Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.107092
Revised: April 16, 2025
Accepted: May 27, 2025
Published online: July 27, 2025
Processing time: 130 Days and 21 Hours
Laparoscopic one anastomosis gastric bypass (OAGB) has grown in popularity in recent years for the treatment of morbid obesity. Despite routine practice, the utility of early postoperative upper gastrointestinal (UGI) swallow studies to detect complications following OAGB has been questioned.
To evaluate the effectiveness and cost-efficiency of performing routine UGI studies on the first postoperative day (POD) after OAGB.
A retrospective cohort analysis of a prospectively collected database was conducted to identify all consecutive patients who underwent OAGB between January 2019 and July 2022. Patient demographics, operative data, and postope
A total of 385 patients were included. All patients had an iodine-based contrast swallow study on the first POD. Abnormal findings were observed in 4 patients (1%), none of which were correlated with postoperative complications. Two patients (0.5%) required reoperation due to complications although both had normal UGI study results. Sensitivity and positive predictive value of UGI studies for detecting complications were 0%, while specificity and negative predictive value were 99% and 98%, respectively. Based on hospital charges the overall cost of all the UGI swallow studies performed in our study was 95865 USD.
The study findings showed that performing routine UGI swallow studies on the first POD after laparoscopic OAGB is ineffective in detecting complications and is not cost effective. Normal UGI studies might mislead clinicians in the postoperative period and thus should be omitted in favor of close clinical monitoring.
Core Tip: Routine upper gastrointestinal swallow studies on the first postoperative day after laparoscopic one anastomosis gastric bypass have long been used to detect complications. However, our study demonstrated that these studies have no diagnostic value, with 0% sensitivity for detecting leaks or other complications. Additionally, the high cost associated with routine upper gastrointestinal studies raises concerns about their cost-effectiveness. Given these findings, reliance on clinical monitoring rather than routine imaging may improve postoperative management while reducing unnecessary healthcare costs.
- Citation: Maden A, Kupietzky A, Zimand Y, Bar-Moshe Y, Dover R, Juster EY, Drayer Lichtman M, Grinbaum R, Mazeh H, Mizrahi I. Utility of routine postoperative upper gastrointestinal swallow studies following laparoscopic one anastomosis gastric bypass. World J Gastrointest Surg 2025; 17(7): 107092
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/107092.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.107092
Obesity has emerged as a major global health challenge, with its prevalence steadily rising over recent decades. According to the World Health Organization, global obesity rates have tripled between 1975 and 2016, now affecting approximately 13% of the world’s population. This condition is strongly linked to various serious health issues, including type 2 diabetes, cardiovascular disease, obstructive sleep apnea, and hypertension. Notably, intentional weight loss has been shown to significantly lower the risk of these comorbidities and in some cases even reverse them[1].
Among all available treatment options, bariatric surgery remains the most effective for achieving substantial and sustained weight loss. Non-surgical interventions, although widely used, have generally failed to produce comparable long-term outcomes in terms of weight control and resolution of associated conditions[2,3]. While the Roux-en-Y gastric bypass (RYGB) has long been considered a standard procedure, the one anastomosis gastric bypass (OAGB) has emerged as a viable alternative, demonstrating similar or superior efficacy in weight reduction and metabolic improvement[4,5]. Another widely performed operation is sleeve gastrectomy, though its long-term outcomes appear to be less favorable than those achieved with either form of gastric bypass[4,6].
Early postoperative complications following gastric bypass, such as bleeding, anastomotic leakage, and obstruction, often necessitate urgent intervention[7]. To address this, many bariatric centers, including ours, routinely perform an upper gastrointestinal (UGI) contrast study on the first day following surgery before initiating oral intake[7,8]. However, several studies have highlighted the limitations of UGI studies, noting their cost, technical challenges in patients with obesity, and questionable diagnostic utility particularly after RYGB[9,10]. While this issue has been investigated in the context of sleeve gastrectomy and RYGB, data specific to OAGB remain limited[11].
This study aimed to evaluate the diagnostic value and cost-effectiveness of routine UGI swallow studies performed on postoperative day (POD) 1 in patients undergoing laparoscopic OAGB.
This retrospective cohort study was conducted using prospectively collected data from all patients who underwent laparoscopic OAGB at our institution between January 2019 and July 2022. Ethical approval was obtained from the local institutional review board, which waived the need for informed consent due to the retrospective design of the study. Patient data, including demographics, operative details, postoperative outcomes, and radiological reports, were retrieved for analysis. Complications were classified according to the Clavien-Dindo grading system. All patients were included; no exclusion criteria were applied.
All surgeries were performed laparoscopically by two experienced bariatric surgeons following a standardized technique similar to the single anastomosis sleeve-jejunal bypass as described in previous literature[12-18]. The procedure involved the use of a 36-Fr bougie to size the gastric pouch followed by construction of a single gastrojejunostomy using a 45-mm linear stapler. A 200-cm biliopancreatic limb was measured from the ligament of Treitz, and the anastomosis was closed with a hand-sewn technique. Prior to closure of the abdominal cavity, a leak test with methylene blue was conducted, hemostasis was ensured, and a closed-suction drain was placed adjacent to the anastomotic site.
On the first POD, all patients underwent a routine UGI contrast swallow study. The imaging protocol included an initial anteroposterior abdominal radiograph followed by administration of approximately 60 mL of water-soluble contrast (Gastrografin). Fluoroscopic evaluation was performed in multiple views (anteroposterior and obliques) to trace contrast flow. Radiographic studies were reviewed and interpreted by an experienced radiologist.
Patients were managed postoperatively under a standardized clinical protocol. After a normal UGI result and routine blood work, a clear liquid diet was introduced. Patients were typically discharged on POD 3 and followed up with a multidisciplinary bariatric team at 10 days, 3 months, 6 months, 12 months, and then annually. Proton pump inhibitors were prescribed during hospitalization and continued for 6 months after discharge. Patients also received dietary and physical activity counseling.
UGI study results were categorized as normal or abnormal based on findings such as leak, delayed transit, reflux, or obstruction. Clinical outcomes and need for reoperation were analyzed in relation to UGI findings. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to assess diagnostic performance. Univariate analysis was conducted using the χ2 test or Fisher’s exact test for ordinal and categorical variables. For continuous variables normality was assessed with the Shapiro-Wilk test followed by either the Mann-Whitney U test or Student’s t-test as appropriate. Statistical analyses were performed using SPSS software (version 26; SPSS, Inc.) with a P value ≤ 0.05 considered statistically significant.
Our cohort included 385 patients who underwent OAGB at our institution between January 2019 and July 2022. Of the entire cohort, 264 patients (69%) were females, the mean age was 41 years (range 17-71), and the mean body mass index was 43 kg/m2 (range 26-70).
OAGB was performed as revisional surgery in 87 patients (22.6%); 44 patients (50.6%) after laparoscopic adjustable gastric banding and 43 patients (49.4%) after a failed laparoscopic sleeve gastrectomy (LSG). Patient demographics and clinical data are detailed in Table 1.
| OAGB, n = 385 | |
| Age (mean), years | 41 (17-70) |
| Female gender, n (%) | 264 (69.0) |
| BMI (mean) | 43 (26-70) |
| Previous bariatric surgery, n (%) | 87 (22.6) |
| Previous LAGB | 44 (50.6) |
| Previous sleeve | 43 (49.4) |
| Smoking, n (%) | 77 (20.0) |
| Diabetes, n (%) | 68 (17.7) |
| Hypertension, n (%) | 83 (21.6) |
| Hyperlipidemia, n (%) | 113 (29.4) |
| Fatty liver, n (%) | 290 (75.3) |
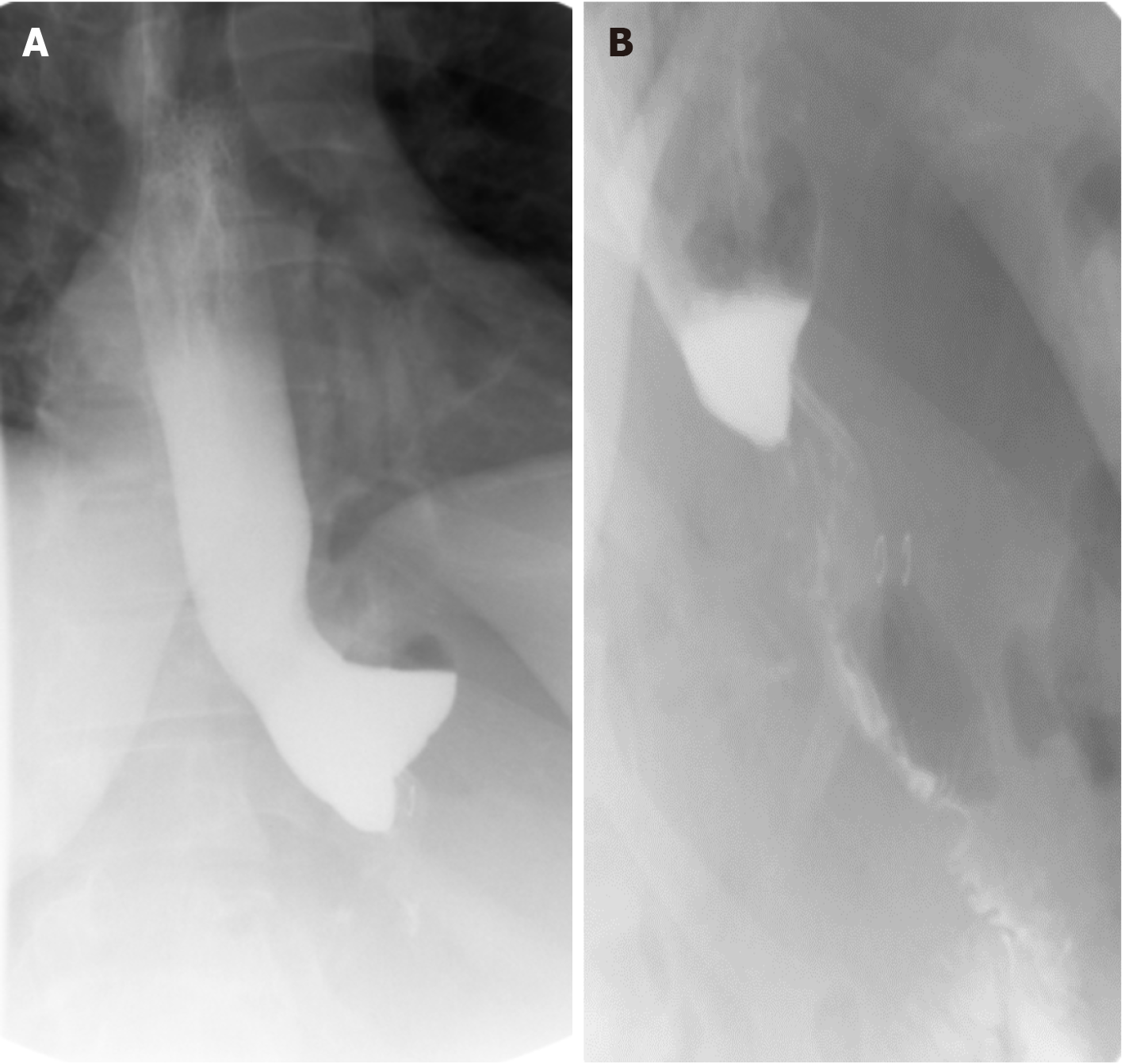
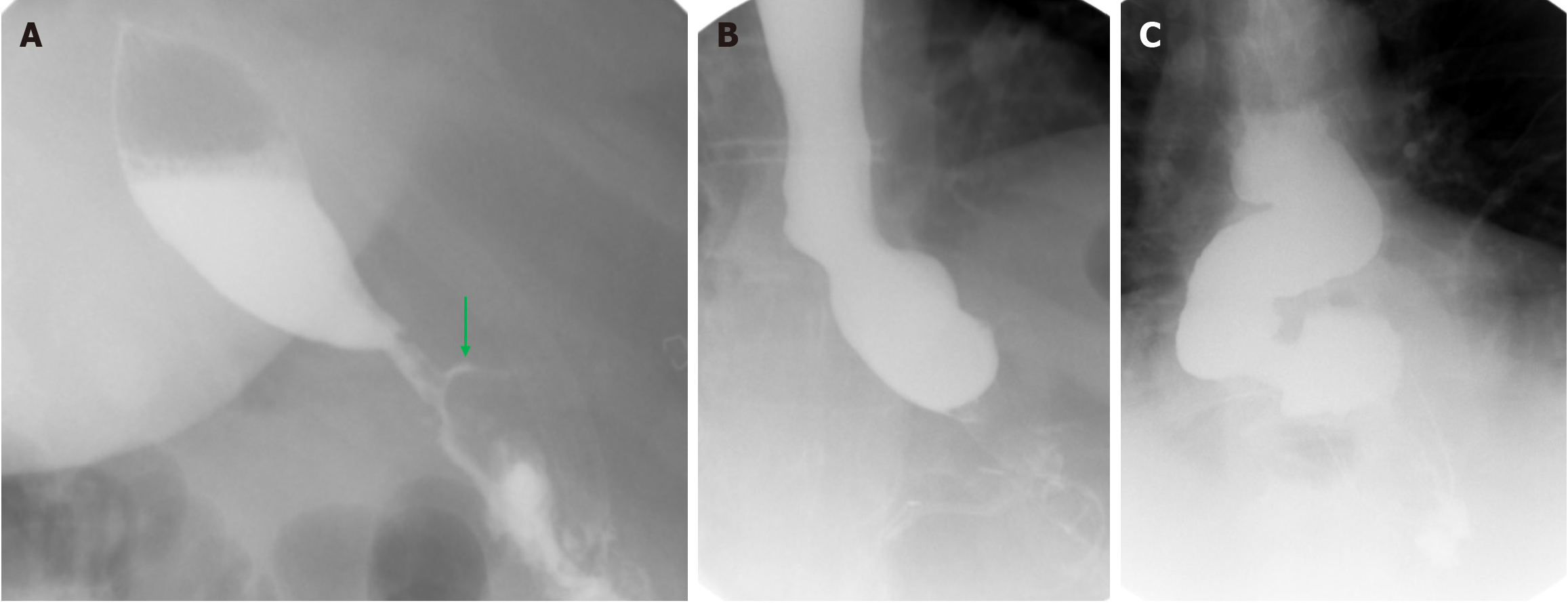
Thirty-seven patients (9.6%) were found to have a hiatal hernia during their operation, and all hernias were repaired at the time of surgery. All patients included in the cohort underwent a UGI swallow study on the first POD. Four patients (1%) had an abnormal UGI study. Three of these patients had slow passage on the UGI study without any clinical complaints, and the repeat UGI study performed on POD 2 appeared normal. The study of the last patient showed an anastomotic leak. This patient showed no concerning clinical signs, and the CT scan was completely normal. Further clinical follow-up identified no signs of related postoperative complications. Data regarding abnormal UGI swallow studies is summarized in Table 2 and Figures 1 and 2.
| Patient | Diagnosis on UGI | Clinical signs | Work-up | Outcome |
| 1 (Figure 1) | Slow passage | None | Repeat UGI swallow study on POD 2 showed normal passage | Discharge on POD 3 |
| 2 (Figure 2A) | Suspected anastomotic leak | None | Immediate CT scan without any pathology | Discharge on POD 3 |
| 3 (Figure 2B) | Slow passage | Nausea | Repeat UGI and endoscopy on same day showed normal passage | Discharge on POD 4 |
| 4 (Figure 2C) | Slow passage | None | None | Discharge on POD 3 |
Two patients (0.5%) required reoperation due to an anastomotic leak. Both patients had a normal UGI swallow study on the first POD. The leaks were diagnosed on POD 2 and 4. Both patients presented with severe epigastric pain and foul-smelling discharge in the drain, prompting immediate diagnostic laparoscopy by the surgeon who performed the initial procedure. In the first patient, a clear leak was observed at the staple line of the gastroenterostomy. A laparoscopic primary repair with a continuous v-loc barbed suture was performed. In the second patient, no distinct leak point was identified. Both patients underwent laparoscopic lavage with drain placement. Neither of the patients who developed leaks were after revisional surgery nor did they have concurrent hiatal hernia repair during their OAGB. Postoperatively, they were kept NPO, initiated on total parenteral nutrition, and started on broad-spectrum antibiotics.
Unfortunately and despite our best efforts, the first patient experienced acute clinical deterioration presenting with severe inflammatory respiratory syndrome and eventually passed away on POD 10. A gastroscopy was performed on the second patient on POD 9, revealing fibrin residue at the gastroenterostomy site and was interpreted as a healed anastomotic leak. No endoscopic intervention was required. The patient showed clinical improvement and was discharged on POD 11 after a CT scan confirmed the absence of any leaks. A summary of data regarding patients who underwent reoperation due to leaks is shown in Table 3.
| POD at presentation | Symptoms | WBC count (5 × 103) | UGI swallow | CT | Treatment | Outcome | |
| Leak 1 | 2 | Epigastric pain radiating to back; Foul discharge in drain | 17.0 | Negative | Not performed | Diagnostic laparoscopy, lavage, drain, intravenous antibiotics, TPN | Mortality |
| Leak 2 | 4 | Epigastric and periumbilical pain; Foul discharge in drain | 14.2 | Negative | Not performed | Diagnostic laparoscopy, lavage, drain, intravenous antibiotics, TPN | Leak resolved; Discharge on POD 11 |
In addition, 8 patients (2%) required reoperation because of bleeding which is clearly not detectable on UGI swallow studies. These patients were diagnosed based on blood in the drain placed during surgery, and reoperation was performed on the first POD for hemostasis.
To determine the efficacy of routine UGI swallow studies after OAGB, sensitivity, specificity, PPV, and NPV were calculated for detecting a leak. The sensitivity and specificity for detecting a leak were 0% and 99.0%, respectively, resulting in a 0% PPV and 98.5% NPV.
The cost of a single UGI swallow study at our institution is approximately 249 USD. The total cost of UGI swallow studies in our study, based on hospital charges, amounted to 95865 USD.
Our study includes the largest cohort to date investigating the efficacy of UGI swallow studies after OAGB surgery. The routine UGI swallow studies conducted on the first POD failed to detect the anastomotic leaks, which occurred in 2 patients. Furthermore, 4 patients whose swallow studies were deemed abnormal due to slow passage, obstruction, or leak were clinically irrelevant as all 4 patients were discharged as planned without any symptoms. Thus, the study clearly showed that performing routine a UGI swallow study on POD 1 is not effective in early detection of complications following OAGB and hence has no cost benefit.
The clinical value of routine postoperative UGI studies remains a topic of debate in the bariatric surgery literature and even more so after gastric bypass surgery. Madan et al[19] studied 245 patients who underwent laparoscopic RYGB with a 3 % anastomotic leak rate. Their study concluded that UGI studies are the most predictive of an early leak and that relying on clinical signs alone is not as useful. In contrast other studies have demonstrated low clinical benefit in performing postoperative UGI studies specifically after gastric bypass surgery[8,20-23].
Findings suggesting ineffectiveness of routine UGI studies after LSG were found in several studies. Mizrahi et al[7] investigated a cohort of 722 patients who underwent sleeve gastrectomy. Five patients were found to have leaks, none of whom were detected by a UGI swallow study, leading to a 0% sensitivity rate. Similarly, Sakran et al[24] investigated a cohort of 2834 patients who underwent LSG with a 1.5% leak rate (44 patients). Routine postoperative UGI studies were performed in 33 patients, and all but one study (3%) failed to detect a leak.
Several reasons may account for the low detection rate of anastomotic leaks seen in our study and in the studies mentioned above. First, all UGI studies were performed routinely on the first POD regardless of their clinical symptoms. However, from a time standpoint leaks probably occur later than POD 1. The leaks in our study were diagnosed on POD 2 and 4. In a study by Spaniolas et al[25] investigating the time leaks were detected postoperatively in 71694 patients who underwent different bariatric procedures (other than OAGB), it was found that leaks were detected on POD 10 (range 5-15 days). Sakran et al[24] showed that leaks after LSG present on POD 7 (median). Thus, it may be that leaks are still not formed on POD 1 or contained by tissue edema expected after surgery. Second, some leaks are microleaks of air bubbles alone that can be seen on CT scans but do not appear on swallow studies as extravasation of contrast. Lastly, our low leak rate (0.5%) may have a negative impact on the sensitivity of UGI studies.
Basic clinical signs and symptoms are probably more suggestive of postoperative complications than any routine imaging study. Previous studies have demonstrated that clinical indicators, such as tachycardia, abdominal pain, respiratory distress, and changes in drain output, are strong predictors of leaks following sleeve gastrectomy and gastric bypass surgery[8,26,27]. Both patients in our study who were diagnosed with a leak had normal UGI studies on POD 1, and leaks were eventually diagnosed on POD 2 and 4. Our patients presented with severe and worsening abdominal pain, tachycardia, elevated WBC count, and foul discharge in the drain. This further supports omitting routine performance of UGI on POD 1 as they might mislead clinicians. Patients who present with signs and symptoms suggestive of a complication should be further evaluated by close clinical monitoring and advanced imaging such as CT scans, which prove to be more sensitive in detecting postoperative complications such as anastomotic leaks[7].
Health care leaders in decision making positions rely on the cost benefit (among other factors) when approving routine examinations such as UGI studies after OAGB. The total cost of UGI swallow studies in our study, based on hospital charges, amounted to 95865 USD. As these studies did not detect any of the leaks, they are clearly unbeneficial from a cost benefit standpoint.
Limitations of this study included its retrospective design and reliance on data from a single medical center, which may limit the generalizability of the findings. The relatively small number of patients who experienced postoperative complications, while consistent with previously reported rates, also constrains broader applicability. Although each UGI swallow study was reviewed by both a senior radiologist and the operating bariatric surgeon, interpretation remains subjective and may be affected by human error. A key limitation was that all UGI studies were performed on POD 1 in accordance with our standard inpatient protocol. Since anastomotic leaks often present later (typically between POD 5 and POD 10), early imaging may reduce sensitivity and lead to false negatives. Future studies incorporating delayed or symptom-triggered imaging could further clarify the diagnostic value of UGI. Lastly, it would be valuable to compare these findings across multiple centers as has been done for other bariatric procedures.
Our data demonstrated that performing routine UGI swallow studies on the first POD after laparoscopic OAGB carries a 0% sensitivity rate at detecting postoperative complications and clearly has no cost benefit. UGI swallow studies should be omitted after OAGB in favor of close clinical monitoring and selective use of CT scan in patients presenting with disturbing signs and symptoms.
| 1. | Wolfe BM, Kvach E, Eckel RH. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ Res. 2016;118:1844-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 476] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 2. | Nudel J, Sanchez VM. Surgical management of obesity. Metabolism. 2019;92:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Guerreiro V, Neves JS, Salazar D, Ferreira MJ, Oliveira SC, Souteiro P, Pedro J, Magalhães D, Varela A, Belo S, Freitas P, Carvalho D; AMTCO Group. Long-Term Weight Loss and Metabolic Syndrome Remission after Bariatric Surgery: The Effect of Sex, Age, Metabolic Parameters and Surgical Technique - A 4-Year Follow-Up Study. Obes Facts. 2019;12:639-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Felsenreich DM, Bichler C, Langer FB, Gachabayov M, Prager G. Sleeve Gastrectomy: Surgical Technique, Outcomes, and Complications. Surg Technol Int. 2020;36:63-69. [PubMed] |
| 5. | Wang FG, Yan WM, Yan M, Song MM. Outcomes of Mini vs Roux-en-Y gastric bypass: A meta-analysis and systematic review. Int J Surg. 2018;56:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Gu L, Huang X, Li S, Mao D, Shen Z, Khadaroo PA, Ng DM, Chen P. A meta-analysis of the medium- and long-term effects of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass. BMC Surg. 2020;20:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 7. | Mizrahi I, Tabak A, Grinbaum R, Beglaibter N, Eid A, Simanovsky N, Hiller N. The utility of routine postoperative upper gastrointestinal swallow studies following laparoscopic sleeve gastrectomy. Obes Surg. 2014;24:1415-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Brockmeyer JR, Simon TE, Jacob RK, Husain F, Choi Y. Upper gastrointestinal swallow study following bariatric surgery: institutional review and review of the literature. Obes Surg. 2012;22:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Rahman U, Docimo S, Pryor AD, Bates A, Obeid NR, Spaniolas K. Routine contrast imaging after bariatric surgery and the effect on hospital length of stay. Surg Obes Relat Dis. 2018;14:517-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Diaz Vico T, Elli EF. Utility of Immediate Postoperative Upper Gastrointestinal Contrast Study in Bariatric Surgery. Obes Surg. 2019;29:1130-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Kermansaravi M, Shahmiri SS, DavarpanahJazi AH, Valizadeh R, Berardi G, Vitiello A, Musella M, Carbajo M. One Anastomosis/Mini-Gastric Bypass (OAGB/MGB) as Revisional Surgery Following Primary Restrictive Bariatric Procedures: a Systematic Review and Meta-Analysis. Obes Surg. 2021;31:370-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Pazouki A, Kermansaravi M. Single Anastomosis Sleeve-Jejunal Bypass: a New Method of Bariatric/Metabolic Surgery. Obes Surg. 2019;29:3769-3770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Salama TMS, Sabry K, Ghamrini YE. Single Anastomosis Sleeve Ileal Bypass: New Step in the Evolution of Bariatric Surgeries. J Invest Surg. 2017;30:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Mahdy T, Al Wahedi A, Schou C. Efficacy of single anastomosis sleeve ileal (SASI) bypass for type-2 diabetic morbid obese patients: Gastric bipartition, a novel metabolic surgery procedure: A retrospective cohort study. Int J Surg. 2016;34:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Mahdy T, Gado W, Alwahidi A, Schou C, Emile SH. Sleeve Gastrectomy, One-Anastomosis Gastric Bypass (OAGB), and Single Anastomosis Sleeve Ileal (SASI) Bypass in Treatment of Morbid Obesity: a Retrospective Cohort Study. Obes Surg. 2021;31:1579-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Mahdy T, Emile SH, Alwahedi A, Gado W, Schou C, Madyan A. Roux-en-Y Gastric Bypass with Long Biliopancreatic Limb Compared to Single Anastomosis Sleeve Ileal (SASI) Bypass in Treatment of Morbid Obesity. Obes Surg. 2021;31:3615-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Mahdy T, Emile SH, Madyan A, Schou C, Alwahidi A, Ribeiro R, Sewefy A, Büsing M, Al-Haifi M, Salih E, Shikora S. Evaluation of the Efficacy of Single Anastomosis Sleeve Ileal (SASI) Bypass for Patients with Morbid Obesity: a Multicenter Study. Obes Surg. 2020;30:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Sewefy AM, Saleh A. The outcomes of single anastomosis sleeve jejunal bypass as a treatment for morbid obesity (Two-year follow-up). Surg Endosc. 2021;35:5698-5704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Madan AK, Stoecklein HH, Ternovits CA, Tichansky DS, Phillips JC. Predictive value of upper gastrointestinal studies versus clinical signs for gastrointestinal leaks after laparoscopic gastric bypass. Surg Endosc. 2007;21:194-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Doraiswamy A, Rasmussen JJ, Pierce J, Fuller W, Ali MR. The utility of routine postoperative upper GI series following laparoscopic gastric bypass. Surg Endosc. 2007;21:2159-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | White S, Han SH, Lewis C, Patel K, McEvoy B, Kadell B, Mehran A, Dutson E. Selective approach to use of upper gastroesophageal imaging study after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Schiesser M, Guber J, Wildi S, Guber I, Weber M, Muller MK. Utility of routine versus selective upper gastrointestinal series to detect anastomotic leaks after laparoscopic gastric bypass. Obes Surg. 2011;21:1238-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Bertelson NL, Myers JA. Routine postoperative upper gastrointestinal fluoroscopy is unnecessary after laparoscopic adjustable gastric band placement. Surg Endosc. 2010;24:2188-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I, Shimonov M, Assalia A. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Spaniolas K, Kasten KR, Sippey ME, Pender JR, Chapman WH, Pories WJ. Pulmonary embolism and gastrointestinal leak following bariatric surgery: when do major complications occur? Surg Obes Relat Dis. 2016;12:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Dallal RM, Bailey L, Nahmias N. Back to basics--clinical diagnosis in bariatric surgery. Routine drains and upper GI series are unnecessary. Surg Endosc. 2007;21:2268-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Hamilton EC, Sims TL, Hamilton TT, Mullican MA, Jones DB, Provost DA. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/