Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.107110
Revised: April 24, 2025
Accepted: May 28, 2025
Published online: July 27, 2025
Processing time: 130 Days and 7.9 Hours
Gastrointestinal (GI) malignancies, including gastric and colorectal cancers, remain one of the primary contributors to cancer-related illness and death globally. Despite the availability of conventional diagnostic tools, early detection and personalized treatment remain significant clinical challenges. Integrated multi-omics methods encompassing genomic, transcriptomic, proteomic, metabo
To investigate the application of multi-omics approaches in the early detection, risk stratification, treatment optimization, and biomarker discovery of GI malignancies.
The systematic review process was conducted in accordance with the PRISMA 2020 guidelines. Five databases, PubMed, ScienceDirect, Scopus, ProQuest, and Web of Science, were searched for studies published in English from 2015 onwards. Eligible studies involved human subjects and focused on multi-omics integration in GI cancers, including biomarker identification, tumor microenvironment analysis, tumor heterogeneity, organoid modeling, and artificial intelligence (AI)-driven analytics. Data extraction included study characteristics, omics modalities, clinical applications, and evaluation of study quality conducted with the Cochrane risk of bias 2.0 instrument.
A total of 17196 initially identified articles, 20 met the inclusion criteria. The findings highlight the superiority of multi-omics platforms over traditional biomarkers (e.g., carcinoembryonic antigen and carbohydrate antigen 19-9 in detecting early stage GI cancers. Key applications include the identification of circulating tumor DNA, extra
Multi-omics approaches offer significant advancements in the early diagnosis, prognostic evaluation, and personalized treatment of GI malignancies. Their integration with AI analytics, organoid biobanking, and microbiota modulation provides a pathway for precision oncology research.
Core Tip: Gastrointestinal malignancies present substantial diagnostic and therapeutic challenges due to their molecular complexity. This systematic review highlights how comprehensive omics strategies incorporating genomic, transcriptomic, proteomic, and metabolomic data and epigenomics, integrated with machine learning and artificial intelligence, can revolutionize precision oncology. These strategies enable early detection through biomarkers like circulating tumor DNA and extracellular vesicles, enhance risk stratification, and uncover mechanisms of drug resistance and tumor heterogeneity, paving the way for individualized gastrointestinal cancer therapies.
- Citation: Koo TH, Lee YL, Leong XB, Hayati F, Zakaria MH, Zakaria AD. Multi-omics perspectives for gastrointestinal malignancy: A systematic review. World J Gastrointest Surg 2025; 17(7): 107110
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/107110.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.107110
Gastrointestinal (GI) cancers including gastric cancer (GC) and colorectal cancer (CRC) represent a major contributor to cancer-related mortality and high incidence globally. Millions of new cases and deaths have been reported annually[1]. For instance, with a five-year survival rate of only 6% in advanced cases, ranked third, GC contributes significantly to mortality and fifth regarding global incidence[2]. Accurate risk assessment and early diagnosis are essential to improve patient outcomes. The majority of conventional diagnostic techniques, including serum-based biomarkers and endoscopy, are frequently insensitive. For large-scale screening, the current gold standards, such as gastroscopy, are intrusive, costly, and unreliable[3].
To provide insights into the underlying molecular biology and clinical features of GI malignancies, multi-omics approaches have emerged as critical tools for integrating these diverse aspects into genomic, transcriptomic, proteomic, metabolomic, and metabolome data[4]. Developments in machine learning (ML) and lipidomics have provided a foundation for the discovery of cancer-specific indicators and the creation of reliable early detection and screening instruments. These techniques, such as liquid biopsy, surpass traditional markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 72-4 (CA72-4), and CA19-9 for cancer diagnosis and prognosis[3].
By focusing on the involvement of limb bud-heart (LBH)-related genes, which further contribute to cancer invasion and dissemination through the regulation of epithelial-mesenchymal transition (EMT) signaling, these techniques have demonstrated the relevance of particular molecular pathways, such as EMT[2]. Blood metabolomics data has enabled investigations into the existence of GI cancers, offering a prognostic perspective for patients with cancer[3]. Transcriptomic and proteomic analyses have revealed the reprogramming of lipid metabolism, further suggesting that certain lipid-related metabolic pathways are dysregulated in patients with GC[3].
Despite promising advancements, some limitations were encountered. Organoid models offer physiologically realistic in vitro models for disease research and individualized treatment because they closely resemble the biological traits of the original malignancy, such as gene expression activity, protein profiles, metabolic dynamic, and other tumor-related variables. In studies by Zhang et al[5], the broad use of organoid research was confronted with several important obstacles, including technical constraints, standardization concerns, and uncontrollable reproducibility issues related to organoids, which further increased the challenges of translating organoid applications into clinical settings. This systematic review aimed to investigate the application of multi-omics approaches in the early detection, risk stratification, treatment optimization, and biomarker discovery of GI malignancies.
This study presents a systematic review of published literature on multi-omics approaches for GI malignancies using the PRISMA 2020 guidelines. A standardized protocol, consistent with the Cochrane Handbook for Systematic Reviews of Interventions and the PRISMA 2009 Checklist, was used to conduct this systematic review.
Studies were eligible for inclusion if they were interventional or non-interventional and satisfied the following eligibility criteria: (1) The article must consider multi-omics approaches or advancements in cancer research with a primary or significant focus on GI malignancies; (2) All types of healthcare environments and locations were considered, without restriction; (3) The study included findings on at least one outcome associated with early detection, stratification at risk, and individualized treatment of GI cancers via multi-omics approaches, incorporating biomarkers, analysis of tumor microenvironment (TME), artificial intelligence (AI)-driven analytics, and/or tumor heterogeneity; and (4) Only studies published in English and concerning human healthcare were included.
Furthermore, studies were excluded when not assigned to a category as belonging to the healthcare sector, unrelated to cancer research or multi-omics applications, or published before 2015. Non-peer-reviewed sources, including conference proceedings, posters presentations, and oral communications, or included in textbooks were not considered in the screening phase. In addition, this review excluded research articles comprising secondary literature, including commentaries, letters to the editor, and editorials pieces, as well as unrelated publications and duplicates.
An extensive literature search was performed using five electronic databases: PubMed, ScienceDirect, Scopus, ProQuest, and Web of Science. Articles published in 2015 were identified through a tailored search approach. Keywords and Boolean terms applied in the search strategy included: (“Multi-omics” OR “Multi-Omic Analysis”) AND (“Gastrointestinal Malignancy” OR “GI Cancer” OR “Gastrointestinal Cancer”) AND (“Genomics” OR “Transcriptomics” OR “Proteomics” OR “Metabolomics” OR “Epigenomics”) AND (“Biomarkers” OR “Molecular Pathways” OR “Biomarker Discovery”) AND (“Precision Oncology” OR “Personalized Medicine”) AND (“Review” OR “Systematic Review” OR “Literature Review”). To minimize the retrieval of irrelevant articles, the search strategy limited the use of keywords and medical subject headings terms within the “Title/Abstract” field.
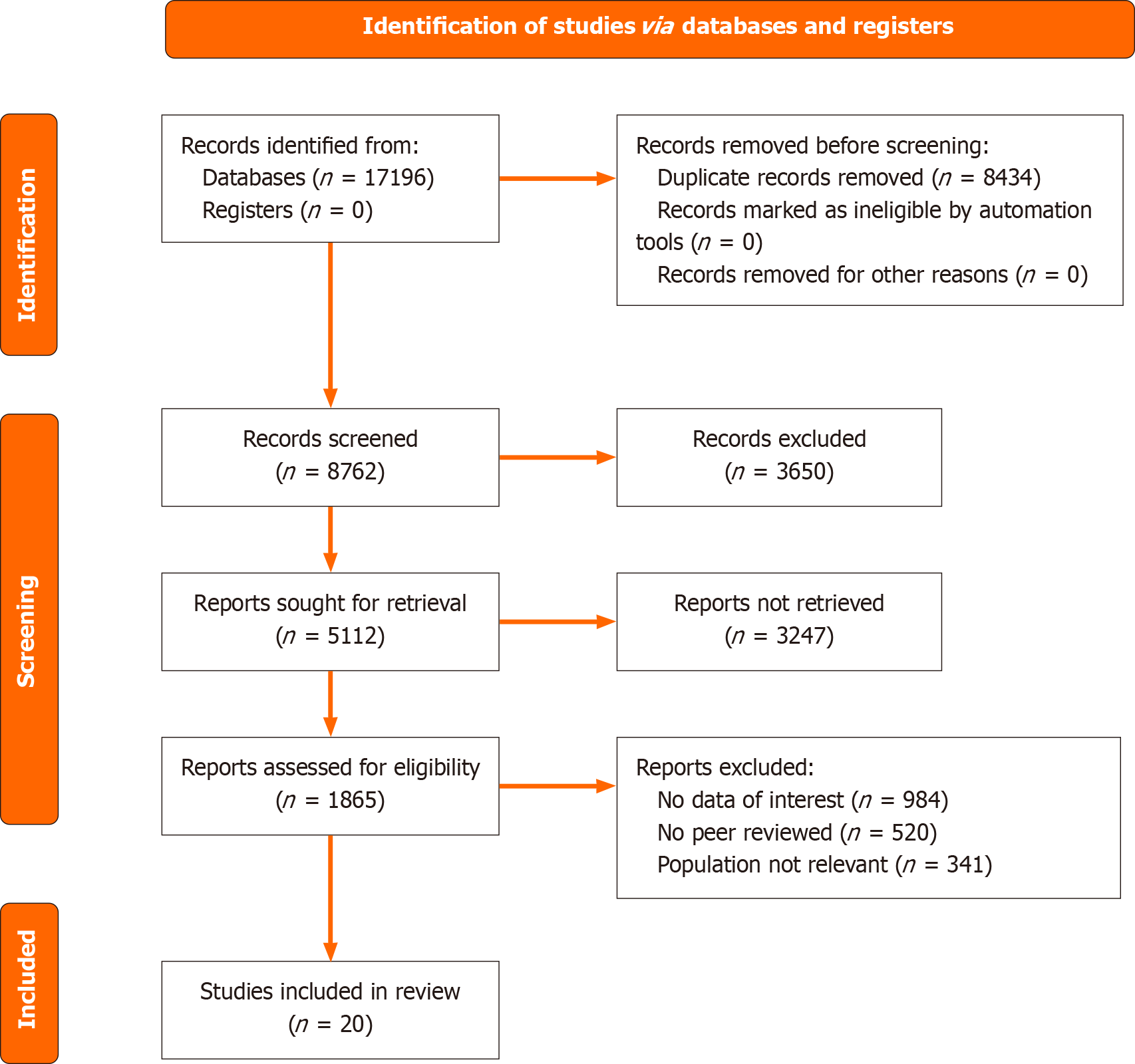
The collected articles were transferred to Microsoft Excel (Microsoft Corp, United States) to facilitate the removal of duplicates and the screening process. Two reviewers independently assessed the titles and abstracts before proceeding to scrutinize the articles with full-text availability. Conflicts in assessment between the reviewers were settled through consultation with a senior reviewer until consensus was achieved. The PRISMA flow diagram (Figure 1) outlines the excluded studies along with the reasons for exclusion.
The primary outcomes of this guideline focus on the early detection and risk stratification of GI malignancies using multi-omics approaches. The diagnostic accuracy is improved by integrating molecular markers such as circulating tumor DNA (ctDNA) and extracellular vesicles (EVs). Additionally, it analyzes TME profiling to enhance immunotherapy and analyzes targeted therapies for cancer therapy for individualized care. The secondary outcomes of this guideline were understanding tumor heterogeneity and molecular subtyping for more effective therapeutic choices, studying metastasis and drug resistance mechanisms, and assessing the role of organoid models in preclinical research. This study also investigated AI and ML integration in multi-omics data analysis, which further refined the identification of biomarkers and predictive analytics to improve clinical outcomes in GI cancer management.
Data regarding these outcomes were extracted from the included studies. Data on primary and secondary outcomes were systematically extracted to support evidence-based recommendations. For early stage identification and risk assessment (primary) outcomes, data were analyzed across multi-layered omics analyses involving genes, transcripts, proteins, and metabolites to identify key biomarkers, such as ctDNA, EVs, and serum lipid metabolic signatures. These findings highlight the utility of machine-learning-based diagnosis models and liquid biopsy approaches for enhancing early detection rates. For TME modulation and therapeutic optimization (secondary) outcomes, data were analyzed to test the relevance of immune markers, such as tumor-infiltrating lymphocytes, EMT regulators, and pyroptosis-associated signatures. Key interventions, such as targeted approaches to personalized medicine based on molecular subtypes and predictive biomarkers, were considered to assess their impact on treatment sensitivity, response to immunotherapy, and patient survival.
A structured and standardized form was used by both two of the authors to extract the necessary data. The extracted data comprised: (1) Study attributed, including name of the author, publication year, geographical origin, and methodological approach; (2) Details of the intervention (type of multi-omics approaches, biomarker integration, TME profiling, and AI-driven analytics for GI malignancy management); and (3) Outcome data (diagnostic accuracy improvements, efficacy of individualized treatments, tumor heterogeneity assessment, and overall clinical impact).
We assess the risk of bias (RoB) of the included studies using established predefined assessment criteria. Prior to analysis, the literature must undergo a quality assessment. The Cochrane RoB 2.0 tool was employed and used for assessing randomization-related bias, deviations from targeted interventions, data attrition, methodology of outcome measurement, reporting bias, and overall bias risk. Articles were assessed by applying the RoB 2.0 assessment framework and categorized to one of three levels: Low risk, some concerns, or high RoB. Any differences in assessments were addressed between reviewers through dialogue until agreement was reached.
The search term “multi-omic” generated 20000 results. The integration of “multi-omic” and “gastrointestinal malignancy” resulted in 21 results, the pairing of “multi-omic”, “gastrointestinal malignancy”, and “precision oncology” generated three results, and the integration of “multi-omic” and “biomarker discovery” contributed to 2780 hits. In total, there are 17196 papers being identified and 8434 repeated titles were identified and excluded.
After excluding subsequent 3650 records, a total of 5112 articles fulfilled the eligibility criteria. Among them, 3247 were excluded for lacking full-text access, and 1865 articles underwent full-text assessment. Upon review, articles with no data of interest (n = 984), those that were not peer-reviewed (n = 520), and those with a population that was not relevant (n = 341) were excluded. Out of all the articles reviewed, only 20 satisfied the selection criteria and were retained for final analysis (Table 1). The PRISMA flowchart summarizing the selection process appears in Figure 1.
| Ref. | Study designs | Location | Sample size | Purpose | Outcomes |
| Qian et al[9], 2024 | Prospective cohort | China | 813 patients | To investigate the proteomic landscape of EOC and identify potential biomarkers for early detection and monitoring | Study discovered genetic pathways of treatment resistance in EOC, identified eight malignancy-associated proteins as putative biomarkers, and created machine learning models for recurrence prediction |
| Wang et al[11], 2024 | Retrospective cohort | China | 1903 patients (925 patients from three public databases and 978 patients from four independent Chinese medical cohorts) | To investigate the role of pyroptosis in tumor immune microenvironment remodeling and its impact on optimizing neoadjuvant immunotherapy for GC | Low pyroptosis risk score suggests a high infiltration of anti-tumor immune cells, good immunotherapy response, and better survival for patients, while pyroptosis risk score can serve as a predictive biomarker for guiding neoadjuvant immunotherapy strategy in GC |
| Sun et al[12], 2024 | Case-control and retrospective cohort | China and United Kingdom | Discovery cohort: 150 CRC cases, 50 controls; validation cohort: 731 CRC cases, 51500 controls | To establish a prediction model for CRC onset by incorporating proteomics, polygenic risk scores, and QCancer-15 | Further improvement in accuracy with respect to the prediction of risk for CRC was achieved after integrating the proteomic biomarkers with polygenic risk scores |
| Wang et al[13], 2024 | Literature review | China | N/A | To summarize the biomarkers, molecular mechanisms and therapeutic strategies for liver metastasis in GC | The importance of molecular biomarkers like TP53, HER2, and MET is outlined in guiding precision therapy for liver metastasis. It has proposed a systemic and targeted therapy with genomic profiling that improves survival outcomes |
| Ai et al[1], 2024 | Retrospective cohort | China | 515 patients | To investigate gastrointestinal tumor heterogeneity through integrated analysis of multimodal data at transcriptomic, imaging, and immune profiles | Highlighted hub genes (e.g., MAGI2-AS3, MALAT1, and SPARC) linked to tumor behavior and therapeutic targets |
| Ye et al[14], 2025 | Retrospective cohort | China | 939 patients | To build a predictive model of CDI for prognosis and treatment in serous ovarian carcinoma | High CDI was associated with poor survival, immunosuppression, and drug resistance. It predicted possible therapy options for high CDI cohorts including trametinib and BMS-754807 |
| Xie et al[15], 2024 | Retrospective cohort | China | 1340 patients | To discuss the role of BAP1-mediated MAFF deubiquitylation in CRC growth and its implications on considerations of patient outcome, particularly focusing mainly on conceptions of protein stability and potential therapeutic interventions | Low expression of both MAFF and low levels of BAP1 correlate with a poor prognosis and at an advanced disease stage of CRC, while BAP1-MAFF axis emerging as a potential therapeutic target |
| Cai et al[3], 2024 | Retrospective cohort | China | 944 participants from four cohorts | To construct a serum lipid metabolic signature towards early detection and prognosis of GC | Serum lipid metabolic signature showed a distinctive performance in effectively discriminating GC patients from healthy donors and was ultra-high effective for early-stage diagnosis and prognosis in GC |
| Gao et al[8], 2024 | Prospective cohort | China | 373 patients (training cohort: 93 CRC patients and 96 healthy controls; validation cohort: 89 CRC patients and 95 healthy controls) | To develop a multi-omics liquid biopsy assay for the early detection of CRC | Multi-omics model attained an AUC, AUC of 0.981 during validation, 94.7% specificity, with high detection rates for stage I at 80% and stage II at 89.2% CRC |
| Yin et al[7], 2024 | Retrospective cohort | China | 949 patients (discovery: 37 cases; training: 338 cases; test: 328 cases; external validation: 246 cases) | To find out the key proteomic biomarkers in serum extracellular vesicles by validating a machine learning-based diagnostic model for CRC diagnosis | PF4 and AACT were identified as top biomarkers. A random forest model attained AUC between 0.960 and 0.963 in the non-invasive diagnosis of CRC, even in its early stages |
| Dai et al[2], 2024 | Scientific report | China | 867 GC tissue samples and 32 normal tissue samples | To investigate ICD and its impact on the tumor microenvironment in GC | Developed ICD-related gene signature for prognosis. LBH was identified as a key gene promoting GC progression via EMT pathway |
| Matsuoka et al[6], 2024 | Review | Japan | N/A | To assess the role of genetic testing and emerging technologies in GI cancer management | Emphasized new technologies in genetic testing (e.g., multi-gene panels, liquid biopsies) and their application in personalized treatment for patients with diverse GI cancers |
| Mazloomnejad et al[4], 2023 | Systematic review | Iran | N/A | To review multi-omics approaches for the identification of upper GI cancers, biomarkers for diagnosis, prognosis, and treatment response | Identified prognostic and diagnostic biomarkers, patient stratifications, and therapeutic targets through omics integration approaches |
| Kiran et al[19], 2024 | Review | India | N/A | To discuss precision medicine advancements in CRC by emphasizing molecular profiling and targeted therapy | Emphasized the importance of biomarkers such as microsatellite instability, KRAS, and targeted therapies like EGFR inhibitors and immune checkpoint inhibitors, emphasizing their transformative role in personalized care |
| Yu et al[17], 2022 | Review | China | 455 patients | To assess the role of patient-derived organoid biobanks’ roles in the advancement of personalized medicine for GI cancers | Highlighted that patient-derived organoids accurately reflect tumor features, aiding chemotherapy, radiotherapy, and targeted therapy decisions, with further proposed improvements for biobank protocols and drug screenings |
| Zhang et al[5], 2024 | Review | China | N/A | To explore CRC organoids as models for clinical research, drug screening, and precision medicine | Focus on organoids as reliable models in the study of tumor heterogeneity, therapeutic response, and gene editing, while discussing challenges like culture standardization and clinical translation |
| Uyar et al[18], 2021 | Observational computational analysis | Germany | 6775 patients from 21 cancer types | To demonstrate how multi-omics integration with deep learning could be used for cancer subtype classification, prognosis, and treatment response prediction | Presented a deep learning model for integrating multi-omics data that reached high accuracy in the classification of cancer and the prediction of personalized therapy |
| Xie et al[10], 2024 | Review | China | N/A | To review progression in multi-omics studies and the survival mechanisms of circulating tumor cells in cancer metastasis | Discusses the genetic, transcriptomic, proteomic, and metabolomic profiles of circulating tumor cells and emphasizing their role in metastasis, drug resistance as targets for therapy in precision medicine |
| Bi et al[16], 2024 | Review | China | N/A | To discuss the role of intratumoral microbiota in gastrointestinal cancer with a particular focusing on its metabolic effects and biomarker potential for diagnosis, prognosis, and treatment | Emphasizing how intratumoral microbiota influences tumor development, immune response, and resistance to therapy, and considers future perspective through advanced omics and clinical studies towards integrating microbiota-based strategies in precision oncology |
| Paverd et al[20], 2024 | Perspective | United Kingdom and Italy | N/A | To explore strategy for integrating radiological imaging with other multi-omics data to help precision oncology | Discusses challenges and developments in the fusion of imaging-omics data, outlining on deep learning, and multimodal integration as promising for enhanced prediction and improved outcomes in cancer treatment |
GI malignancies comprise 30% of all tumors worldwide and are responsible for more than 3.5 million deaths annually, constituting approximately 37% of all cancer-related mortalities. This demonstrates their importance to human health as a major global challenge[6]. It is worth mentioning that the present review applied a publication time filter, including studies from 2015 onwards. This criterion was established based on the recognition that multi-omics research, particularly in GI malignancies, has evolved rapidly over the past decade. Significant advancements in mass spectrometry-based proteomics and large-scale sequencing technologies, metabolomics platforms, and integrative bioinformatics tools have largely emerged post-2015. These developments have enabled more comprehensive, clinically relevant, and technically robust studies to better reflect the current landscape of precision oncology. Nevertheless, we acknowledge that certain landmark studies published prior to 2015 have facilitated the conceptual development of multi-omics approaches. To address this, we have carefully reviewed and selectively referenced pivotal earlier studies, in which their contributions remain directly relevant and irreplaceable to the field. This approach aimed to balance methodological rigor with comprehensiveness, while ensuring the inclusion of both cutting-edge evidence and an essential historical context.
GI cancers are generally clinically silent during the initial period, leading to late-stage presentations with high recurrence rates. In addition, only 10%-20% of pancreatic or stomach cancers may be considered for surgery when diagnosed. The recurrence rate over five years can be reaching up to 80%-90% in pancreatic cancers and observed 60%-70% in gastric malignancies[4].
At present, the combination of gastroscopy and histopathological examination is the gold standard for diagnosis, but it is an expensive and invasive procedure that depends heavily on the expertise of endoscopists. Hence, it cannot be applied in mass screening[3]. Likewise, despite being the most reliable method for CRC detection, colonoscopy also fails to reach the masses owing to its invasive nature and the inconvenience of repeated screening[7].
Traditional biomarkers such as CEA and CA19-9 are valuable for monitoring treatment responses in patients with CRC, but they lack sufficient sensitivity for early CRC detection[8]. As documented in a study by Yin et al[7], analysis of receiver operating characteristic curves in the training set revealed that platelet factor 4 (PF4) with an area under the curve (AUC) of 0.926 and alpha-1-antichymotrypsin (AACT) with an AUC of 0.770 demonstrated superior performance compared to CEA, which had an AUC of 0.623, and CA19-9, which achieved an AUC of 0.676. Notably, combining PF4 and AACT significantly improved diagnostic accuracy, yielding an outstanding AUC of 0.950[7]. By convention, the ability to correctly identify true positives and true negatives of the CEA test in a multi-omics model in the study by Gao et al[8] were 53.9% and 82.4%, respectively, with the following detection rates according to CRC stage: 26.7% (stage I), with prevalence increased from 62.2% in stage II to 100% in stage IV, with an intermediate value of 55.6% in stage III. These results reflect the superior performance of the multi-omics-based model for CRC detection compared with plasma CEA testing, suggesting that its clinical use could be further expanded[8].
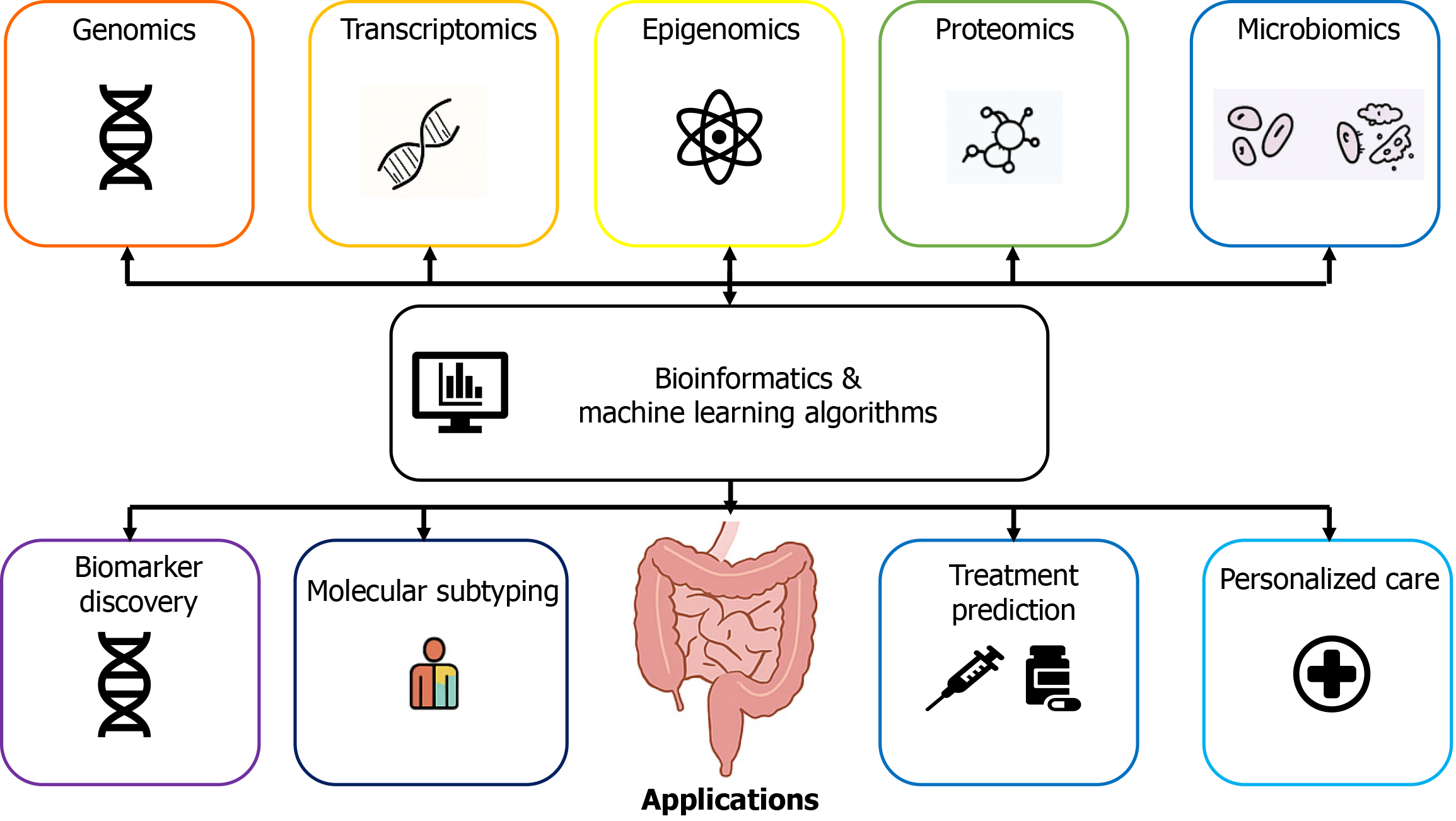
The integration of multi-omics data ranges from the genomic and transcriptomic levels to epigenomic, proteomic, and metabolomic data, along with microbiome information (Figure 2). This improves the classification of tumors, stratification of patients, evaluation of microbial activity and host responses, and prediction of treatment responses[4]. EVs in liquid biopsy, which function to detect ctDNA, have become one of the most promising noninvasive strategies for the detection of GI cancers[3,7].
In research carried out by Yin et al[7], a ML-based diagnostic model using EV-related random forest algorithms achieved the most effective diagnostic performance, as reflected by demonstrating high diagnostic performance with AUCs of 0.960 and 0.963 respectively. This study also identified key proteins, such as PF4 and AACT, using 4D-data-independent acquisition proteomics, which exceeded the performance of conventional biomarkers[7]. A study carried out by Cai et al[3] demonstrated the possibility of integrating data from lipidomics with ML techniques and realizing serum lipid metabolic signatures, which presented a high ability to distinguish early stage GC from controls[3]. A number of proteomic analyses performed to date on epithelial ovarian cancer have identified a series of biomarkers that could be useful for assessing malignancy risk, classifying histological subtypes, stratifying patients, predicting prognosis, and identifying putative drug targets[9]. Compared to the current single-cell RNA sequencing techniques, single-unit cell proteomics techniques achieve greater accuracy. By enabling high-throughput and rapid profiling of tumor proteins, this technology enables the detection of multiple protein markers with significant diagnostic potential[10].
The low existence of tumor-infiltrating lymphocytes within the TME is one of the most substantial obstacles to effective antitumor immunotherapy. The increase in immune cell infiltration will be highly useful in enhancing the immune activities of “cold” tumors and significantly improving their responsiveness to immunotherapy, which would be a major advance in cancer treatment[11]. Additionally, the EMT pathway is a major driver of tumor progression. Dai et al[2] identified the LBH-related gene as a key EMT regulator in GC, further contributing to poor prognosis and enhanced tumor invasion[2]. In contrast, in healthy individuals, the concentration of the trefoil factor 1 (TFF1) in patients with CRC was significantly higher. Furthermore, the expression of TFF1 mRNA was detected in higher abundance in CRC tissues compared to matched adjacent normal tissues. Moreover, for colon cancer, the TFF1 promotes EMT[12].
Reprogramming of lipid metabolism is essential for the onset and progression of tumors[3]. With their predictive value in GC patient survival, GC prognostic subtypes demonstrate clinical utility, whereas subtype-specific genes enable individualized treatment strategies for GC[3]. Pyroptosis is generally defined as an inflammatory programmed cell death (PCD) mechanism that serves an essential function in cancer immunotherapy[11]. The pyroptosis risk score has been developed as a reliable indicator for immunotherapy responsiveness of patient. Low pyroptosis risk score is associated with higher infiltration of CD4+ T cells, effector T cells, and tumor-associated macrophages, making it a potential tool for effective immunotherapeutic strategies in patients with GC[11]. In the study by Dai et al[2], five core regulatory genes, SMAP2, TXNIP, TNFAIP8, LBH, and PIK3IP1, were identified as the principal genes contributing to risk within the immunogenic cell death-related gene signature. Of these, LBH has been recognized as an important factor influencing prognosis in GC[2]. Patient who has CRC with higher latent transforming growth factor-β binding protein 2 levels tend to have worse overall survival, indicating the potential of these genes as both oncogenes in CRC progression and prognostic biomarkers[13].
Differences in gene expression, along with molecular, epigenetic, and immune differences, reflect the intrinsic diversity of GI tumors. These variations are fundamental to understanding why different tumor subtypes respond differently to treatment and have varying prognoses. Tumor-intrinsic variability affects immune responses, causing increased difficulty in predicting and standardizing treatments, leading to further challenges in clinical decision making[1]. A strategy focused on improving cancer prognosis and predictive, preventive, and personalized medicine emphasizes PCD as a key target for enhancing both therapy and prognostic evaluation[14]. Transcriptomic and imaging data analysis has identified key genes, such as MAGI2-AS3, MALAT1, and SPARC, which are implicated in tumor metastasis and invasion[1].
In a study by Xie et al[15], v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog basic leucine zipper transcriptional factor F (MAFF) was underscored as a critical regulator of the ubiquitin-proteasome in CRC progression. Abnormal ubiquitination causes its downregulation and exacerbates disease progression[15]. By stabilizing MAFF, the BRCA1-associated protein 1-MAFF-dual specificity phosphatase 5 axis in this signaling pathway could be a promising target for treating CRC. More extensive exploration using MAFF-knockout mice will help validate the condition when MAFF is completely removed, which could lead to new strategies for CRC treatment[15]. Ye et al[14] conducted studies leveraging the PCD-related gene model to compare cell index death levels in patients. This model demonstrated significant potential for assessing drug sensitivity, immune response, and overall prognosis in cancer patients, establishing it as a crucial tool for precision oncology[14]. The results indicated that immunosuppression was observed in patients with serous ovarian carcinoma with high cell index death, leading to poor responses to immunotherapy[14]. This study also mentioned the need for further research to validate the effectiveness of PCD-related genes in treating cancers other than serous ovarian carcinoma[14].
GI cancers, such as gastric, colorectal, and esophageal cancers, frequently metastasize to distant organs including liver, lungs, peritoneum, and lymph nodes[13,16]. Among these, GC has a remarkable tendency to metastasize to the liver in 4%-14% of cases. Once liver metastasis develops, the prognosis deteriorates considerably, with only 5%-19% of patients surviving beyond five years[13].
Defining it as a disruption in normal equilibrium of microbial communities, dysbiosis can significantly impact the structure and metabolic functions of the host microbiota and influencing tumor metabolism. These disturbances in microbial communities commonly cause a shift in some essential metabolic pathways including glycolysis, fatty acid metabolism, and amino acid metabolism, which can facilitate facilitation of energy requirements from cancerous cells and thus promote their accelerated growth[16]. The provision of key amino acids, such as glutamine and arginine, in TME can be regulated by certain bacterial species, which influence both cancer metabolism and immune cell activity. Additionally, disruptions in microbial balance can alter amino acid metabolism, resulting in the production of immunosuppressive compounds such as kynurenine. This can hinder antitumor immune responses and promote immune evasion in cancer[16].
Bi et al[16] showed that Fusobacterium nucleatum, which is generally present in esophageal squamous cell carcinoma and CRC, promotes metastasis by facilitating immune escape and chemoresistance. Shifts in microbial diversity include an increased abundance of Methylobacterium in GC, which further correlates with poor prognosis, partly because of decrease in CD8+ tissue-resident memory T cells[16]. This indicates that Methylobacterium contributes to tumor development by affecting the expression of transforming growth factor-β and lowering the immune status of the body[16].
Beyond compositional changes, microbiota exert profound functional influences on tumor development and immune modulation through the biosynthesis of microbial products like short-chain fatty acids, secondary bile acids, and tryptophan metabolites[16]. These compounds can shape the TME by regulating immune cell differentiation, cytokine production, and epithelial barrier integrity. For instance, short-chain fatty acids such as butyrate have been shown to enhance regulatory T-cell function and exert anti-inflammatory effects, while dysregulated bile acid metabolism may promote carcinogenesis through pro-inflammatory signaling pathways[13,16].
Moreover, microbial-associated molecular patterns, including lipopolysaccharides and peptidoglycans, can activate pattern recognition receptors, such as toll-like receptors and nucleotide-binding oligomerization domain-like receptors, which co-localized on malignant and immune cell types[12-16]. This activation can either stimulate anti-tumor immunity or, conversely, promote immune evasion, depending on the microbial context and tumor characteristics.
Certain species such as Fusobacterium nucleatum possess the ability to facilitate immune evasion by inhibiting natural killer cell activity and promoting myeloid-derived suppressor cell recruitment within the TME[17]. Helicobacter pylori infection is associated with gastric carcinogenesis through chronic inflammation and immune dysregulation, whereas enterotoxigenic Bacteroides fragilis has been implicated in CRC initiation via T helper cell 17-mediated inflammation[11,15].
Importantly, emerging evidence indicates that microbiota composition may significantly affect patient response to immune checkpoint inhibitors[12,15]. Enrichment of specific taxa, such as Akkermansia muciniphila or Bifidobacterium spp., has been correlated with improved therapeutic outcomes, whereas dysbiosis or antibiotic exposure may impair immunotherapy efficacy[15-17]. These findings suggest that microbiota modulation strategies, including dietary interventions, probiotic supplementation, or fecal microbiota transplantation, may represent promising adjunctive approaches to optimize the immunotherapy response in GI malignancies.
Wang et al[13] demonstrated that tumor protein p53 (TP53) mutations are present in both primary gastric tumors and their matched liver metastases, emphasizing their key role in tumorigenesis. This underscores their crucial roles in cancer progression[13]. However, metastasis is seldom influenced by a single genetic alteration. Instead, mutations in genes such as APC, LRP1B, PIK3CA, and ADAMTS20 work together with TP53 mutations to facilitate the spread of cancer to the liver[13]. EMT plays an essential role in this process, allowing cancer cells to become invasive and migrate through pathways involving matrix metalloproteinases, Wnt signaling, chemokines, and glutamate-cysteine ligase modulatory subunit-related factors[13]. Moreover, patients with TP53-mutated GC who also exhibit human epidermal growth factor receptor 2 and mesenchymal-epithelial transition amplifications might have a greater possibility of liver metastasis, making these genetic changes viable options for therapeutic intervention[13].
The frequently embraced Matrigel-based 3D culture method mimics the extracellular matrix for organoid self-organization, enhances cell-matrix interactions, and almost accurately replicates the condition of the in vivo tumor environment. Organoids can also be used to study tumor heterogeneity by comparing diverse tumor cell subtypes in response to treatments, and they are essential for the further development of personalized therapeutic approaches[5].
The ability of patient-derived organoids (PDOs) to retain the genetic, morphological, and phenotypic characteristics of original tumors makes them invaluable for drug screening and precision medicine applications for tailoring radiotherapy, immunotherapeutics choices, targeted therapy and chemotherapy[17]. Living biobanks for GI cancers, including CRC, primary liver cancer, pancreatic cancer, esophageal cancer, biliary cancer, and GC, have been developed to support the extended preservation and maintenance of PDOs while preserving their morphological, histological, pathological, genetic, and phenotypic similarities with the original tumors[17].
Despite these advantages, several challenges have hindered widespread clinical translation of PDOs. One major challenge mentioned in the studies by Yu et al[17] refers to the duration between biopsy or surgery for functional preparation of the characteristics; however, this can be resolved by utilizing single-cell PDOs from early passages to enhance the efficiency of drug testing for GI cancers. This method reduces the time required for organoid development in clinical applications[17]. Furthermore, the integration of data and record analysis through the application of advanced analytical tools for the clinical information of organoid cultures, passage, and cryopreservation is necessary for the proper assessment of growth and development. Moreover, these tools are important for accelerating organoid technologies for clinical applications[15]. Advances in microfluidic systems may also improve the predictive accuracy of drug evaluation and toxicity assessment in PDO models, because blood flow and nutrient transport in vivo can be mimicked[5].
The lack of standardization in culture protocols, including medium composition, scaffold materials, and sampling requirements, tends to reduce reproducibility, contributing to inter-laboratory variability and inconsistencies in PDO-derived research findings[5]. Good-quality samples are only possible if regular quality checks are performed, such as DNA stability or cell survival evaluation, and consent from patients is ensured. This makes the current study both valid and ethical[5].
AI, particularly ML, is transforming cancer diagnosis and treatment planning by leveraging extensive medical data, such as imaging and multi-omics analysis. Advanced ML approaches are crucial for streamlining high-dimensional data while identifying complex, yet biologically meaningful biomarkers[18]. Integrating multi-omics data remains a fundamental challenge in precision oncology because of the high dimensionality, heterogeneity, and modality imbalance of datasets originating from genomic, transcriptomic, proteomic, epigenomic, and metabolomic platforms[18]. Several computational strategies have been developed to address these challenges, which can be broadly categorized into early integration, intermediate integration, late integration, and deep-learning-based approaches[18]. Early integration methods involved the concatenation of features from different omics layers into a unified dataset, followed by the application of ML approaches like support vector machines and random forest classifiers or elastic net regression[18,19]. Although straightforward, these methods are sensitive to data imbalance and noise.
Intermediate integration methods aim to transform each omics dataset into latent representations before fusion. Examples include canonical correlation analysis, multi-omics factor analysis, and sparse generalized canonical correlation analysis, which allows identification of shared biological signals while preserving the unique contribution of each omics layer[18,19]. For instance, multi-omics factor analysis has been applied effectively in cancer subtype classification and risk stratification. Late integration methods adopt model-level fusion, where predictive models are built independently for each omics layer, and their outputs are subsequently combined using ensemble learning techniques[18]. This approach enhances the model interpretability and flexibility in handling missing or incomplete data.
Recently, deep learning-based integration has emerged as a powerful strategy capable of capturing complex nonlinear interactions across multiple omics layers. Autoencoders, variational autoencoders, and graph neural networks have been successfully employed to perform unsupervised integration of multi-omics data. For example, Uyar et al[18] developed a multi-omics auto-encoder framework that facilitated the stratification of microsatellite instability status in GI cancers and improved survival prediction. Similarly, in the context of GI malignancies, the integration of proteomic and lipidomic data using ML models has demonstrated improved accuracy in early GC detection[3], whereas multi-omics signatures related to pyroptosis and immunogenic cell death have enhanced immunotherapy response prediction in GC[2,11]. Unlike conventional diagnostic models, ML enhances the precision and efficiency by detecting complex patterns and extracting essential biomarkers from liquid and tissue biopsies. These capabilities make ML a valuable tool for improving cancer detection, guiding treatment strategies, and refining prognostic assessments[7].
Yin et al[7] proposed an EV-based random forest model for CRC diagnosis. The authors identified key protein markers using an ML-driven pipeline and confirmed their relevance using enzyme-linked immunosorbent assay detection. Uyar et al[18] discussed a multi-omics auto-encoder for unsupervised integration-based multi-omic signatures that presented a wide array of biological variations across tumor samples from 21 The Cancer Genome Atlas cohorts in a compelling manner by determining key features associated with survival outcomes of interest. These features allow for the prediction and classification of molecular cancer subtypes, including microsatellite instability status in GI cancers across multiple sites and different tissue-based classifications in non-small cell lung cancers. This can be simplified to decision trees to enhance interpretability and practical use. For instance, Uyar et al[18] characterized the reaction from patients with metastatic urothelial cancer undergoing anti-programmed death-ligand 1 immunotherapy and identified well-known biomarkers associated with chemotherapy resistance in patients with glioblastoma multiforme.
Whereas conventional ML requires manually selected radiomics features, often obtained using software such as pyradiomics, deep learning autonomously extracts features from raw medical images[19]. By utilizing convolutional neural networks and vision transformers, deep learning enables a more automated and efficient pathology feature extraction. AI and ML leverage large-scale biological data analyses to identify potential drug candidates and assess their efficacy in defined patient populations[19,20]. Furthermore, this could lead to advancements in targeted therapies for CRC and accelerate drug development. As mentioned by Kiran et al[19], protecting data privacy, complying with regulatory standards, and rigorously validating AI algorithms are essential steps for the implementation of AI and ML[19].
Unlike deep learning methods that often lack interpretability, traditional approaches, such as logistic regression, offer greater transparency by deriving quantitative explanations from feature coefficients[20]. To enhance the interpretability of deep learning, techniques such as saliency and attention maps are implemented to enable the visualization of decision-making processes in radiological images[20].
Looking ahead, future research should focus on translating multi-omics discoveries into clinically actionable strategies for the precision treatment of GI malignancies. This requires the development and validation of robust multi-omics-based predictive models that integrate genomic, transcriptomic, proteomic, and metabolomic signatures to guide individualized therapeutic decisions. Additionally, expanding the application of PDO biobanks and integrating them with multi-omics profiling will provide valuable platforms for preclinical drug screening, functional validation of biomarkers, and tailoring treatment regimens based on patient-specific tumor characteristics.
To realize the full potential of multi-omics in clinical practice, several critical challenges must be addressed. These include the standardization of omics data generation and analysis pipelines, harmonization of computational integration methods, validation of biomarkers in multicenter prospective cohorts, and improvement in the interpretability and transparency of AI-driven models. Furthermore, ensuring ethical governance, patient data privacy, and the development of regulatory frameworks for clinical multi-omics applications are essential for safe and effective implementation in healthcare settings. With continued technological innovation, interdisciplinary collaboration, and the incorporation of multiple omics layers with clinical parameters, the future of precision oncology for GI malignancies holds considerable promise for improving patient outcomes and revolutionizing personalized cancer care.
In conclusion, GI malignancies pose significant global health challenges. While traditional diagnostic methods such as endoscopy and standard biomarkers are not ideal, multi-omics has emerged as a breakthrough method that integrates genomic, transcriptomic, proteomic, and metabolomic data. The integration thereof would enable enhanced early detection by ML processing of markers, such as ctDNA and EVs, further enhancing risk stratification and enabling personalized therapy through the identification of specific molecular signatures and complex tumor microenvironmental interactions. Furthermore, multi-omics enables the understanding of tumor heterogeneity, metastasis, and drug resistance. Microbial imbalances pose another level of complexity, but new developments in organoid models and AI analytics offer promising strategies for treatment specificity, including standardization, reproducibility, data privacy, and ethical concerns that await resolution.
| 1. | Ai D, Du Y, Duan H, Qi J, Wang Y. Tumor Heterogeneity in Gastrointestinal Cancer Based on Multimodal Data Analysis. Genes (Basel). 2024;15:1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Dai SL, Pan JQ, Su ZR. Multi-omics features of immunogenic cell death in gastric cancer identified by combining single-cell sequencing analysis and machine learning. Sci Rep. 2024;14:21751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Cai ZR, Wang W, Chen D, Chen HJ, Hu Y, Luo XJ, Wang YT, Pan YQ, Mo HY, Luo SY, Liao K, Zeng ZL, Li SS, Guan XY, Fan XJ, Piao HL, Xu RH, Ju HQ. Diagnosis and prognosis prediction of gastric cancer by high-performance serum lipidome fingerprints. EMBO Mol Med. 2024;16:3089-3112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Mazloomnejad R, Ahmadi A, Piroozkhah M, Omranian AZ, Zabihi MR, Nazemalhosseini-mojarad E, Kavousi K, Salehi Z. Multi-omics data integration in upper gastrointestinal cancers research: A review of concepts, approaches, and application. 2023 Preprint. Available from: Research Square. [DOI] [Full Text] |
| 5. | Zhang Y, Meng R, Sha D, Gao H, Wang S, Zhou J, Wang X, Li F, Li X, Song W. Advances in the application of colorectal cancer organoids in precision medicine. Front Oncol. 2024;14:1506606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Matsuoka T, Yashiro M. Current status and perspectives of genetic testing in gastrointestinal cancer (Review). Oncol Lett. 2024;27:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Yin H, Xie J, Xing S, Lu X, Yu Y, Ren Y, Tao J, He G, Zhang L, Yuan X, Yang Z, Huang Z. Machine learning-based analysis identifies and validates serum exosomal proteomic signatures for the diagnosis of colorectal cancer. Cell Rep Med. 2024;5:101689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Gao Y, Cao D, Li M, Zhao F, Wang P, Mei S, Song Q, Wang P, Nie Y, Zhao W, Wang S, Yan H, Wang X, Jiao Y, Liu Q. Integration of multiomics features for blood-based early detection of colorectal cancer. Mol Cancer. 2024;23:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Qian L, Zhu J, Xue Z, Zhou Y, Xiang N, Xu H, Sun R, Gong W, Cai X, Sun L, Ge W, Liu Y, Su Y, Lin W, Zhan Y, Wang J, Song S, Yi X, Ni M, Zhu Y, Hua Y, Zheng Z, Guo T. Proteomic landscape of epithelial ovarian cancer. Nat Commun. 2024;15:6462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 10. | Xie Q, Liu S, Zhang S, Liao L, Xiao Z, Wang S, Zhang P. Research progress on the multi-omics and survival status of circulating tumor cells. Clin Exp Med. 2024;24:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Wang JB, Gao YX, Ye YH, Zheng QL, Luo HY, Wang SH, Zhang T, Jin QW, Zheng CH, Li P, Lin JX, Chen QY, Cao LL, Yang YH, Huang CM, Xie JW. Comprehensive multi-omics analysis of pyroptosis for optimizing neoadjuvant immunotherapy in patients with gastric cancer. Theranostics. 2024;14:2915-2933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Sun J, Liu Y, Zhao J, Lu B, Zhou S, Lu W, Wei J, Hu Y, Kong X, Gao J, Guan H, Gao J, Xiao Q, Li X. Plasma proteomic and polygenic profiling improve risk stratification and personalized screening for colorectal cancer. Nat Commun. 2024;15:8873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Ding G, Chu C, Cheng XD, Qin JJ. Genomic biology and therapeutic strategies of liver metastasis from gastric cancer. Crit Rev Oncol Hematol. 2024;202:104470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Ye L, Long C, Xu B, Yao X, Yu J, Luo Y, Xu Y, Jiang Z, Nian Z, Zheng Y, Cai Y, Xue X, Guo G. Multiomics identification of a novel signature for serous ovarian carcinoma in the context of 3P medicine and based on twelve programmed cell death patterns: a multi-cohort machine learning study. Mol Med. 2025;31:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Xie Z, Lin H, Huang Y, Wang X, Lin H, Xu M, Wu J, Wu Y, Shen H, Zhang Q, Chen J, Deng Y, Xu Z, Chen Z, Lin Y, Han Y, Lin L, Yan L, Li Q, Lin X, Chi P. BAP1-mediated MAFF deubiquitylation regulates tumor growth and is associated with adverse outcomes in colorectal cancer. Eur J Cancer. 2024;210:114278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 16. | Bi X, Wang J, Liu C. Intratumoral Microbiota: Metabolic Influences and Biomarker Potential in Gastrointestinal Cancer. Biomolecules. 2024;14:917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | Yu YY, Zhu YJ, Xiao ZZ, Chen YD, Chang XS, Liu YH, Tang Q, Zhang HB. The pivotal application of patient-derived organoid biobanks for personalized treatment of gastrointestinal cancers. Biomark Res. 2022;10:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (77)] |
| 18. | Uyar B, Ronen J, Franke V, Gargiulo G, Akalin A. Multi-omics and deep learning provide a multifaceted view of cancer. 2021 Preprint. Available from: bioRxiv: 462364. [DOI] [Full Text] |
| 19. | Kiran NS, Yashaswini C, Maheshwari R, Bhattacharya S, Prajapati BG. Advances in Precision Medicine Approaches for Colorectal Cancer: From Molecular Profiling to Targeted Therapies. ACS Pharmacol Transl Sci. 2024;7:967-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Paverd H, Zormpas-Petridis K, Clayton H, Burge S, Crispin-Ortuzar M. Radiology and multi-scale data integration for precision oncology. NPJ Precis Oncol. 2024;8:158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/