Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.107002
Revised: April 15, 2025
Accepted: May 21, 2025
Published online: July 27, 2025
Processing time: 132 Days and 23.4 Hours
Gastroesophageal junction (GEJ) or gastrointestinal stromal tumor (GIST) are located in unfavorable parts of the stomach, due to the anatomical complexity of these regions, protecting the cardia while ensuring R0 resection is a major cha
Two cases of GEJ stromal tumors were reported. Abdominal computed tomo
The gastric tube-guided robotic-assisted laparoscopic resection is a safe and effective method for tumor resection while preserving the cardia, and it is worth further promotion in clinical practice.
Core Tip: Protecting the cardia is a considerable challenge in the surgical management of esophagogastric junction gastrointestinal (GI) stromal tumors. This study aims to investigate and summarize the application value of gastrostomy-guided and robot-assisted techniques in safeguarding the cardia during the resection of GI stromal tumors situated in anatomically unfavorable gastric locations.
- Citation: Su QL, Yuan SL, Chen P, Wang HD, Liu J, Jiang W, Jiang ZW, Dai HS, Liu XX. Gastric tube-guided and robot-assisted laparoscopic resection of gastroesophageal junction stromal tumors: Two case reports. World J Gastrointest Surg 2025; 17(7): 107002
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/107002.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.107002
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal (GI) tract, with approximately 60% occurring in the stomach[1]. GISTs at the gastroesophageal junction (GEJ) or cardia sphincter present significant challenges due to their complex anatomy, complicating surgical resection. Therefore, achieving R0 resection while preserving the function of the cardia sphincter remains a significant challenge for surgeons[2].
In traditional laparoscopic surgery, the limited operating space and lack of tactile feedback complicate the intraoperative accurate assessment of tumor margins, which can lead to injuring the cardia sphincter[3]. Warner and Sasse[4] have emphasized that nasogastric or calibration tubes not only help in assessing gastric capacity but also protect the cardia sphincter. Recently, robotic surgery has gained recognition because of the flexible robot arms and high-definition visualization, which provide more precise manipulation and better tissue dissection, reducing the incidence of such complications[5]. Solaini et al[6] has shown that the robotic approach offers significant advantages in fine dissection and suturing. Additionally, a single-center study by Furbetta et al[7] demonstrated the role of robotic surgery in resecting GISTs in anatomically complex areas. By combining the advantages of nasogastric tube guidance with robotic surgery, GISTs can be more accurately resected in challenging gastric locations while preserving the function of the cardia sphincter. Inspired by the use of calibration tubes in sleeve gastrectomy[8], here, we report introducing a nasogastric tube guidance technique to enhance tactile feedback and more accurately assess tumor boundaries.
Case 1: A 44-year-old woman who presented with a 2-year history of severe anemia, accompanied by intermittent upper abdominal discomfort, gastroesophageal reflux, and heartburn that began 1 year ago.
Case 2: A 59-year-old male patient presented with upper abdominal pain for over half a month.
Case 1: The patient has been receiving iron supplementation for the past 2 years without significant improvement in her anemia. She began experiencing upper abdominal discomfort, acid reflux, and heartburn 1 year ago.
Case 2: He experienced occasional upper abdominal discomfort, acid reflux, heartburn, nausea, and frequent post
No past medical history.
No past medical history and no personal and family history.
Case 1: On physical examination, the vital signs were as follows: Body temperature, 36.3 °C; blood pressure, 108/60 mmHg; heart rate, 72 beats per minute; respiratory rate, 18 breaths per minute. Additionally, the patient appeared pale (indicating anemia), with mild tenderness in the upper abdomen. The abdomen was soft, without rebound tenderness or muscle rigidity.
Case 2: On physical examination, the vital signs were as follows: Body temperature, 36.3 °C; blood pressure, 112/70 mmHg; heart rate, 73 beats per minute; respiratory rate, 17 breaths per minute. Additionally, there was mild tenderness in the upper abdomen. The abdomen was soft, without rebound tenderness or muscle rigidity.
Case 1: Hemoglobin level: 46 g/L; red blood cell count: 2.03 × 10¹²/L; Fecal occult blood: Positive (+); serum tumor markers: Normal; liver function tests, renal function tests, and urinalysis: Within normal limits.
Case 2: Laboratory examinations including complete blood count, urinalysis, fecal routine, liver function tests, and renal function tests, revealed no significant abnormalities.
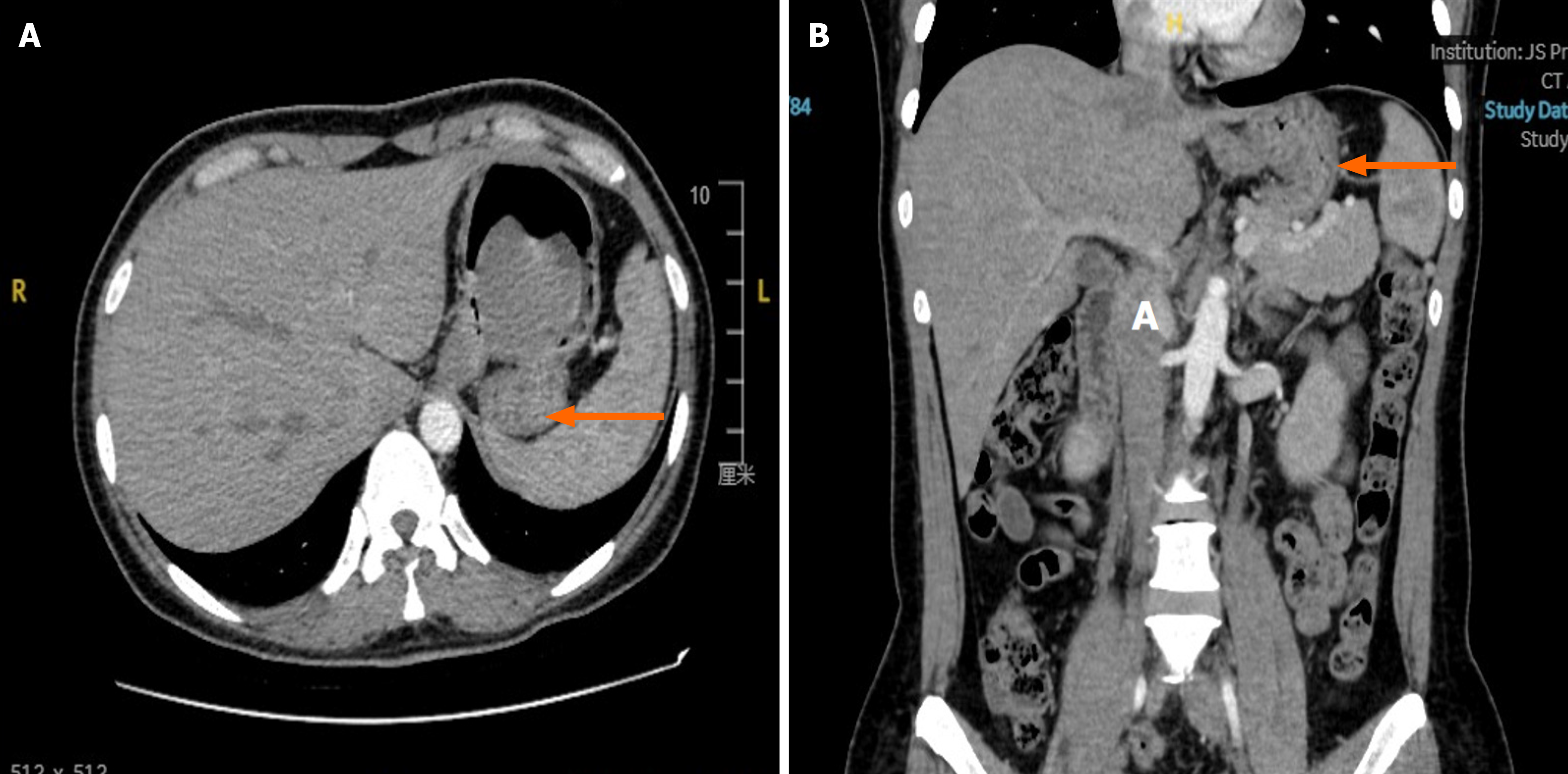
Case 1: Gastroscopy: A 5.5-cm protrusion was identified on the posterior wall of the gastric fundus, with an associated ulcer and fresh bleeding.
Contrast-enhanced computed tomography (CT) (Figure 1): A soft tissue density mass measuring approximately 5.0 cm × 4.8 cm was detected in the gastric fundus. The mass exhibited clear margins with mild enhancement.
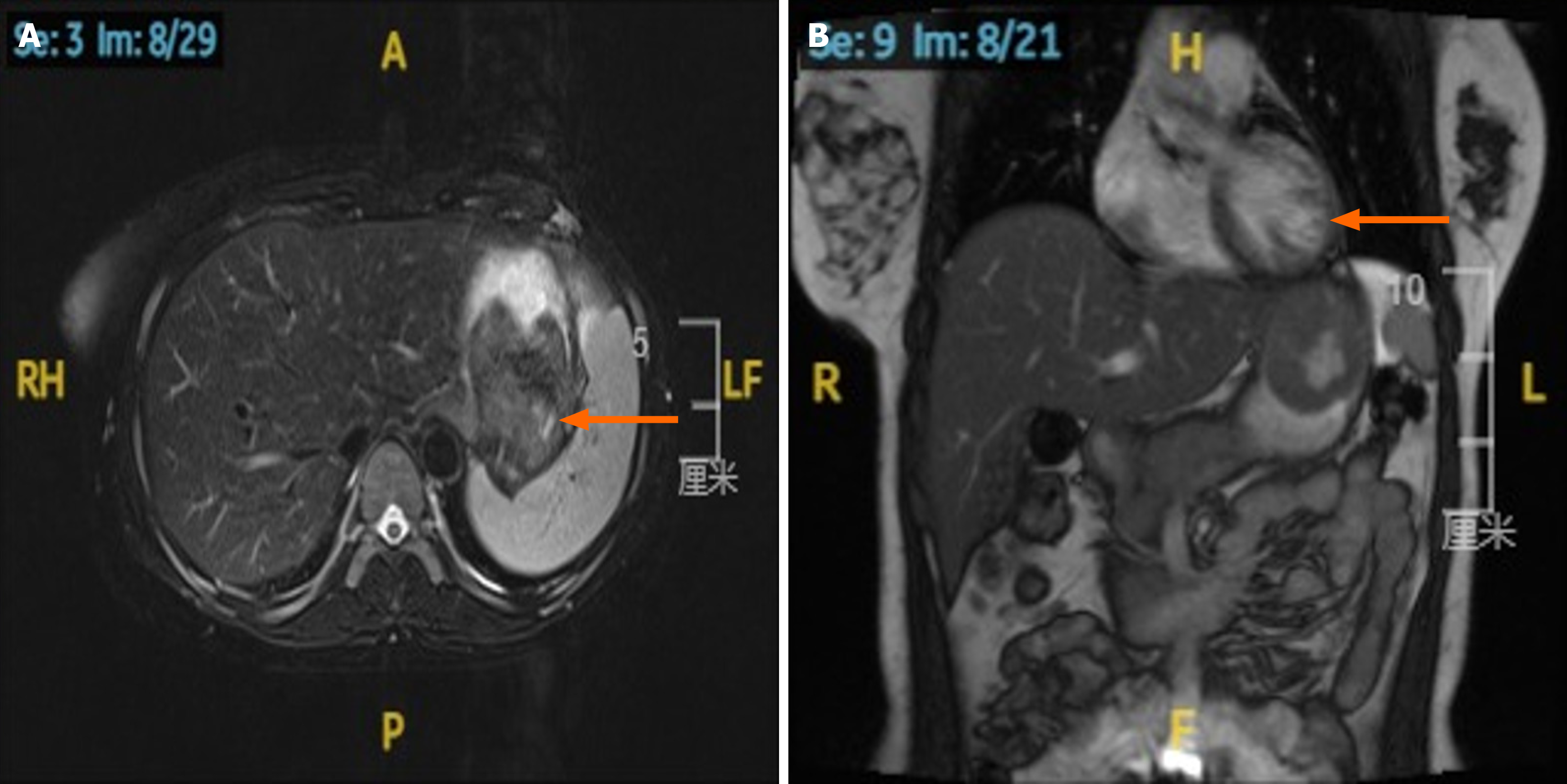
Abdominal magnetic resonance imaging (Figure 2): A slightly low-signal filling defect was observed in the gastric fundus, measuring approximately 50 mm × 46 mm. The lesion demonstrated a slightly high signal intensity on diffusion-weighted imaging.
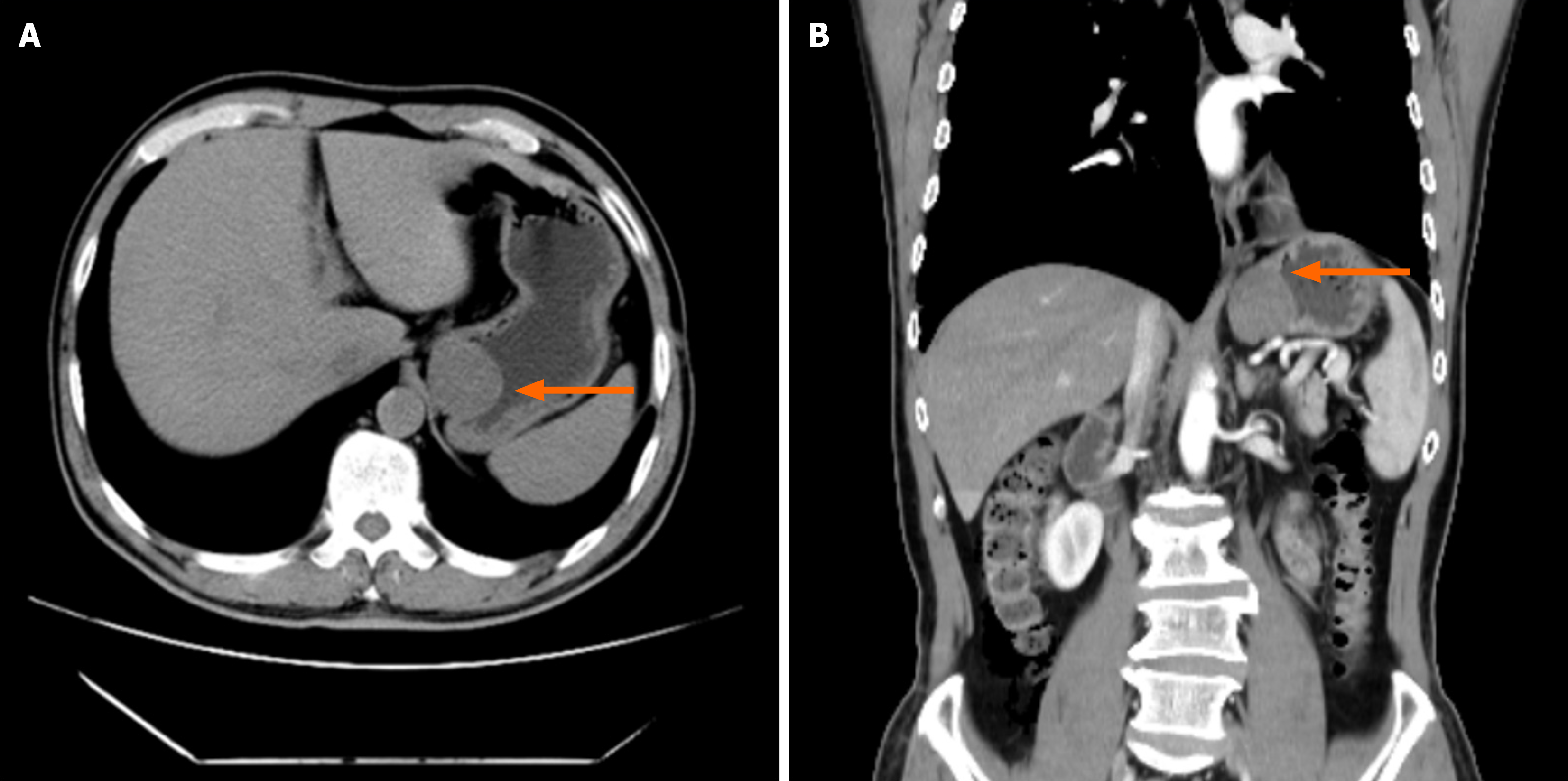
Case 2: Contrast-enhanced CT (Figure 3) showed a well-defined submucosal mass at the cardia, measuring approximately 2.5 cm × 2.7 cm.
Endoscopic ultrasound revealed a 4-cm hypoechoic, oval-shaped submucosal protrusion extending from the cardia to the gastric fundus, likely originating from the muscularis propria.
Considering the symptoms, physical examination, and ancillary tests, a GIST of the GEJ is highly suspected.
Following the exclusion of surgical contraindications, both patients underwent robotic-assisted laparoscopic gastric wedge resection.
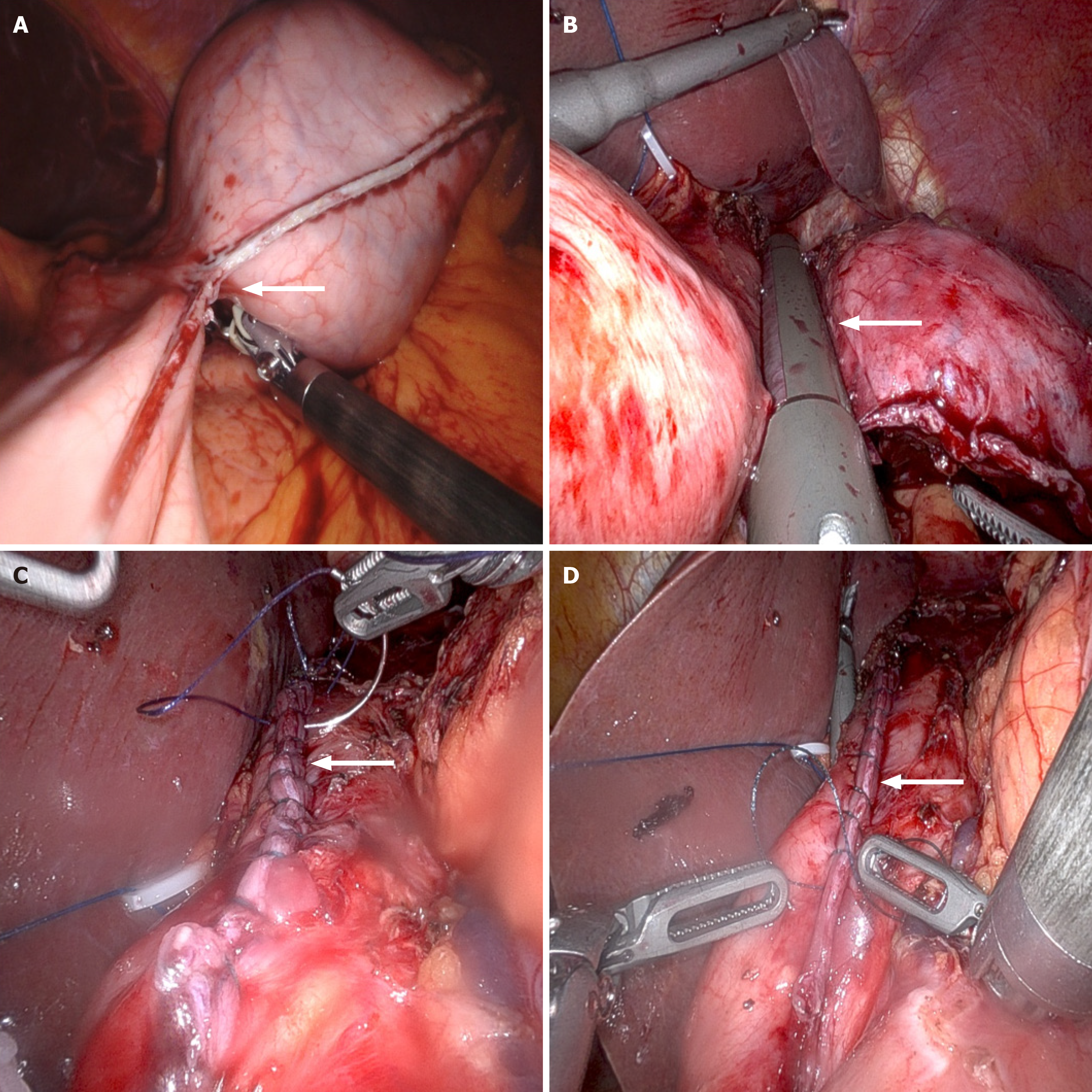
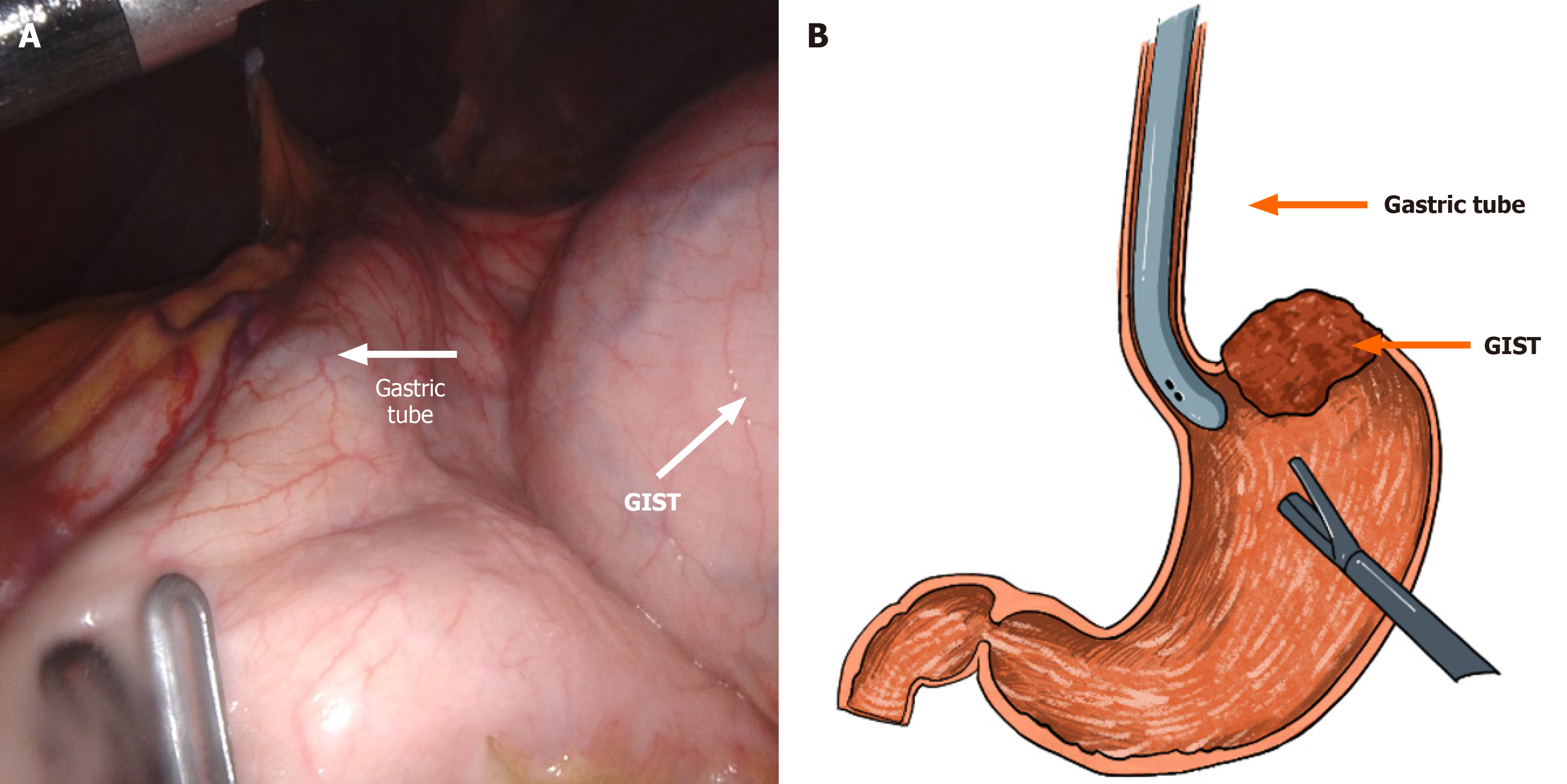
Under general anesthesia, the patient was placed in a supine position with legs abducted in a V-shape. A 1-cm transverse incision was made slightly to the right of the midline below the umbilicus to establish the observation port. The abdominal cavity was insufflated to a pressure of 12 mmHg. Intraoperative exploration revealed no metastatic lesions. A 5.5 cm × 5.0 cm mass was palpated on the posterior wall near the cardia of the upper gastric body. An 8-mm robotic trocar was inserted at the right and left midclavicular lines at the costal margin, and a 12-mm trocar was positioned at the left midclavicular line at the umbilical level to serve as an assistant port (Figure 4). The omentum was retracted cephalad to expose the operative field. The gastroepiploic artery was carefully dissected and ligated. The short gastric and posterior gastric arteries were dissected and ligated using a vascular clip and ultrasonic scalpel. The gastrosplenic ligament was completely mobilized up to the left side of the cardia. After palpating the mass, a nasogastric tube (36F Bougie Tube) was inserted to provide full support to the GEJ and the gastric wall adjacent to the tumor (Figure 5). Gentle dissection was meticulously performed to clearly delineate the tumor margins, with careful preservation of the cardia. A wedge resection of the GIST was executed using an endoscopic stapler. The gastric wall was subsequently reinforced with a continuous suture using a 3-0 barbed suture material (Figure 6). The resected specimen was placed into a retrieval bag and removed in a completely intact manner. One abdominal drain was positioned in the left upper quadrant, and another was placed in the right upper quadrant. After confirming the absence of bleeding and leakage, the trocar sites and surgical incisions were meticulously closed with sutures, thereby completing the procedure.
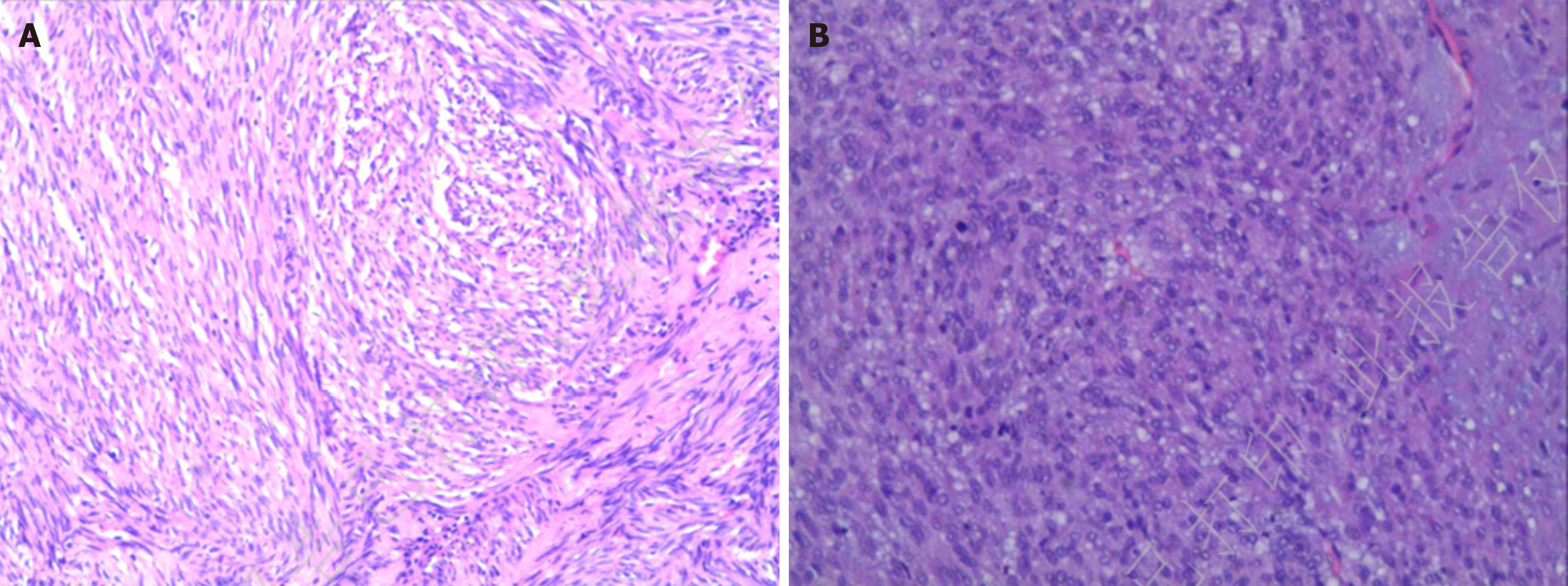
After a brief period of postoperative treatment with intravenous fluids, antibiotics, and other routine interventions, the drainage tubes were removed on postoperative days 3 to 5. The patients were allowed to resume oral liquid diets without experiencing abdominal pain or bloating, and with normal flatus and bowel movements. They were discharged once their conditions stabilized. Postoperative pathology results: Case 1: Gastric tumor resection specimen GIST. Postoperative risk stratification: Intermediate. No residual tumor was identified at the resection margins. Immunohistochemical results (magnification: × 200): Tumor cells were positive for CD34(+), CD117(+), and DOG1(+), and negative for SMA(-), desmin(-), S-100(-). Normal expression of SDHB was observed, and the proliferation index Ki67 was approximately 5% positive (Figure 7A). Case 2: Gastric tumor resection specimen GIST. Postoperative risk stratification: High. No residual tumor was identified at the resection margins. Immunohistochemical results: Tumor cells were strongly positive for CD117(++), CD34(++), and DOG1(+++), focally positive for SMA(+), and negative for desmin(-). Scattered cells were positive for S-100(+). Normal expression of SDHB was retained, and the proliferation index Ki67 was approximately 10% positive (Figure 7B).
GISTs are a common type of mesenchymal tumor[1]. GISTs in the lesser curvature of the stomach, the posterior wall of the stomach, and the GEJ are referred to as gastric GISTs in unfavorable locations due to their anatomical characteristics, which make them difficult to resect via laparoscopy or endoscopy[3]. Surgery is the preferred treatment for gastric GISTs, with the choice of surgical approach determined by a comprehensive consideration of the tumor's anatomical location, size, growth pattern (e.g., intraluminal, extraluminal, or intramural), and the potential impact on postoperative gastric function. The primary routes of metastasis for GISTs are hematogenous metastasis and peritoneal seeding, with common metastatic sites including the liver, mesentery, and retroperitoneal space. Lymph node metastasis is extremely rare, hence routine lymphadenectomy is not performed during surgery[9].
GEJ GISTs are defined as GISTs located within 5 cm above or below the GEJ[10,11]. For GEJ GISTs, the current recommended surgical approach is laparoscopic wedge resection of the stomach. However, surgeries in this area face numerous challenges: The complex anatomical structure, the density of important structures, and the complexity of GI reconstruction[12]. During laparoscopic surgery, exposure is difficult, which is not conducive to accurately determining the tumor margins, potentially leading to excessive resection of gastric tissue or tumor residue[13]. Moreover, repeated intraoperative manipulation of the tumor may cause tumor rupture, increasing the risk of peritoneal seeding and metastasis. Given these complexities, a high level of surgical expertise, particularly in suturing, is required. Postoperative complications may include anastomotic leakage, anastomotic stricture, and gastroesophageal reflux. Achieving R0 resection while preserving the cardia and maximizing gastric tissue retention remains a significant challenge for surgeons. Compared with traditional laparoscopic surgery, robotic surgery, with its high-definition imaging, high precision in operation, and high flexibility, has gradually gained recognition for its ability to reduce postoperative complications[14]. Lwin et al[5] also noted that robotic surgery can improve the effectiveness and safety of procedures for gastric GISTs in anatomically challenging locations.
To address the challenge of cardia preservation during wedge resection of GEJ GISTs, we report two cases of gastric GISTs located at the GEJ. During both procedures, we guided the operation by placing a large diameter nasogastric tube routine used in sleeve gastrectomy, and utilized the flexible robotic arms to increase the surgeon's tactile perception of the tumor, facilitating more accurate determination of tumor margins and better preservation of gastric cardia function. Therefore, we not only ensured the R0 resection of the tumor but also preserved the cardia sphincter and retained the maximum amount of gastric tissue. None of the two surgeries was converted to open surgery. The flexible robotic arms played a significant role in GI reconstruction and suturing, enabling the successful completion of the surgeries. In terms of postoperative complications, no patients experienced anastomotic leakage, anastomotic stricture, or gastroesophageal reflux. Regarding operative time, although robotic surgery may take slightly longer than traditional laparoscopic surgery, with continuous technological advancements and increasing proficiency of the surgical team, the operative time is expected to be shortened[15].
In conclusion, robotic-assisted gastric wedge resection guided by nasogastric tube placement achieved R0 resection of the tumor while successfully preserving the cardia and retaining the maximum amount of gastric tissue. This surgical method is warrants further consideration for broader clinical application.
The gastric tube-guided robotic-assisted laparoscopic resection is a safe and effective method for tumor resection while preserving the cardia, and it is worth further promotion in clinical practice.
Thanks to Professor Dai HS and Jiang ZW for his perioperative guidance on the use of “enhanced recovery after surgery”, and Wei Jiang and Liu Jiang and Professor Liu XX for precise surgery.
| 1. | Serrano C, Martín-Broto J, Asencio-Pascual JM, López-Guerrero JA, Rubió-Casadevall J, Bagué S, García-Del-Muro X, Fernández-Hernández JÁ, Herrero L, López-Pousa A, Poveda A, Martínez-Marín V. 2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther Adv Med Oncol. 2023;15:17588359231192388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 2. | Li J, Khajoueinejad N, Sarpel U. Surgical Management of Gastric Gastrointestinal Stromal Tumors. Surg Clin North Am. 2025;105:109-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Zheng GL, Zhang B, Wang Y, Liu Y, Zhu HT, Zhao Y, Zheng ZC. Surgical resection of esophagogastric junction stromal tumor: How to protect the cardiac function. World J Gastroenterol. 2021;27:854-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Warner DL, Sasse KC. Technical Details of Laparoscopic Sleeve Gastrectomy Leading to Lowered Leak Rate: Discussion of 1070 Consecutive Cases. Minim Invasive Surg. 2017;2017:4367059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lwin TM, Fong ZV, Narayan RR, Wang SJ, Wang J. Robotic Function-Preserving Resection of Gastric Gastrointestinal Stromal Tumor. J Surg Res. 2023;290:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Solaini L, Cavaliere D, Fico V, Milone M, De Pascale S, Desiderio J, Vitali G, Parisi A, Fumagalli Romario U, De Palma GD, D'Ugo D, Ercolani G. Open versus laparoscopic versus robotic gastric gastrointestinal stromal tumour resections: A multicentre cohort study. Int J Med Robot. 2021;17:e2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Furbetta N, Palmeri M, Guadagni S, Di Franco G, Gianardi D, Latteri S, Marciano E, Moglia A, Cuschieri A, Di Candio G, Mosca F, Morelli L. Gastrointestinal stromal tumours of stomach: Robot-assisted excision with the da Vinci Surgical System regardless of size and location site. J Minim Access Surg. 2019;15:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Rosenthal RJ; International Sleeve Gastrectomy Expert Panel, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M, Galvao-Neto M, Higa KD, Himpens J, Hutchinson CM, Jacobs M, Jorgensen JO, Jossart G, Lakdawala M, Nguyen NT, Nocca D, Prager G, Pomp A, Ramos AC, Rosenthal RJ, Shah S, Vix M, Wittgrove A, Zundel N. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 733] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 9. | von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol. 2018;36:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 10. | Maric R, Cheng KK. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1999;86:1098-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 936] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Liu X, Zheng Z, Yin J, Zhang J. Safety, efficacy, and selection strategy of laparoscopic local gastrectomy for gastrointestinal stromal tumors in the esophagogastric junction. Front Surg. 2022;9:1015126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Xiong W, Xu Y, Chen T, Feng X, Zhou R, Wan J, Li Y, Li G, Wang W. Laparoscopic vs. open surgery for gastrointestinal stromal tumors of esophagogastric junction: A multicenter, retrospective cohort analysis with propensity score weighting. Chin J Cancer Res. 2021;33:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Kawka M, Fong Y, Gall TMH. Laparoscopic versus robotic abdominal and pelvic surgery: a systematic review of randomised controlled trials. Surg Endosc. 2023;37:6672-6681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Ceccarelli G, Costa G, De Rosa M, Codacci Pisanelli M, Frezza B, De Prizio M, Bravi I, Scacchi A, Gallo G, Amato B, Bugiantella W, Tacchi P, Bartoli A, Patriti A, Cappuccio M, Komici K, Mariani L, Avella P, Rocca A. Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/