Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.107085
Revised: April 23, 2025
Accepted: May 23, 2025
Published online: July 27, 2025
Processing time: 130 Days and 23.3 Hours
Enhanced recovery after surgery (ERAS), a multidisciplinary and multimodal perioperative care protocol, has been widely used in several surgical fields. However, the effect of this care protocol on liver transplant recipients with end-stage liver disease remains unclear.
To compare the clinical outcomes of the ERAS protocol and standard care (SC) for liver transplant recipients with end-stage liver disease.
PubMed, Web of Science, Cochrane Library, and EMBASE databases were systematically searched to identify literature reporting the effects of the ERAS protocol on clinical outcomes in patients undergoing liver transplant recipients with end-stage liver disease. All articles published to January 1, 2025 were searched, followed by data extraction of the included literature and independent quality assessment. Then pooled mean difference (MD) and odds ratio (OR) with a 95% confidence interval (CI) were calculated by either a random-effects or fixed-effects model.
Overall, eight relevant studies (including two randomized controlled trials, two prospective cohort studies, and four retrospective cohort studies) involving 1220 patients (704 patients in the ERAS group and 516 patients in the SC group). The primary outcomes evaluated included intensive care unit (ICU) stay duration, hospital length of stay, overall complication rates, mortality, and 30-day readmission rates. Our findings showed that ERAS protocols significantly reduced ICU stay duration (MD: -1.21 days, 95%CI: -2.08 to -0.34; P = 0.006), hospital length of stay (MD: -4.91 days, 95%CI: -7.45 to -2.37; P = 0.0002), overall complication rates (OR = 0.32, 95%CI: 0.22–0.46; P < 0.0001), and mortality (OR = 0.57, 95%CI: 0.33–0.98; P = 0.04). However, ERAS was associated with an increased 30-day readmission rate (OR = 3.20, 95%CI: 1.54–6.67; P = 0.003).
The current meta-analysis indicated that ERAS protocols can significantly improve short-term clinical outcomes in liver transplant recipients, although the increased readmission rate requires further investigation. Future studies should aim to refine ERAS protocols and explore their long-term efficacy and underlying mechanisms.
Core Tip: The enhanced recovery after surgery (ERAS) program represents an integrated, multidisciplinary approach to perioperative care, which has been widely adopted across various surgical specialties. However, the clinical outcomes of ERAS in liver transplant recipients remain inconclusive. The results of this meta-analysis will strengthen the existing evidence base in support of ERAS protocols in liver transplantation and guide future research and clinical practices.
- Citation: Zheng YJ, Pan Y, Li DL, Zhang JC, Tao JL, Li PC, Liu XD, An CG, Luo GS. Efficacy and safety of enhanced recovery after surgery protocol on liver transplantation: A meta-analysis. World J Gastrointest Surg 2025; 17(7): 107085
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/107085.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.107085
Liver transplantation is a crucial treatment option for those with end-stage liver disease, providing a potential cure and significantly enhancing survival rates[1]. However, this procedure comes with considerable morbidity, prolonged hospital stays, and substantial healthcare costs[2-5]. Despite significant advancements in surgical techniques and perioperative management, liver transplantation recipients frequently encounter complications like infections, graft failure, and prolonged recovery periods. These problems highlight the necessity for new methods to improve patient outcomes and reduce the financial burden on healthcare systems. Enhanced recovery after surgery (ERAS) protocols, which have been successfully implemented in various surgical specialties[6-10], present a promising approach to tackle these challenges in the context of liver transplantation.
ERAS protocols are comprehensive perioperative care pathways that aim to accelerate recovery by reducing surgical stress and enhancing physiological function[11]. These protocols generally include preoperative patient education, optimized anesthetic techniques, early mobilization, and prompt enteral feeding, among other elements[12-16]. In the realm of liver transplantation, ERAS protocols are designed to reduce postoperative complications, reduce the duration of hospital stays, and enhance overall patient outcomes. Research in other surgical domains has shown that ERAS protocols are effective in reducing morbidity rates, accelerating recovery, and reducing healthcare costs[6-10]. However, the implementation of ERAS protocols in liver transplantation is still in its nascent stages, with limited evidence to guide clinical guidelines.
The existing literature on ERAS protocols in liver transplantation is characterized by a limited number of high-quality randomized controlled trials (RCTs)[3,17], and most evidence[2] comes from retrospective and prospective cohort studies[18-22]. Although these investigations offer important insights, they often show methodological shortcomings, such as restricted sample sizes, inconsistent application of protocols, and diverse outcome measures. This emphasizes the need for a thorough synthesis of current evidence to evaluate the effectiveness and safety of ERAS protocols in liver transplantation. To address this gap, a meta-analysis is essential to consolidate existing data and establish a stronger foundation for clinical decision-making.
This study's main objective was to carry out a systematic review and meta-analysis to determine the influence of ERAS protocols on clinical results in liver transplant recipients. Specifically, we assessed the effects on intensive care unit (ICU) and hospital stay lengths, overall complication rates, mortality, and other relevant outcomes. By synthesizing the available evidence, this research provides a detailed evaluation of the efficacy and safety of ERAS protocols in liver transplantation. The results of this meta-analysis will strengthen the existing evidence base in support of ERAS protocols in liver transplantation and guide future research and clinical practices. Ultimately, this study hopes to improve patient outcomes and reduce the economic burden of liver transplantation by improving perioperative care strategies.
This meta-analysis was reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement[23] and the Assessing the Methodological Quality of Systematic Reviews Guidelines[24]. Moreover, the study is registered with PROSPERO (CRD420250654697).
A comprehensive literature search was conducted across multiple electronic databases including PubMed, Web of Science, Cochrane Library, and EMBASE, covering all available records to January 1, 2025. The search identified all published investigations evaluating the impact of ERAS protocols on clinical outcomes following hepatic transplantation procedures. The search strategy employed various combinations of key terms and medical subject headings: (1) ERAS; (2) Enhanced recovery after surgery; (3) Enhanced recovery after bariatric surgery; (4) Fast track; (5) Liver transplantation; and (6) Liver transplantation surgery. To ensure thoroughness, we performed manual screening of reference sections from retrieved articles to locate potentially relevant studies that might have been missed in the initial database search. This systematic review was limited to publications available in the English language.
Three authors independently assessed the article eligibility, with any disagreements resolved through group discussion. The inclusion criteria were: (1) RCTs and retrospective cohort studies reporting; (2) ERAS protocols incorporating at least eight items from the recommended guidelines; (3) Comparative studies evaluating ERAS protocol versus standard care (SC) for patients undergoing liver transplantation; and (4) Studies with available full texts. For duplicate studies from the same institution, the most recent and comprehensive data were included. The exclusion criteria were: (1) Reviews; (2) Case reports; (3) Conference abstracts and meeting reports; (4) Studies lacking a control group; and (5) Studies without clearly defined ERAS protocol components.
Two independent investigators conducted a comprehensive evaluation of potentially relevant articles through full-text assessment, followed by systematic data collection according to established protocols. Discrepancies in evaluation were resolved through discussion until consensus agreement was reached. The collected dataset encompassed the following aspects: (1) Methodological characteristics, including geographical location, research methodology, and participant numbers in comparative groups; (2) Demographic and clinical profiles of participants, specifically age, sex, body mass index, comorbidity, and type of surgery; (3) Components of enhanced recovery protocols; and (4) Key clinical outcomes, such as hospital length of stay, ICU duration, overall complications, readmissions within 30 days, and mortality.
The methodological quality of the included studies was independently assessed by two researchers using the Cochrane collaboration tool across seven different aspects[25]. The included trials were graded as low, high, or moderate quality primarily based on the potential of bias: (1) Low quality was assigned if either randomization or allocation concealment was assessed as having a high risk of bias, regardless of other criteria; (2) High quality was assigned when both randomization and allocation concealment were assessed as having a low risk of bias and all other criteria were assessed as having low or unclear risk of bias; and (3) Moderate quality was assigned if the trial did not meet the criteria for either high or low risk. The methodologic quality of the included non-randomized prospective study was assessed using the Newcastle-Ottawa Scale (NOS), based on selection of the study population, comparability of the groups under study, and outcome assessment. The maximum score on the scale was 9, and studies scoring > 5 were considered to be of high methodological quality[26]. Disagreements were resolved by consensus among the researchers.
For continuous and categorical outcome measures, the pooled effect sizes were quantified using mean difference (MD) and odds ratio (OR), respectively, accompanied by their corresponding 95% confidence interval (CI). Interstudy heterogeneity was assessed through the χ² test and quantified using the I2 statistic, with substantial heterogeneity defined as P < 0.10 or I2 > 50%. When significant heterogeneity was detected, a random-effects model was employed for meta-analysis; otherwise, a fixed-effects model was used. To evaluate result robustness, sensitivity analyses were performed by systematically excluding individual studies and recalculating the pooled estimates. Where appropriate, subgroup analyses were conducted to investigate potential sources of observed heterogeneity. Publication bias was assessed using contour-enhanced funnel plots. All statistical analyses were performed using Review Manager (RevMan version 5.3; The Cochrane Collaboration, Oxford, England), with statistical significance set at P < 0.05.
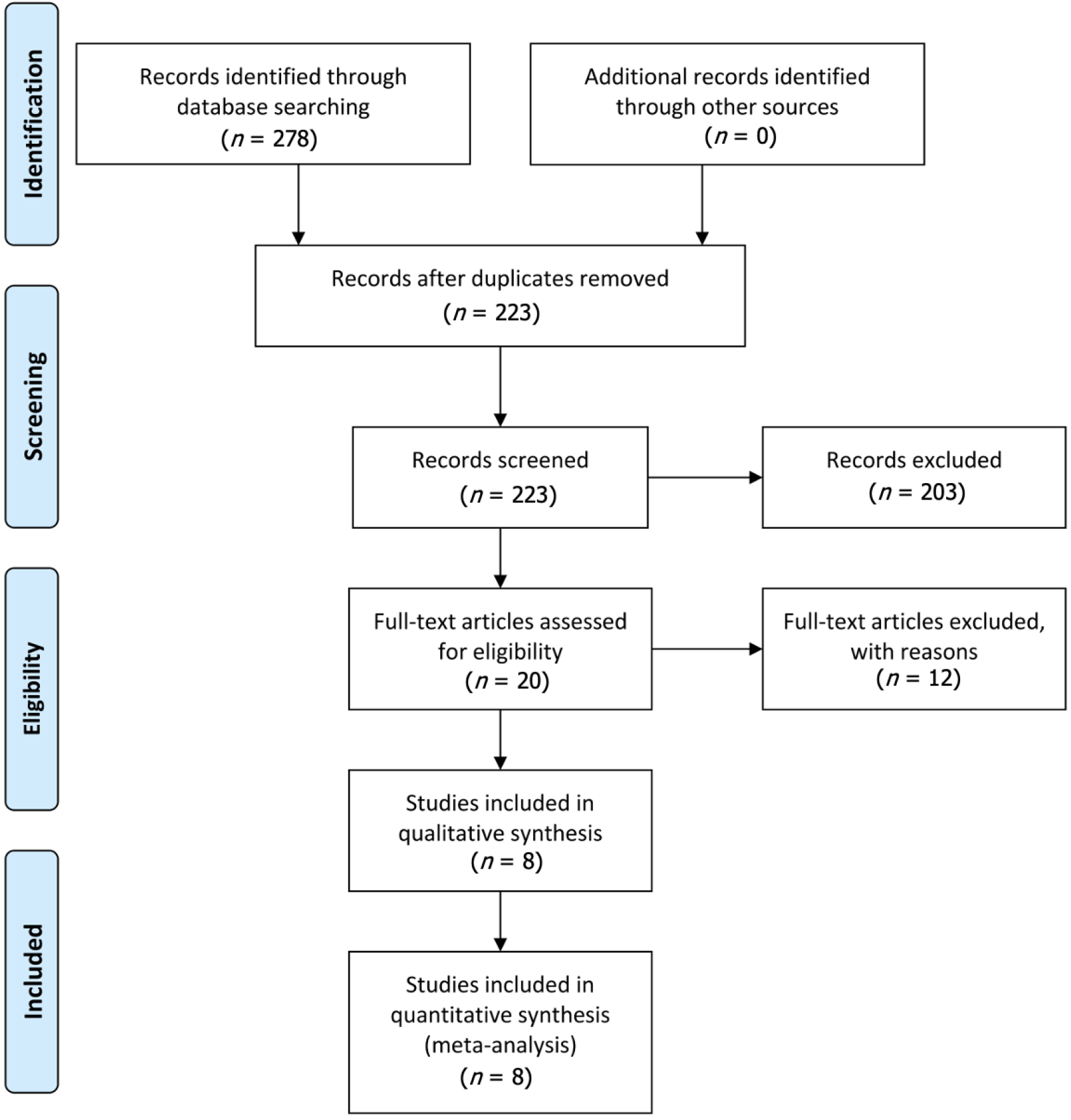
A total of 278 relevant citations were identified through the initial search strategy. Among these, 55 articles were excluded due to duplication, while 203 were eliminated after title and abstract screening. Following a comprehensive full-text review and evaluation, twelve additional articles were excluded for failing to meet the inclusion criteria. Resulting from this evaluation process, eight eligible studies[2,3,17-22] were used in this meta-analysis (two RCTs), two prospective cohort studies and four retrospective cohort study. Data on a total of 1220 liver transplantation patients was collected from the eight studies. This including 704 ERAS cases and 516 SC cases. Figure 1 describes the PRISMA flow diagram of the literature selection process. Table 1 lists the characteristics of the included studies[2,3,17-22].
| Ref. | Region | Design | Intervention | Sample size | Age (years) | Sex (male/female) | Follow-up | Outcomes | Newcastle-Ottawa Scale score |
| Brustia et al[2] | France | PCS | ERAS | 10 | 60.1± 3.4 | 8/2 | > 1 year | (1), (2), (3), (4), (5) | 7 |
| SC | 20 | 58.2 ± 3.18 | 17/3 | ||||||
| Findlay et al[17] | United States | RCT | ERAS | 39 | 51.0 ± 11.5 | 22/17 | NR | (1) | - |
| SC | 39 | 53.1 ± 8.5 | 24/15 | ||||||
| Hillings et al[20] | Denmark | RCS | ERAS | 270 | 48.3 ± 12 | 158/112 | 329 days ± 87 days | (1), (3), (4) | 8 |
| SC | 64 | 51 ± 10 | 39/25 | ||||||
| Jin et al[19] | China | RCS | ERAS | 130 | 49 ± 10.25 | 82/48 | 1 year | (3), (4) | 8 |
| SC | 120 | 45 ± 11 | 68/52 | ||||||
| Kara et al[18] | Turkey | RCS | ERAS | 20 | 42 ± 3.28 | 12/8 | NR | (1), (2), (3); | 9 |
| SC | 40 | 48 ± 2.57 | 27/13 | ||||||
| King et al[22] | United States | PCS | ERAS | 141 | 58 ± 2.5 | 78/28 | 1 year | (1), (2), (5) | 8 |
| SC | 106 | 58 ± 2.25 | 40/14 | ||||||
| Rao et al[3] | China | RCT | ERAS | 54 | 52.4 ± 15.2 | 40/14 | 3 months | (1), (2), (3), (4), (5) | - |
| SC | 74 | 55.8 ± 14.3 | 58/16 | ||||||
| Xu et al[21] | China | RCS | ERAS | 40 | 49.5 ± 4.2 | 35/5 | 2 months | (1), (2), (3), (4), (5) | 8 |
| SC | 53 | 53 ± 3 | 46/7 |
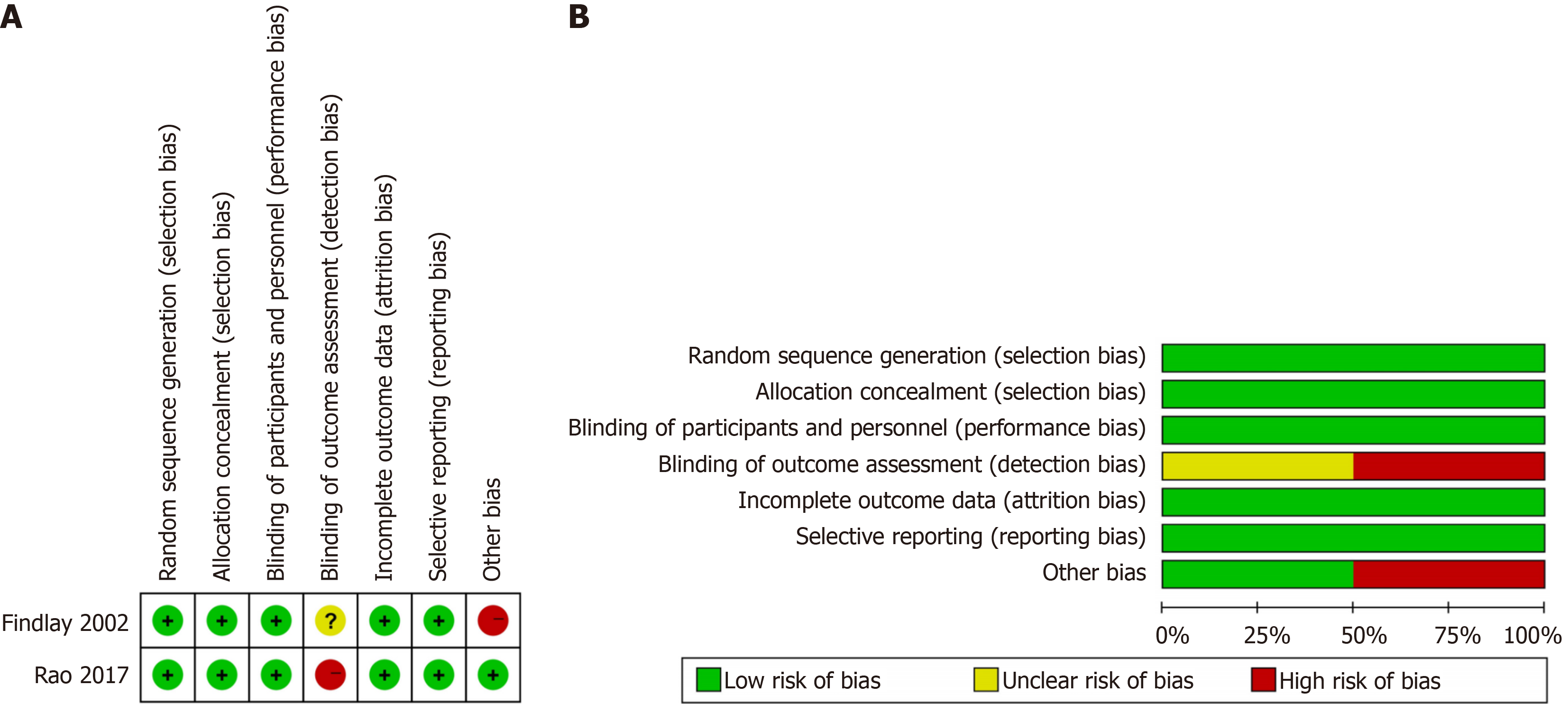
Six high-quality nonrandomized trials were identified, with NOS scores ≥ 7. The detailed NOS scores for the selected studies are presented in Table 1[2,3,17-22]. Furthermore, based on the Cochrane Collaboration's risk of bias tool, all included RCTs clearly described their random sequence generation methods. The bias assessment results for all RCTs are summarized in Figure 2.
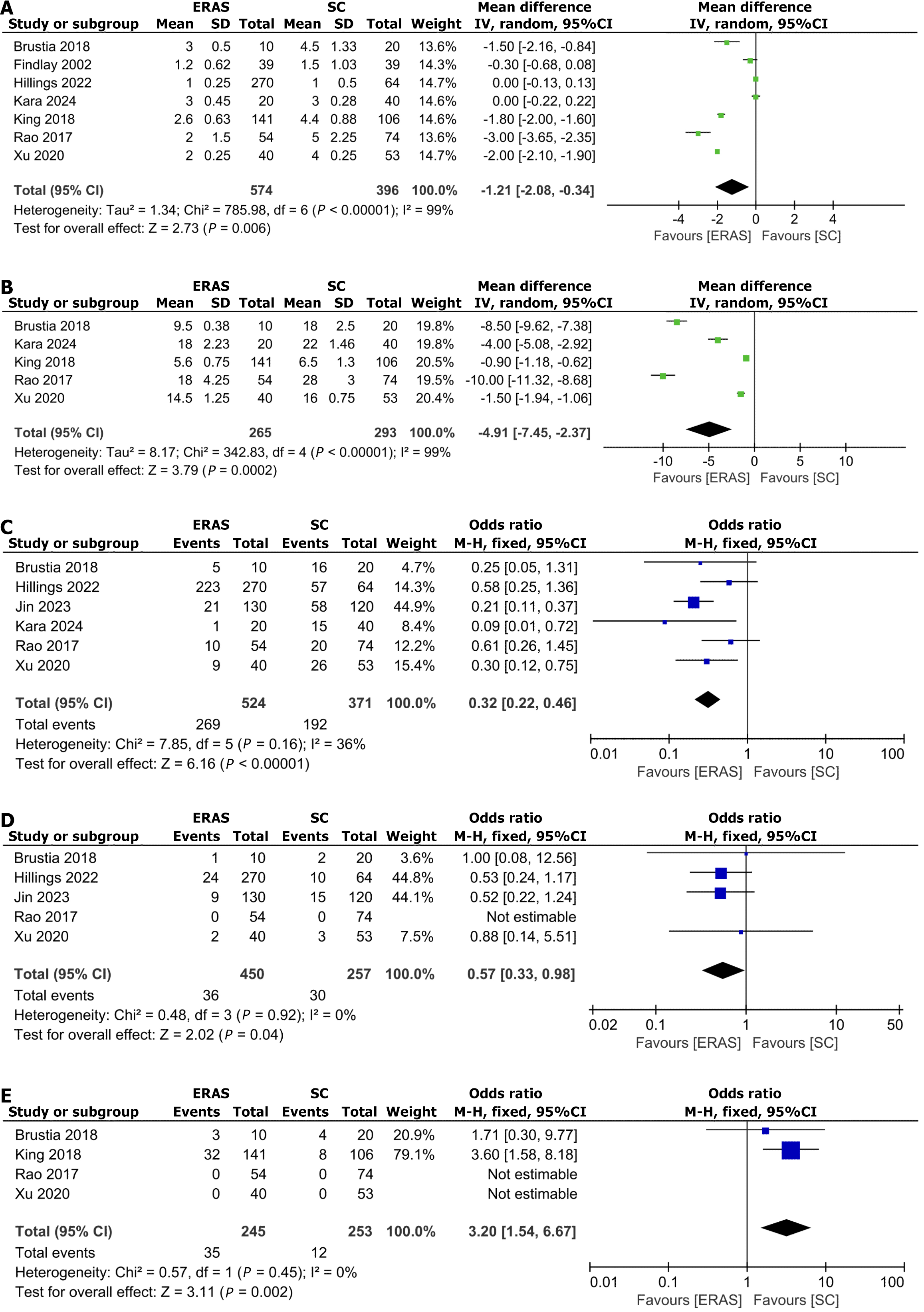
ICU duration: Our meta-analysis incorporating seven clinical studies[2,3,17,18,20-22] with an aggregate sample size of 790 participants evaluated ICU length of stay. The pooled analysis employing a random-effects model demonstrated a statistically significant reduction in ICU stay for patients undergoing ERAS protocols (MD: -1.21, 95%CI: -2.08 to -0.34; P = 0.006) (Figure 3A).
Length of hospitalization: A analysis of hospitalization duration was conducted across five studies[2,3,18,21,22], involving a total of 558 participants. Employing a random-effects model, the results demonstrated a statistically significant reduction in hospital stay duration for patients undergoing ERAS protocols (MD: -4.91, 95%CI: -7.45 to -2.37; P = 0.0002) (Figure 3B).
Overall complications: Six studies[2,3,18-21] involving 895 patients reported the incidence of overall complications. Based on the fixed-effects model, the incidence of overall complications was significantly lower in the ERAS group (OR = 0.32; 95%CI: 0.22–0.46; P < 0000.1) (Figure 3C).
Mortality: Five studies[2,3,19-21] involving 707 patients reported the mortality. Based on the fixed-effects model, the Mortality was significantly lower in the ERAS group (OR = 0.57, 95%CI: 0.33–0.98; P = 0.04) (Figure 3D).
Readmissions within 30 days: Four studies[2,3,21,22] involving 498 patients reported the operation time. Based on the fixed-effects model, the readmissions within 30 days was significantly higher in the ERAS group (OR = 3.20; 95%CI: 1.54–6.67; P = 0.003) (Figure 3E).
Liver transplantation is a crucial therapeutic method for patients with end-stage liver disease, providing a potential cure for conditions that would otherwise be fatal[1]. However, this procedure comes with significant challenges, such as prolonged hospital stays, high rates of postoperative complications, and substantial financial burdens on healthcare systems. Traditional perioperative care strategies often struggle to address these complex issues, resulting in suboptimal patient outcomes[2,3,5].
The ERAS protocol was formally established in 2001 with the founding of the ERAS Society in Europe, creating an evidence-based paradigm for perioperative management[27]. This multidisciplinary approach combines expertise across surgical, anesthetic, nursing, and nutritional disciplines to holistically optimize perioperative care. By implementing standardized clinical pathways, ERAS protocols significantly mitigate surgical stress responses, lower postoperative complications, enhance recovery rates, shorten hospital stays, and improve overall clinical outcomes[12,13]. The protocol represents a paradigm shift in perioperative medicine through its integrated, multidisciplinary approach to patient management. These protocols focus on minimizing physiological stress, accelerating recovery, Given the high complexity and resource-intensive nature of liver transplantation, there is an urgent need to rigorously evaluate the effectiveness of ERAS protocols in this context to improve both patient care and resource allocation.
This meta-analysis systematically evaluates the impact of ERAS protocols on clinical outcomes in liver transplant recipients, synthesizing data from various studies to provide a comprehensive assessment. By combining evidence from RCTs and cohort studies, this study provides solid insights into the potential advantages and disadvantages of ERAS protocols in the context of liver transplantation. The results show significant improvements in critical outcomes, such as reductions in ICU stay duration, hospital length of stay, and overall complication rates. Additionally, the analysis identifies specific areas where ERAS protocols may not offer significant benefits. The following discussion examines these findings in detail, exploring their clinical significance and potential to improve perioperative care strategies in liver transplantation.
Our findings have great clinical significance. The obvious reduction in both ICU and hospital stay durations means that ERAS protocols can significantly reduce the financial burden related to liver transplantation, which is a crucial consideration in the context of increasing global healthcare expenditures. Only one study showed that the hospitalization costs of the ERAS group were lower than those of the SC group in liver transplant patients, as reported in the eight articles we analyzed[19]. Moreover, the observed decrease in overall complication and mortality rates shows the potential of ERAS to improve patient outcomes and quality of life. These findings strongly support the inclusion of ERAS protocols in standard clinical guidelines for liver transplantation. But, we found that the ERAS protocols significantly increased the 30 day readmission rate, which may be due to the complexity and difficulty of the surgery. The author also believes that it may be attributed to early discharge policies inherent to ERAS, and lead to premature discharge before patients are fully stabilized. In the future, exploring this possibility and discussing potential strategies to mitigate readmission risks, such as enhanced post-discharge monitoring or patient education, would add significant value to the study. Additionally, the lack of discussion on long-term outcomes beyond 30 days in the literature we included suggests, so we should focus more on studying long-term outcomes in the future. Healthcare policymakers and providers should consider these results when developing strategies to optimize post-transplant care and also take them into account when implementing these strategies.
However, our study had limitations. The main concern was the heterogeneity among the included studies, which may have affected the generalizability of our findings. Although we used random-effects models to address this variability, differences in patient demographics, surgical techniques, and ERAS protocol implementations across studies could still have influenced the results. Additionally, most of the included studies were retrospective cohort studies, which are inherently prone to selection bias and confounding factors. Future research should prioritize the inclusion of more RCTs to provide higher quality evidence. Moreover, the long-term effects of ERAS on patient outcomes remain unknown, as most studies in our analysis had relatively short follow-up periods. Addressing these limitations in future research will be crucial for refining ERAS protocols and maximizing their benefits in liver transplantation.
In conclusion, this comprehensive meta-analysis revealed that ERAS protocols can significantly enhance clinical outcomes in liver transplant recipients, particularly by shortening the length of stay in the ICU and the overall hospital stay, reducing the frequency of complications, and increasing the survival rate. It is crucial to note that the high 30-day readmission rates observed in this study emphasize the significance of implementing strict post-operative surveillance and follow-up care strategies. Although our results strongly advocate for the integration of ERAS protocols in liver transplantation settings, some methodological limitations call for further investigation through large-scale, well-designed RCTs to confirm these findings and assess the long-term effects. Future studies should focus on improving ERAS protocols to tackle challenges like readmissions, while also exploring their applicability across diverse patient populations and healthcare systems. Further emphasis is placed on strategies to overcome obstacles and optimize outcomes, providing practical recommendations for implementing ERAS programs in liver transplant centers globally.
| 1. | Bismuth H, Castaing D, Ericzon BG, Otte JB, Rolles K, Ringe B, Sloof M. Hepatic transplantation in Europe. First Report of the European Liver Transplant Registry. Lancet. 1987;2:674-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 141] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Brustia R, Monsel A, Conti F, Savier E, Rousseau G, Perdigao F, Bernard D, Eyraud D, Loncar Y, Langeron O, Scatton O. Enhanced Recovery in Liver Transplantation: A Feasibility Study. World J Surg. 2019;43:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Rao JH, Zhang F, Lu H, Dai XZ, Zhang CY, Qian XF, Wang XH, Lu L. Effects of multimodal fast-track surgery on liver transplantation outcomes. Hepatobiliary Pancreat Dis Int. 2017;16:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;CD007635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 5. | Levy MF, Greene L, Ramsay MA, Jennings LW, Ramsay KJ, Meng J, Hein HA, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB. Readmission to the intensive care unit after liver transplantation. Crit Care Med. 2001;29:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Nelson G, Bakkum-Gamez J, Kalogera E, Glaser G, Altman A, Meyer LA, Taylor JS, Iniesta M, Lasala J, Mena G, Scott M, Gillis C, Elias K, Wijk L, Huang J, Nygren J, Ljungqvist O, Ramirez PT, Dowdy SC. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int J Gynecol Cancer. 2019;29:651-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 492] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 7. | Offodile AC 2nd, Gu C, Boukovalas S, Coroneos CJ, Chatterjee A, Largo RD, Butler C. Enhanced recovery after surgery (ERAS) pathways in breast reconstruction: systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2019;173:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 8. | Xiong J, Szatmary P, Huang W, de la Iglesia-Garcia D, Nunes QM, Xia Q, Hu W, Sutton R, Liu X, Raraty MG. Enhanced Recovery After Surgery Program in Patients Undergoing Pancreaticoduodenectomy: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016;95:e3497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 654] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Yang J, Chen X, Du L, Li K, Zhou Y. Enhanced recovery after surgery on multiple clinical outcomes: Umbrella review of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;99:e20983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1818] [Article Influence: 62.7] [Reference Citation Analysis (4)] |
| 12. | Lee A, Chiu CH, Cho MW, Gomersall CD, Lee KF, Cheung YS, Lai PB. Factors associated with failure of enhanced recovery protocol in patients undergoing major hepatobiliary and pancreatic surgery: a retrospective cohort study. BMJ Open. 2014;4:e005330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Holubar SD, Hedrick T, Gupta R, Kellum J, Hamilton M, Gan TJ, Mythen MG, Shaw AD, Miller TE; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond). 2017;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Edwards MR, Forbes G, MacDonald N, Berdunov V, Mihaylova B, Dias P, Thomson A, Grocott MP, Mythen MG, Gillies MA, Sander M, Phan TD, Evered L, Wijeysundera DN, McCluskey SA, Aldecoa C, Ripollés-Melchor J, Hofer CK, Abukhudair H, Szczeklik W, Grigoras I, Hajjar LA, Kahan BC, Pearse RM; OPTIMISE II investigators. Optimisation of Perioperative Cardiovascular Management to Improve Surgical Outcome II (OPTIMISE II) trial: study protocol for a multicentre international trial of cardiac output-guided fluid therapy with low-dose inotrope infusion compared with usual care in patients undergoing major elective gastrointestinal surgery. BMJ Open. 2019;9:e023455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Hajibandeh S, Hajibandeh S, Bill V, Satyadas T. Meta-analysis of Enhanced Recovery After Surgery (ERAS) Protocols in Emergency Abdominal Surgery. World J Surg. 2020;44:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Willcutts KF, Chung MC, Erenberg CL, Finn KL, Schirmer BD, Byham-Gray LD. Early Oral Feeding as Compared With Traditional Timing of Oral Feeding After Upper Gastrointestinal Surgery: A Systematic Review and Meta-analysis. Ann Surg. 2016;264:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Findlay JY, Jankowski CJ, Vasdev GM, Chantigian RC, Gali B, Kamath GS, Keegan MT, Hall BA, Jones KA, Burkle CM, Plevak DJ. Fast track anesthesia for liver transplantation reduces postoperative ventilation time but not intensive care unit stay. Liver Transpl. 2002;8:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kara S, Ozturk G, Demir Yetis Z, Korkut E, Aksungur N, Altundas N, Dogan N, Ozden K. The Effect of Enhanced Recovery After Surgery Protocol on Surgical Site Infections in Liver Transplantation. Surg Infect (Larchmt). 2024;25:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Jin B, Gu Y, Xi S, Liu X, Wu X, Li G. Application of enhanced recovery after surgery following liver transplantation. World J Surg Oncol. 2023;21:248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Hillingsø JG, Rostved AA, Dengsø KE, Sørensen CL, Frederiksen HJ, Krohn PS, Petersen CR, Larsen PN, Fukumori D, Burgdorff SK, Kehlet H, Schultz NA. Enhanced recovery after surgery is feasible and safe in liver transplantation: a cohort study. HPB (Oxford). 2022;24:2022-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Xu Q, Zhu M, Li Z, Zhu J, Xiao F, Liu F, Wang Y, Liu C. Enhanced recovery after surgery protocols in patients undergoing liver transplantation: A retrospective comparative cohort study. Int J Surg. 2020;78:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | King AB, Kensinger CD, Shi Y, Shotwell MS, Karp SJ, Pandharipande PP, Wright JK, Weavind LM. Intensive Care Unit Enhanced Recovery Pathway for Patients Undergoing Orthotopic Liver Transplants Recipients: A Prospective, Observational Study. Anesth Analg. 2018;126:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 5227] [Article Influence: 1045.4] [Reference Citation Analysis (1)] |
| 24. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 6528] [Article Influence: 725.3] [Reference Citation Analysis (0)] |
| 25. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26325] [Article Influence: 1755.0] [Reference Citation Analysis (4)] |
| 26. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 13644] [Article Influence: 852.8] [Reference Citation Analysis (1)] |
| 27. | Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017;152:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 2434] [Article Influence: 270.4] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/