proximal sigmoid colon. The anastomosis is performed extra-corporeally, after which the anastomosed segment is repositioned into the abdominal cavity through the anus[37].
The second approach encompasses transanal pull-through techniques, which begin with abdominal mobilization of the rectum, followed by transanal dissection and transection distal to the tumor. After the mobilized rectum is exteriorized, the procedure culminates in proximal resection and coloanal anastomosis. This approach has evolved into two principal variants: The two-stage Bacon procedure and the single-stage Parks procedure[38]. The Bacon procedure is characterized by initial coloanal anastomosis with temporary exteriorization of the redundant colon, necessitating a second-stage resection of the exteriorized segment. In contrast, the Parks procedure accomplishes complete mobilization, pull-through, and immediate manual coloanal anastomosis in a single stage[37].
Contemporary surgical practice has witnessed the emergence of various modifications to the Parks procedure[39]. These modern adaptations incorporate technological innovations, particularly laparoscopic approaches, and mechanical anastomotic devices, which have successfully addressed many of the technical limitations associated with the traditional Parks procedure, especially concerning low pelvic dissection and anal anastomosis[40]. These technological and methodological advances have significantly improved sphincter preservation rates and enhanced surgical outcomes.
Transanal TME (TaTME) represents an innovative sphincter-preserving surgical approach that has emerged in contemporary colorectal surgery. Despite its promising potential, the technique remains in its evolutionary phase, and standardization of the anatomical approach and procedural steps is still ongoing due to its technical complexity and relatively recent introduction into surgical practice.
The fundamental concept of TaTME involves a bottom-up approach to mesorectal excision, contrasting with the traditional top-down dissection. While the anatomical principles established in conventional TME serve as a reference point, the reverse dissection technique presents unique challenges, primarily due to the altered perspective and absence of conventional anatomical landmarks. This technical complexity necessitates advanced surgical expertise and a thorough understanding of pelvic anatomy from a novel viewpoint.
Evidence from multicenter studies and systematic reviews has been encouraging, demonstrating that TaTME achieves oncological outcomes comparable to conventional anterior resection[41] Similarly, postoperative functional outcomes and quality of life measures have shown promising results[42]. A notable advantage of this technique lies in its elimination of the need for surgical stapling devices during the distal transection, potentially reducing both anastomotic leak rates and associated healthcare costs.
Conformal sphincter preservation operation (CSPO) represents a novel surgical approach developed in response to the enhanced understanding of sphincter dysfunction mechanisms following ISR. This innovative technique is characterized by two fundamental principles: Preservation of the intersphincteric space to minimize neurological damage and implementation of a tumor-specific conformal resection pattern that maximizes preservation of healthy tissue on the contralateral aspect of the tumor.
The distinctive feature of CSPO lies in its anatomical approach to the anastomosis, where the proximal colon is strategically anastomosed to the rectum, prioritizing maximal tissue preservation on the tumor-opposite side. This technical modification aims to establish the anastomotic line as distant as possible from the dentate line[43]. The procedure encompasses two primary variants: The pull-through technique and the transanal conformal resection approach[44].
Although CSPO has not yet achieved widespread adoption, comparative analyses with conventional procedures such as LAR and APR have yielded promising results. Studies demonstrate that CSPO achieves comparable safety profiles in carefully selected cases while enabling sphincter preservation at lower anatomical levels. Notably, functional outcomes are equivalent to those achieved with LAR[45]. Additional research has confirmed favorable anal function and quality of life outcomes compared to ISR[46].
The clinical significance of CSPO has been formally recognized through its inclusion in the Chinese Expert Consensus on Digestive Tract Reconstruction for Mid- and Low-lying Rectal Cancer (2021 Edition)[47], underscoring its emerging role in contemporary colorectal surgery.
The development of minimally invasive surgical techniques represents a pivotal milestone in contemporary surgical practice. In the domain of sphincter-preserving surgery for low rectal cancer, the evolution from initial local resection to current sophisticated approaches has introduced new therapeutic possibilities[48]. A watershed moment occurred in 1990 when Professor Leahy performed the first laparoscopic Dixon procedure, marking the beginning of a new era in minimally invasive colorectal surgery[49]. Through more than three decades of refinement, laparoscopic surgery has established itself as a standard therapeutic approach in rectal cancer management.
The field of rectal cancer surgery has entered a phase of diversified development, with the emergence of multiple innovative minimally invasive techniques. These include TaTME, natural orifice transluminal endoscopic surgery (NOTES), ISR, and single-port laparoscopic surgery[50]. Among these innovations, TaTME has demonstrated particular efficacy in treating mid and low-rectal cancer through its unique bottom-up approach to TME. At the same time, NOTES technology continues to expand the boundaries of minimally invasive surgery. Introducing the da Vinci robotic surgical system, with its enhanced three-dimensional visualization and superior instrument articulation, has shown significant advantages in complex pelvic procedures.
Implementing these innovative technologies has not only diversified the surgical approaches available for rectal cancer treatment but also enabled more personalized therapeutic strategies based on individual patient characteristics. However, it is crucial to emphasize that successfully applying these advanced techniques necessitates rigorous standardized training programs and robust quality control measures to ensure optimal surgical outcomes and patient safety[48]. The subsequent sections will examine the specific applications and progress of NOTES technology and the da Vinci robotic surgical system in rectal cancer treatment.
NOTES represents an innovative advancement in minimally invasive surgical techniques. This approach involves accessing the abdominal cavity through natural orifices (oral, vaginal, anal, or urethral) using endoscopic guidance to perform various procedures including cholecystectomy, nephrectomy, rectal TME resection, and gynecological surgeries. The surgical specimens are subsequently extracted through these natural orifices[51]. When compared to conventional surgical approaches, NOTES demonstrates superior outcomes in terms of reduced surgical trauma and enhanced cosmetic results. The feasibility of performing TME surgery for rectal cancer via natural orifices has been extensively validated through clinical research[52].
Significant evidence supporting the efficacy of NOTES comes from a multicenter randomized controlled trial conducted by Serra-Aracil and colleagues. Their findings demonstrated that NOTES-based rectal cancer TME, when compared to traditional LAR, offers several advantages: Lower conversion rates, accelerated postoperative recovery, and reduced overall recurrence rates[53]. However, the implementation of pure NOTES procedures remains limited in clinical practice due to their technical complexity and associated surgical risks[54]. In the Chinese healthcare context, hybrid NOTES procedures for rectal cancer, incorporating laparoscopic assistance, have emerged as a more practical and reliable approach[55].
The current spectrum of laparoscopic NOTES encompasses various techniques, including ISR, CSPO, pull-out anastomosis, and TaTME. This hybrid surgical paradigm successfully preserves the benefits of minimally invasive surgery while ensuring both surgical safety and favorable oncological outcomes through standardized procedural protocols[56].
Despite laparoscopic technology's advancement over conventional open surgery, inherent limitations including compromised stability, diminished haptic feedback, and technical challenges in performing precise microsurgical maneuvers such as suturing have constrained its application in complex surgical procedures. The advent of da Vinci robot-assisted laparoscopic surgery has effectively addressed these limitations, demonstrating superior operational precision and enhanced surgical maneuverability. This technological advancement has significantly simplified complex laparoscopic procedures, including suturing and anastomosis, consequently improving the sphincter preservation rates in low rectal cancer surgeries[57].
Clinical evidence demonstrates comparable therapeutic outcomes between robotic rectal resection and conventional laparoscopic approaches. While initial capital investment and operational costs remain higher, post-learning curve analyses reveal reduced operative times and equivalent clinical efficacy to traditional laparoscopic procedures[58]. Furthermore, empirical studies indicate an abbreviated learning curve associated with robotic rectal cancer surgery compared to conventional approaches[57].
Multiple investigations have substantiated the superior capability of robotic surgical systems in preserving pelvic autonomic nerve structures through their articulated robotic arms, resulting in significantly reduced postoperative genitourinary dysfunction rates[59,60]. Nevertheless, comprehensive longitudinal studies evaluating long-term oncological outcomes and cost-effectiveness analyses remain imperative for definitive validation of robotic surgical approaches[61].
In the gracilis muscle flap transplantation context, multiple configurational approaches can be implemented based on the anatomical defect characteristics. The surgical options encompass α-type configuration, γ-type configuration, and bilateral gracilis muscle flap reconstruction utilizing the "camera shutter" technique[20,62]. These diverse reconstructive methodologies facilitate individualized approaches to anal sphincter functional restoration.
Clinical evidence demonstrates that selecting appropriate configurational techniques tailored to patient-specific anatomical and physiological parameters significantly influences therapeutic outcomes[63].
The utilization of single vascular pedicle perfusion characteristics facilitates the en bloc transfer of multiple tissue components, enabling simultaneous sphincter functional reconstruction and posterior vaginal wall defect restoration[64]. This composite tissue transfer methodology optimizes vascular perfusion while enhancing operative efficiency. Clinical investigations demonstrate the superiority of composite tissue transfer in cases necessitating concurrent reconstruction of multiple anatomical structures[65].
This synergistic approach leverages independent vascular supply networks, substantially enhancing reconstructive flexibility and reliability[66]. The technique demonstrates particular efficacy in complex cases requiring comprehensive posterior vaginal wall reconstruction. The autonomous vascular systems ensure optimal tissue viability while facilitating customized reconstructive strategies.
A systematically structured biofeedback training protocol incorporates a progressive ‘sandwich’ model methodology and an evidence-based training intensity stratification system[62]. Clinical follow-up investigations demonstrate that systematic rehabilitation protocols facilitate the restoration of anal sphincter functional parameters, including maximum systolic pressure, sustained contraction duration, resting pressure, and high-pressure zone length. These parameters frequently approximate or achieve normative values[64,65]. The implementation of standardized postoperative rehabilitation protocols proves instrumental in optimizing functional outcomes.
Surgery-centered comprehensive intervention represents the established therapeutic paradigm in rectal cancer management[67]. Neoadjuvant chemoradiotherapy has emerged as a significant therapeutic modality, particularly for patients with low rectal malignancies where sphincter preservation is indicated[68].
The implementation of preoperative neoadjuvant chemoradiotherapy demonstrates multifaceted therapeutic benefits through tumor downstaging and volume reduction, including enhanced sphincter preservation rates, decreased local recurrence incidence, and improved overall survival metrics[69]. Notably, a subset of patients achieves complete or near-complete clinical response following neoadjuvant chemoradiotherapy, facilitating the transition to a watch-and-wait protocol, thus enabling rectal preservation in cases initially indicated for sphincter excision[70].
The evolution of therapeutic modalities, encompassing immunotherapeutic approaches, refined neoadjuvant radiotherapy protocols, and advanced transanal local excision techniques, is anticipated to mitigate the technical challenges associated with sphincter-preserving procedures in low rectal malignancies. These advancements suggest rectal preservation may become an increasingly viable therapeutic option for appropriately selected patients[71].
Function-preserving perineal reconstruction represents a paradigm shift in contemporary surgical approaches, transcending traditional radical tumor management to emphasize postoperative functional outcomes. Various reconstructive techniques, including isolated gracilis flap transplantation and composite gracilis myocutaneous flap reconstruction, combined with systematic rehabilitation protocols, demonstrate significant improvement in patient outcomes. This approach is particularly pertinent in cases requiring posterior pelvic exenteration, where functional preservation can be optimized while maintaining oncological principles.
The evolution of the bio-psycho-social medical model has heightened focus on anal function and quality of life following sphincter-preserving procedures. Systematic investigations demonstrate a substantial prevalence of LARS, with meta-analyses indicating a pooled incidence of major LARS at 44% (95%CI: 40%-48%)[72]. This functional impairment persists beyond the immediate postoperative period, with documented symptomatology extending minimally one year postoperatively[73]. International multicenter analyses reveal significantly compromised quality-of-life metrics across multiple domains in patients with severe LARS[74].
Surgical parameters significantly influence LARS development. Extended neoadjuvant radiotherapy and TME have been identified as primary risk factors[72]. Systematic reviews emphasize the necessity of incorporating long-term functional outcomes in treatment algorithms, particularly regarding the impact of preoperative radiotherapy on intestinal function. A large-scale analysis encompassing 5102 patients identified distal tumor location, low anastomotic position, and postoperative complications as significant predictors of LARS[75].
The increasing incidence of rectal cancer in younger populations, projected to constitute 23% of cases by 2030[76], necessitates comprehensive consideration of not only oncological outcomes and anal function but also urogenital function, sexual function, and quality of life. Given the heterogeneity of low rectal cancer presentations[77], treatment strategies increasingly emphasize individualization, incorporating patient-specific factors and surgical expertise.
Despite significant advances, sphincter-preserving surgery for low rectal cancer faces ongoing challenges. Primary concerns include the refinement of surgical indication criteria[78], longitudinal evaluation of functional reconstruction outcomes, standardization of novel surgical techniques, and the development of comprehensive postoperative rehabilitation protocols. Studies have shown that blockchain technology provides technical support for the credibility of surgical research and the security of clinical data management, promoting the field toward a more transparent and efficient direction[79,80].
The management of low rectal cancer is evolving toward individualized precision medicine[81]. Enhanced patient outcomes are anticipated through multidisciplinary collaboration, technological innovation, and standardized management protocols, reflecting the patient-centered paradigm of contemporary surgical practice.
The evolution of sphincter-preserving surgery in low rectal cancer management represents a fundamental paradigm shift from purely oncological outcomes to integrated functional preservation. This transformation has yielded a diverse surgical armamentarium, encompassing local excision, Dixon procedure, ISR, CSPO, and TaTME, facilitating individualized therapeutic approaches based on patient-specific parameters.
The integration of minimally invasive technologies, particularly laparoscopic and robotic platforms, alongside NOTES, has enhanced surgical precision while optimizing postoperative recovery trajectories. Furthermore, innovations in function-preserving perineal reconstruction have established novel approaches for patients requiring posterior pelvic exenteration, reflecting the contemporary emphasis on quality-of-life outcomes in surgical oncology.
Nevertheless, significant challenges persist in sphincter-preserving surgery for low rectal cancer. These include the refinement and standardization of surgical indications, management of postoperative functional impairment-particularly LARS-and the necessity for comprehensive long-term outcome assessment of novel surgical techniques and rehabilitation protocols. Clinical and imaging feature models or machine learning models can be used to predict LARS following laparoscopic anterior resection, enabling early intervention and improving quality of life[82,83].
The increasing incidence of rectal cancer in younger populations has expanded therapeutic objectives beyond oncological radicality to encompass functional preservation and quality-of-life considerations. This evolution necessitates individualized treatment algorithms based on thorough patient assessment within a multidisciplinary framework.
The trajectory of sphincter-preserving surgery in low rectal cancer continues toward minimally invasive, personalized, and precise interventions. Through technological advancement, conceptual innovation, and standardized rehabilitation protocols, improved outcomes for patients with low rectal cancer are anticipated. This progression embodies the patient-centered paradigm of contemporary medicine and exemplifies the evolutionary direction of surgical oncology.
| 1. | Roshandel G, Ghasemi-Kebria F, Malekzadeh R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers (Basel). 2024;16:1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 186] [Reference Citation Analysis (1)] |
| 2. | Klimeck L, Heisser T, Hoffmeister M, Brenner H. Colorectal cancer: A health and economic problem. Best Pract Res Clin Gastroenterol. 2023;66:101839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 3. | Krishnamurty DM, Blatnik J, Mutch M. Stoma Complications. Clin Colon Rectal Surg. 2017;30:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Thyø A, Christensen P, Gögenur I, Krogsgaard M, Lauritzen MB, Laursen BS, Mikkelsen AH, Drewes AM, Juul T. The decline of male sexual activity and function after surgical treatment for rectal cancer. Acta Oncol. 2025;64:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Marcellinaro R, Spoletini D, Grieco M, Avella P, Cappuccio M, Troiano R, Lisi G, Garbarino GM, Carlini M. Colorectal Cancer: Current Updates and Future Perspectives. J Clin Med. 2023;13:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 6. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1388] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 7. | Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, Abbott CR, Scott N, Finan PJ, Johnston D, Quirke P. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 546] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Santucci C, Mignozzi S, Malvezzi M, Boffetta P, Collatuzzo G, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2024 with focus on colorectal cancer. Ann Oncol. 2024;35:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 9. | Bordeianou L, Maguire LH, Alavi K, Sudan R, Wise PE, Kaiser AM. Sphincter-sparing surgery in patients with low-lying rectal cancer: techniques, oncologic outcomes, and functional results. J Gastrointest Surg. 2014;18:1358-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Baird P, J D Steinke, H S Minnaar, Stewart AJ. Assessment of Quality of Life in Rectal Cancer with Organ-Preservation Treatment: Are We There yet? Clin Oncol (R Coll Radiol). 2023;35:e110-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Dimitriou N, Michail O, Moris D, Griniatsos J. Low rectal cancer: Sphincter preserving techniques-selection of patients, techniques and outcomes. World J Gastrointest Oncol. 2015;7:55-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Luvisetto F, Shamali A, Rutgers MLW, Flashman K, Khan JS. Sphincter preservation in patients with low rectal cancer: striking the right oncological balance. Discov Oncol. 2021;12:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Collard M, Lefevre JH. Ultimate Functional Preservation With Intersphincteric Resection for Rectal Cancer. Front Oncol. 2020;10:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Varela C, Kim NK. Surgical Treatment of Low-Lying Rectal Cancer: Updates. Ann Coloproctol. 2021;37:395-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Hakiman H, Boostrom S, Fleshman J. Total Mesorectal Excision with Autonomic Nerve Preservation: “Optimized Surgery”. In: Longo, W, Reddy, V, Audisio, R. (eds) Modern Management of Cancer of the Rectum. London, United Kingdom: Springer. [DOI] [Full Text] |
| 16. | Dulucq JL, Wintringer P, Stabilini C, Mahajna A. Laparoscopic rectal resection with anal sphincter preservation for rectal cancer: long-term outcome. Surg Endosc. 2005;19:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Emile SH, Barsom SH, Elfallal AH, Wexner SD. Comprehensive literature review of the outcome, modifications, and alternatives to double-stapled low pelvic colorectal anastomosis. Surgery. 2022;172:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Kitaguchi D, Ito M. Optimal anastomotic technique in rectal surgery to prevent anastomotic leakage. Ann Coloproctol. 2023;39:97-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 19. | De Robles MS, Young CJ. Triple-Staple Technique Effectively Reduces Operating Time for Rectal Anastomosis. Ann Coloproctol. 2021;37:16-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Chang Y, Jiang HH, Li AJ, Ding HB, Tang EJ, Huang L, Tang C, Sun CY, Fang YP, Huang J, Zhang XJ, Wang S, Lin Y, Liu HL, Feng SQ, Lin MB. [Analysis of 6 cases of functional-preserving perineal reconstruction after posterior pelvic exenteration in women]. Zhongguo Shiyong Waike Zazhi. 2025;45:220-226. [DOI] [Full Text] |
| 21. | Binda C, Secco M, Tuccillo L, Coluccio C, Liverani E, Jung CFM, Fabbri C, Gibiino G. Early Rectal Cancer and Local Excision: A Narrative Review. J Clin Med. 2024;13:2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Mohammed H, Mohamed H, Mohamed N, Sharma R, Sagar J. Early Rectal Cancer: Advances in Diagnosis and Management Strategies. Cancers (Basel). 2025;17:588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Jiang SX, Zarrin A, Shahidi N. T1 colorectal cancer management in the era of minimally invasive endoscopic resection. World J Gastrointest Oncol. 2024;16:2284-2294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 24. | Dekkers N, Dang H, van der Kraan J, le Cessie S, Oldenburg PP, Schoones JW, Langers AMJ, van Leerdam ME, van Hooft JE, Backes Y, Levic K, Meining A, Saracco GM, Holman FA, Peeters KCMJ, Moons LMG, Doornebosch PG, Hardwick JCH, Boonstra JJ. Risk of recurrence after local resection of T1 rectal cancer: a meta-analysis with meta-regression. Surg Endosc. 2022;36:9156-9168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Qu H, Du YF, Li MZ, Zhang YD, Shen J. Laparoscopy-assisted posterior low anterior resection of rectal cancer. BMC Gastroenterol. 2014;14:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ding H, Li J, Chen Y, Yang Z, Peng Z, Liao X. Anal function and quality of life analysis after laparoscopic modified Parks for ultra-low rectal cancer patients. World J Surg Oncol. 2020;18:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Bullock M, Nasir IUI, Hemandas A, Qureshi T, Figueiredo N, Heald R, Parvaiz A. Standardised approach to laparoscopic total mesorectal excision for rectal cancer: a prospective multi-centre analysis. Langenbecks Arch Surg. 2019;404:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Martin ST, Heneghan HM, Winter DC. Systematic review of outcomes after intersphincteric resection for low rectal cancer. Br J Surg. 2012;99:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (3)] |
| 29. | Yamada K, Ogata S, Saiki Y, Fukunaga M, Tsuji Y, Takano M. Long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum. 2009;52:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Yamada K, Ogata S, Saiki Y, Fukunaga M, Tsuji Y, Takano M. Functional results of intersphincteric resection for low rectal cancer. Br J Surg. 2007;94:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 31. | Saito N, Moriya Y, Shirouzu K, Maeda K, Mochizuki H, Koda K, Hirai T, Sugito M, Ito M, Kobayashi A. Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience. Dis Colon Rectum. 2006;49:S13-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Shen Y, Yang T, Zeng H, Meng W, Deng X, Wei M, Wang Z. Low anterior resection syndrome and quality of life after intersphincteric resection for rectal cancer: a propensity score-matched study. Tech Coloproctol. 2023;27:1307-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Zhang B, Zhuo GZ, Liu FF, Zhao YJ, Cao Y, Xiang JB, Ding JH. Assessing Severity of Low Anterior Resection Syndrome After Intersphincteric Resection for Ultralow Rectal Cancer: A Pilot Study Using an Exploratory Instrument. Dis Colon Rectum. 2024;67:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Cura Pales CG, An S, Cruz JP, Kim K, Kim Y. Postoperative Bowel Function After Anal Sphincter-Preserving Rectal Cancer Surgery: Risks Factors, Diagnostic Modalities, and Management. Ann Coloproctol. 2019;35:160-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Jung WB. Beyond survival: a comprehensive review of quality of life in rectal cancer patients. Ann Coloproctol. 2024;40:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Perdawood SK. History of Rectal Cancer Surgery. In: Dapri G, Marks JH, editors. Surgical Techniques in Rectal Cancer: Transanal, Laparoscopic and Robotic Approach. Tokyo, Japan: Springer, 2018. [DOI] [Full Text] |
| 37. | Breen RE, Garnjobst W. Surgical procedures for carcinoma of the rectum. A historical review. Dis Colon Rectum. 1983;26:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Lange MM, Rutten HJ, van de Velde CJ. One hundred years of curative surgery for rectal cancer: 1908-2008. Eur J Surg Oncol. 2009;35:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Lu W, Huang S, Ye H, Xiang S, Zeng X. Application of laparoscopic modified Bacon operation in patients with low rectal cancer and analysis of the changes in anal function: A retrospective single-center study. Front Oncol. 2023;13:1087642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Liu Z, Tao K, Xu C, Xiong F, Zhao Y, He F, Pei F, Yao Q, Huang J. 121TiP Comparison of postoperative anal function between parks and bacon techniques in lower rectal cancer: A multicenter, prospective, randomized control study. Ann Oncol. 2024;35:S1447-S1448. [DOI] [Full Text] |
| 41. | Simo V, Tejedor P, Jimenez LM, Hernan C, Zorilla J, Arrredondo J, Lapuente F, Pastor C. Oncological safety of transanal total mesorectal excision (TaTME) for rectal cancer: mid-term results of a prospective multicentre study. Surg Endosc. 2021;35:1808-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Koedam TW, van Ramshorst GH, Deijen CL, Elfrink AK, Meijerink WJ, Bonjer HJ, Sietses C, Tuynman JB. Transanal total mesorectal excision (TaTME) for rectal cancer: effects on patient-reported quality of life and functional outcome. Tech Coloproctol. 2017;21:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 43. | Sun G, Lou Z, Zhang H, Yu GY, Zheng K, Gao XH, Meng RG, Gong HF, Furnée EJB, Bai CG, Zhang W. Retrospective study of the functional and oncological outcomes of conformal sphincter preservation operation in the treatment of very low rectal cancer. Tech Coloproctol. 2020;24:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Lou Z, Zhang W. [Transanal conformal resection for super low rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:246-249. [PubMed] [DOI] [Full Text] |
| 45. | Sun G, Lou Z, Zheng K, Chen Y, Zhang H, Wen R, Gao X, Meng R, Gong H, Bai C, Furnée EJB, Zhang W. Comparison of functional and oncological outcome of conformal sphincter preservation operation, low anterior resection, and abdominoperineal resection in very low rectal cancer: a retrospective comparative cohort study with propensity score matching. Langenbecks Arch Surg. 2023;408:208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Sun G, Zang Y, Ding H, Chen Y, Groothof D, Gong H, Lou Z, Meng R, Chen Z, Furnee E, Xiang J, Zhang W. Comparison of anal function and quality of life after conformal sphincter preservation operation and intersphincteric resection of very low rectal cancer: a multicenter, retrospective, case-control analysis. Tech Coloproctol. 2023;27:1275-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Zheng K, Hu Q, Yu G, Zhou L, Yao Y, Zhou Y, Wang H, Hao L, Yu E, Lou Z, Zhang Y, Qiu H, Meng R, Zhang W. Trends of sphincter-preserving surgeries for low lying rectal cancer: A 20-year experience in China. Front Oncol. 2022;12:996866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 48. | Künzli BM, Friess H, Shrikhande SV. Is laparoscopic colorectal cancer surgery equal to open surgery? An evidence based perspective. World J Gastrointest Surg. 2010;2:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Li XX, Wang RJ. Core value of laparoscopic colorectal surgery. World J Gastrointest Endosc. 2015;7:1295-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Chadi SA, Berho M, Wexner SD. Surgeon perspectives on the use and effects of neoadjuvant chemoradiation in the treatment of rectal cancer: a comprehensive review of the literature. Langenbecks Arch Surg. 2015;400:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Zorron R, Palanivelu C, Galvão Neto MP, Ramos A, Salinas G, Burghardt J, DeCarli L, Henrique Sousa L, Forgione A, Pugliese R, Branco AJ, Balashanmugan TS, Boza C, Corcione F, D'Avila Avila F, Arturo Gómez N, Galvão Ribeiro PA, Martins S, Filgueiras M, Gellert K, Wood Branco A, Kondo W, Inacio Sanseverino J, de Sousa JA, Saavedra L, Ramírez E, Campos J, Sivakumar K, Rajan PS, Jategaonkar PA, Ranagrajan M, Parthasarathi R, Senthilnathan P, Prasad M, Cuccurullo D, Müller V. International multicenter trial on clinical natural orifice surgery--NOTES IMTN study: preliminary results of 362 patients. Surg Innov. 2010;17:142-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 52. | Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 550] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 53. | Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Caro-Tarrago A, Gomez-Diaz CJ, Navarro-Soto S. Transanal endoscopic surgery in rectal cancer. World J Gastroenterol. 2014;20:11538-11545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 54. | Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. October 2005. Surg Endosc. 2006;20:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 532] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 55. | Zhu Y, Xiong H, Chen Y, Liu Z, Jiang Z, Huang R, Gao F, Zhang Q, Wang M, Jin Y, Qiao T, Ma T, Hu H, Wang X, Tang Q, Wang G. Comparison of natural orifice specimen extraction surgery and conventional laparoscopic-assisted resection in the treatment effects of low rectal cancer. Sci Rep. 2021;11:9338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Lacy AM, Tasende MM, Delgado S, Fernandez-Hevia M, Jimenez M, De Lacy B, Castells A, Bravo R, Wexner SD, Heald RJ. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg. 2015;221:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 57. | Flynn J, Larach JT, Kong JCH, Waters PS, McCormick JJ, Warrier SK, Heriot A. Patient-Related Functional Outcomes After Robotic-Assisted Rectal Surgery Compared With a Laparoscopic Approach: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2022;65:1191-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 58. | Jeon Y, Park EJ, Baik SH. Robotic Surgery for Rectal Cancer and Cost-Effectiveness. J Minim Invasive Surg. 2019;22:139-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | TengTeng L, HaiXiao F, Wei F, Xuan Z. Robotic surgery versus laparoscopic surgery for rectal cancer: a comparative study on surgical safety and functional outcomes. ANZ J Surg. 2025;95:156-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Luca F, Craigg DK, Senthil M, Selleck MJ, Babcock BD, Reeves ME, Garberoglio CA. Sexual and urinary outcomes in robotic rectal surgery: review of the literature and technical considerations. Updates Surg. 2018;70:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Kim HJ, Choi GS, Park JS, Park SY, Yang CS, Lee HJ. The impact of robotic surgery on quality of life, urinary and sexual function following total mesorectal excision for rectal cancer: a propensity score-matched analysis with laparoscopic surgery. Colorectal Dis. 2018;20:O103-O113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Jones CS, Nowers J, Smart NJ, Coelho J, Watts A, Daniels IR. Pelvic floor reconstruction with bilateral gracilis flaps following extralevator abdominoperineal excision - a video vignette. Colorectal Dis. 2017;19:1120-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Schoene MI, Schatz S, Brunner M, Fuerst A. Gracilis muscle transposition in complex anorectal fistulas of diverse types and etiologies: long-term results of 60 cases. Int J Colorectal Dis. 2023;38:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 64. | Shahzad F, Ray E. Pelvic and Perineal Reconstruction. Plast Reconstr Surg. 2024;154:803e-816e. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 65. | Bustos SS, Yan M, Forte AJ, Moran SL, Manrique OJ. Options and outcomes of soft tissue reconstruction in patients undergoing surgery for locally recurrent rectal cancer. Semin Colon Rectal Surg. 2020;31:100766. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Insua-Pereira I, Ferreira PC, Teixeira S, Barreiro D, Silva Á. Fournier's gangrene: a review of reconstructive options. Cent European J Urol. 2020;73:74-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Glynne-Jones R, Hall M. Radiotherapy and locally advanced rectal cancer. Br J Surg. 2015;102:1443-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Maes-Carballo M, Gómez-Fandiño Y, García-García M, Martín-Díaz M, De-Dios-de-Santiago D, Khan KS, Bueno-Cavanillas A. Colorectal cancer treatment guidelines and shared decision making quality and reporting assessment: Systematic review. Patient Educ Couns. 2023;115:107856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Fokas E, Rödel C, Smith JJ, Garcia-Aguilar J, Buyse M, Glynne-Jones R. Disease-free survival as the endpoint in multimodal rectal cancer trials: have we got this right? Lancet Gastroenterol Hepatol. 2023;8:962-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 70. | Aschele C, Glynne-Jones R. Selecting a TNT Schedule in Locally Advanced Rectal Cancer: Can We Predict Who Actually Benefits? Cancers (Basel). 2023;15:2567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 71. | Donnelly M, Ryan OK, Ryan ÉJ, Creavin B, O'Reilly M, McDermott R, Kennelly R, Hanly A, Martin ST, Winter DC. Total neoadjuvant therapy versus standard neoadjuvant treatment strategies for the management of locally advanced rectal cancer: network meta-analysis of randomized clinical trials. Br J Surg. 2023;110:1316-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 72. | Juul T, Ahlberg M, Biondo S, Espin E, Jimenez LM, Matzel KE, Palmer GJ, Sauermann A, Trenti L, Zhang W, Laurberg S, Christensen P. Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum. 2014;57:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 73. | Sun R, Dai Z, Zhang Y, Lu J, Zhang Y, Xiao Y. The incidence and risk factors of low anterior resection syndrome (LARS) after sphincter-preserving surgery of rectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2021;29:7249-7258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 74. | Cornish JA, Tilney HS, Heriot AG, Lavery IC, Fazio VW, Tekkis PP. A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann Surg Oncol. 2007;14:2056-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Ye L, Huang M, Huang Y, Yu K, Wang X. Risk factors of postoperative low anterior resection syndrome for colorectal cancer: A meta-analysis. Asian J Surg. 2022;45:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 76. | Spaander MCW, Zauber AG, Syngal S, Blaser MJ, Sung JJ, You YN, Kuipers EJ. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023;9:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 174] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 77. | Lang D, Ciombor KK. Diagnosis and Management of Rectal Cancer in Patients Younger Than 50 Years: Rising Global Incidence and Unique Challenges. J Natl Compr Canc Netw. 2022;20:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 78. | You YN, Dozois EJ, Boardman LA, Aakre J, Huebner M, Larson DW. Young-onset rectal cancer: presentation, pattern of care and long-term oncologic outcomes compared to a matched older-onset cohort. Ann Surg Oncol. 2011;18:2469-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Engel A. Could Blockchain technology add value to surgical outcomes research? Colorectal Dis. 2018;20:369-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Wang S, Du G, Dai S, Miao M, Zhang M. Efficient information exchange approach for medical IoT based on AI and DAG-enabled blockchain. Heliyon. 2025;11:e41617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 81. | Saad El Din K, Loree JM, Sayre EC, Gill S, Brown CJ, Dau H, De Vera MA. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 82. | Qin Q, Huang B, Wu A, Gao J, Liu X, Cao W, Ma T, Kuang Y, Guo J, Wu Q, Shao B, Guan Q, Yao H, Zhang X, Wang H; Chinese Radiation Intestinal Injury Research Group. Development and Validation of a Post-Radiotherapy Prediction Model for Bowel Dysfunction After Rectal Cancer Resection. Gastroenterology. 2023;165:1430-1442.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Wang Z, Shao SL, Liu L, Lu QY, Mu L, Qin JC. Machine learning model for prediction of low anterior resection syndrome following laparoscopic anterior resection of rectal cancer: A multicenter study. World J Gastroenterol. 2023;29:2979-2991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/