Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.106767
Revised: March 30, 2025
Accepted: May 19, 2025
Published online: July 27, 2025
Processing time: 139 Days and 3.8 Hours
Esophageal cancer is one of the most difficult cancers to treat since it is often at an advanced stage at the time of symptom presentation. For locally advanced esophageal cancer, treatment options include multidisciplinary treatment such as surgery or definitive chemoradiotherapy. Surgery has a high local control rate because it involves excision of the cancer along with the surrounding organs; however, it is still highly invasive, although advances in surgery have reduced the burden on patients. On the other hand, chemoradiotherapy may also be applicable in cases in which surgery is inoperable owing to complications or distant lymph node metastasis. However, chemoradiotherapy using X-ray irradiation can cause late toxicities, including those to the heart. Proton beam therapy is widely used to treat esophageal cancer because of its characteristics, and some comparisons between proton beam therapy and X-ray therapy or surgery have recently been reported. This review discusses the role of proton beam therapy in esophageal cancer in comparison to X-ray therapy and surgery.
Core Tip: Despite medical advances, esophageal cancer has a poor prognosis. Recently, there have been many reports on proton beam therapy combined with chemotherapy. Compared to X-ray therapy, proton beam therapy can reduce toxicity and may lead to long-term survival while maintaining the quality of life. Some reports suggest that it may be possible to achieve results comparable to those of surgery, although this is premised on an environment in which appropriate post-recurrence treatment can be performed. Proton beam therapy plays a significant role in the treatment of esophageal cancer in an aging society.
- Citation: Ono T, Koto M. Proton beam therapy for esophageal cancer compared to existing treatments, including X-ray therapy and surgery. World J Gastrointest Surg 2025; 17(7): 106767
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/106767.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.106767
Esophageal cancer (EC) is the 11th most commonly diagnosed cancer and the seventh leading cause of cancer-related deaths in 2022[1]. The burden of EC is increasing due to an aging society, with 307.4 thousand deaths and 7.2 million disability-adjusted life-years. This is particularly problematic in Asia, where approximately 75% of EC cases and deaths occur[2]. Squamous cell carcinoma (SCC), and adenocarcinoma are the main EC pathologies. With advances in medical science, the 5-year overall survival (OS) rates of SCC and adenocarcinoma of the esophagus increased from 3.6% to 21.1% and 5.4% to 24.2%, respectively, between 1973 and 2010[3]. However, EC persists with a poor prognosis. If left untreated, the five-year OS rate is equal to or less than 10%, even including stage I cases with good general conditions where surgery is possible[4].
Therefore, radical treatment for EC is necessary, even if the patient is elderly, has comorbidities, or is in poor general condition. The standard treatment for very early-stage cancer is endoscopic resection, and for locally advanced cancer, preoperative chemoradiotherapy (CRT) followed by surgery is the standard treatment[5,6]. Conversely, the standard treatment in Japan is triplet chemotherapy (cisplatin, 5-luorouracil, and docetaxel), followed by surgery. This approach stems from clinical trials where surgery was performed after preoperative chemotherapy due to concerns about increased toxicity from radiotherapy[7,8]. CRT is indicated in cases where surgery is not possible or denied. In particular, in an aging society, many patients cannot undergo surgery because of comorbidities, even if the patient wishes to undergo surgery[5-7].
Although radiotherapy alone may be curative, it is advisable to combine it with chemotherapy whenever possible[5-7,9]. Although CRT has shown better results compared to surgery alone[10], the recurrence rate is inevitably higher than that of preoperative therapy followed by surgery[5-7]. Even if there is a local recurrence, endoscopic treatment including photodynamic therapy is possible if the disease is not locally advanced[11,12], but surgery is required if the disease progresses. Some clinical trials have shown that the mortality rate of salvage surgery is not high but is higher than that of the initial surgery and should be avoided if possible[13,14]. Other problems are late toxicity, including cardiopulmonary toxicity, which, if encountered, can result in reduced quality of life (QOL).
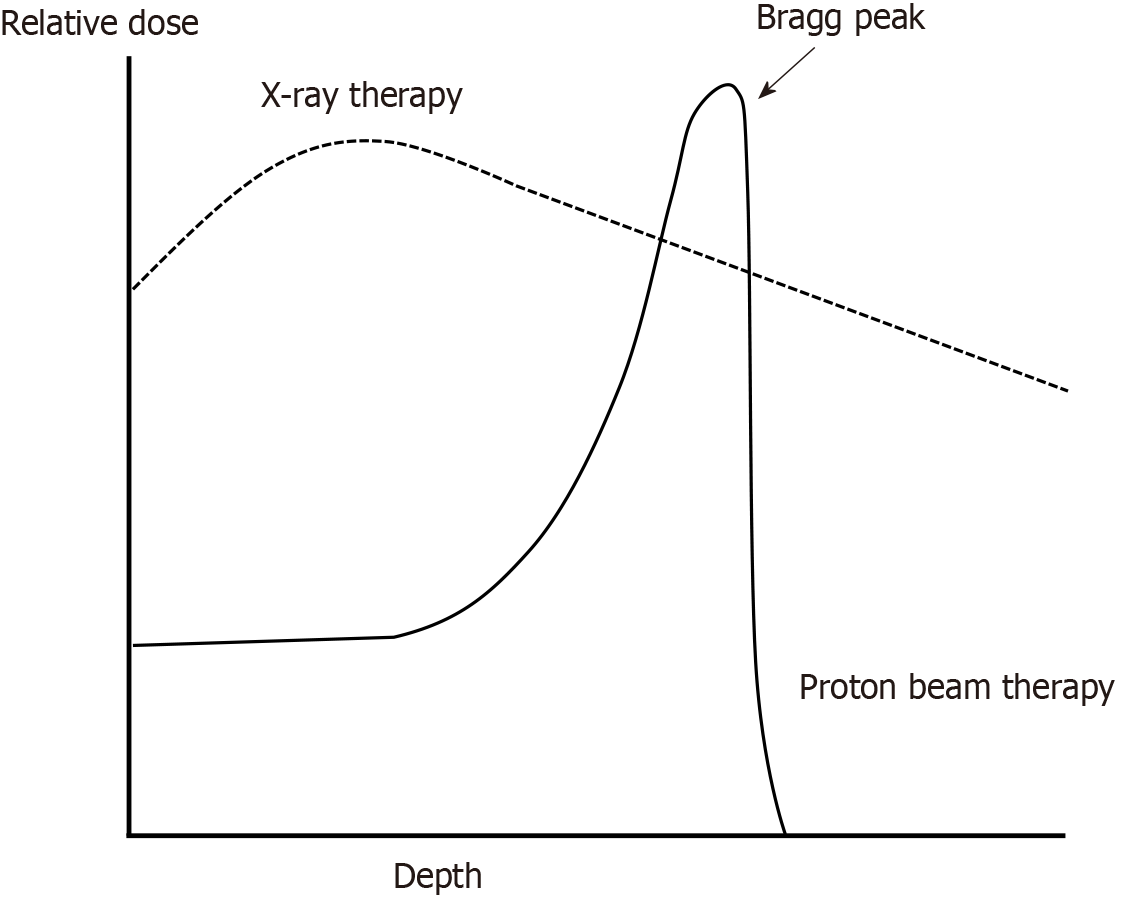
Recently, particle beam therapy has been used, and it is expected that damage to the surrounding organs can be reduced owing to its unique physical characteristics. As shown in Figure 1, particle beam therapy exhibits a phenomenon known as the Bragg peak, where the radiation dose rises sharply and then rapidly decreases near the end of the beam. In contrast, X-ray therapy demonstrates a gradual increase in dose after irradiation, peaking before gradually declining, which inevitably allows low-to-medium doses to spread to the surrounding tissues[15]. Proton beam therapy (PBT) and carbon-ion radiotherapy are used clinically. PBT has been widely used in combination with chemotherapy for EC, and many reports have been published on its clinical advantages and superior dose distribution compared to X-ray therapy[16-40]. Recently, a comparative study was conducted between definitive PBT and adjuvant chemotherapy followed by surgery[41]. There have also been reports of curative treatment with carbon-ion radiotherapy for EC; however, these are limited to stage I[42], and there have been no reports of curative carbon-ion radiotherapy combined with chemotherapy.
In this narrative review of treatment modalities, we explain PBT for EC, which has been increasingly reported, particularly as a curative treatment, and compare it with the existing X-ray therapy and surgery.
This section focuses on simulation studies that compare dose distributions, followed by a detailed comparison of actual clinical toxicity in the next section. The heart and lungs are the main organs for which dose reduction by PBT is expected, and many reports have been published on the effectiveness of this reduction[16-28]. Makishima et al[17] compared the dose volume between PBT and X-ray three-dimensional conformal radiotherapy (3DCRT). In this study, the organ volume irradiated at least xGy (VxGy), mean dose, and changes in normal tissue complication probability (NTCP), which is the probability of toxicity occurring calculated using a formula, were examined. Lung V5-20 Gy, mean lung dose (MLD), and heart V30-50 Gy were significantly lower in the PBT plan. Moreover, the NTCP was lower in the PBT plan than in the X-ray plan in all cases. In the case of the heart, the NTCP, which had a maximum of over 80%, was reduced to approximately 20% in the PBT plan; in many other cases, the probability was reduced to just a few percentages. The maximum NTCP level in the lungs was approximately 12%; however, it was reduced to only a few percentages in the PBT plan. The irradiated area depends on EC progression; however, this shows that it is possible to deliver a dose to the tumor while significantly reducing cardiopulmonary doses. Of note, among patients who received X-ray therapy, the incidence rates of grade 2 or higher radiation pneumonitis and pericardial effusion were 21% and 52.6%, respectively, compared with 0% and 4%, respectively, in patients who received PBT. In addition to 3DCRT, Hirano et al[18] compared the dose-volume parameters of PBT with those of intensity-modulated radiotherapy (IMRT), an X-ray therapy method that adjusts the dose intensity and direction from various directions. In this report, even compared to IMRT, the MLD, lung V5-20 Gy, and heart V20-40 Gy were significantly lower than those of the IMRT plan. Compared with IMRT, which allows for better dose adjustment than 3DCRT, PBT facilitates a good dose distribution.
A small irradiation field is a problem in the conventional irradiation system of PBT. Therefore, it is difficult to achieve a wide range of > 300 mm square, such as elective nodal irradiation (ENI)[16]. Although a patch-field irradiation technique in which multiple proton irradiation fields are connected is used, some institutions use X-ray therapy for ENI[43]. Sasaki et al[16] compared hybrid dose distribution using PBT and volumetric modulation arc therapy (VMAT), which is a rotating IMRT. When ENI was irradiated with VMAT and boost irradiation was performed with PBT, the dose reduction effect was smaller than that when both were irradiated with VMAT. The heart V30 Gy was reduced from 27.4% to 15.1%; however, the lung dose reduction effect was small. In contrast, when both were irradiated with PBT, the lung dose was also greatly reduced from 9.0 Gy to 5.9 Gy in MLD. Regarding the cardiac dose, studies have evaluated dose reduction in various parts of the heart, such as the left anterior descending artery (LAD). Shiraishi et al[19] reported that dose reduction was achieved with PBT compared with IMRT in the atrium, ventricle, and major coronary arteries, including the LAD. Regarding lung cancer, it has been pointed out that the radiation dose to the cardiovascular system, such as the LAD, may contribute to cardiotoxicity and, ultimately, survival prognosis[44]. In this report, V15 Gy of LAD ≥ 10% was associated with an increased risk of all-cause mortality (2-year OS was 47% vs 67%). Considering this threshold, Shiraishi et al[19] showed that even in the low-dose range of V5 Gy, the PBT group had a value of 8%, which was less than 10%. However, in the IMRT group, even in the high-dose range of V30 Gy, the value was 15.8%, which was significantly higher than 10%, making it extremely difficult to maintain the LAD threshold shown in a lung cancer study. Therefore, reducing the radiation dose to the vascular system, especially the LAD, would be more easily achieved using PBT than X-ray therapy.
In addition to the lungs and heart, analysis is being conducted on the effect of reducing bone marrow suppression. Warren et al[29] reported that only PBT plans showed significant sparing of the bone V10 Gy and bone mean dose, especially in patients with a larger irradiated field. This also means that the more advanced the cancer, the greater the significance of using PBT, as the irradiation area will be wider, even if the irradiation is focused on the cancer lesion without ENI.
As mentioned above, simulations show that radiation doses to the heart, lungs, and bones can be reduced using PBT compared to X-ray therapy; however, it is ultimately important to determine whether similar results can be achieved when treating actual patients in clinical situations. Table 1 summarizes the previous reports comparing the toxicities of PBT and X-ray therapies. Currently, there is only one phase 2 randomized controlled trial (RCT). Lin et al[30] reported the total toxicity burden (TTB), which is the cumulative severity of multiple toxicities in patients with EC after CRT with or without surgery. For example, grade 3 toxicities, including radiation pneumonitis, pleural effusion, and pericardial effusion weighed 60 points, whereas myocardial infarction weighed 70 points. In this report, the mean TTB of IMRT was 2.3 times higher than that of PBT, and the mean postoperative complication score of IMRT was 7.6 times higher than that of PBT. On the other hand, there have been only retrospective studies comparing IMRT and PBT in a few cases in which only definitive CRT was administered, and they reported no significant difference in toxicity between IMRT and PBT. However, the incidence rates of pneumonitis, pericardial effusion, and treatment-related deaths are lower in PBT[28]. Currently, most reports are retrospective comparisons, but a meta-analysis that included one RCT, as mentioned above, concluded that the incidence of grade 2 or higher pneumonitis and pericardial effusion was significantly lower in the PBT group compared to the X-ray therapy group with grade 2 or higher radiation pneumonitis was 2%, grade 2 or higher pleural effusion was 4%, grade 2 or higher pericardial effusion was 3%[31]. Therefore, PBT for EC can reduce cardiac and pulmonary toxicity, as demonstrated by its superior dose distribution in the simulation study. In addition, the benefit of a reduced dose is thought to be greater with a wider irradiation area, and PBT may offer the possibility of treatment in cases in which treatment would normally be hesitant owing to toxicity.
| Ref. | Modality | G ≥ 3 pericardial effusion | G ≥ 3 pneumonia | G ≥ 3 pleural effusion | G4 lymphocytopenia |
| Makishima et al[17] | 3DCRT | 0 | 5.3 | 5.3 | - |
| PBT | 0 | 0 | 0 | - | |
| Shiraishi et al[19] | IMRT | - | - | - | 40 |
| PBT | - | - | - | 18 | |
| Choi et al[25] | IMRT | - | - | - | 12.5 |
| PBT | - | - | - | 20.0 | |
| Xi et al[28] | IMRT | 2.4 | 2.9 | 1.9 | - |
| PBT | 0.8 | 1.6 | 0.8 | - | |
| Lin et al[30] | IMRT | 2 | 3 | 10 | - |
| PBT | 0 | 0 | 2 | - | |
| Wang et al[32] | IMRT | - | - | - | 52.5 |
| PBT | - | - | - | 27.3 | |
| PBT | 0 | - | 0 | 40.7 | |
| Fang et al[33] | IMRT | - | - | - | 60.5 |
| PBT | - | - | - | 39.5 | |
| Routman et al[37] | XRT | - | - | - | 60 |
| PBT | - | - | - | 40 | |
| Zhu et al[38] | IMRT | - | - | - | 46.2 |
| PBT | - | - | - | 22.0 |
There have been many comparative clinical reports on bone marrow suppression, particularly regarding lymphopenia[32-38]. Both the sub-analyses of the aforementioned RCT and meta-analysis reported that grade 4 or higher lymphopenia was significantly less likely to occur with PBT than with X-ray therapy, which coincides with the simulation results of the dose distribution advantage[31,32]. A sub-analysis of this RCT found that when baseline lymphocyte counts were less than 1.02 × 103/μL, grade 4 or higher lymphopenia would occur in 100% of cases, regardless of whether IMRT or PBT was used. However, when the irradiation volume of the tumor was 358 mL or more and the baseline lymphocyte count was 1.95 × 103/μL, there was a significantly large difference in the incidence of grade 4 or higher lymphopenia, with 35% for PBT and 70% for IMRT[32]. Therefore, the larger the irradiated field of the tumor, the greater the contribution of PBT to reducing lymphopenia, which is consistent with the results of the dose-volume analysis. In addition, according to a study using machine learning, reductions in grade 4 or higher lymphopenia risk were maximized with PBT in older patients with lower baseline absolute lymphocyte counts and higher lung and heart doses[38]. Although the volume of the irradiated tumor did not remain the most significant factor, it was surmised that high doses to the lungs and heart are more likely to occur when the tumor is large and a wide area must be irradiated. This report supports the results of the sub-analysis of phase 2 RCT. Furthermore, the effectiveness of PBT in elderly people, as shown in this study, suggests that PBT may play a larger role in an aging society in the future.
Currently, the effect of PBT on improving prognosis in the treatment of EC remains controversial. Clinical results are summarized for cases in which OS and progression-free survival (PFS) are reported in the text (Table 2). The phase 2 RCT showed no significant differences in OS or PFS[30]. However, several factors may have influenced the results of this phase 2 RCT, such as the fact that only half of the cases were assumed to be definitive settings, most underwent surgery and the PBT group had significantly more cases with a performance status of 1 or higher, despite being randomized. In addition, when evaluating the clinical results, it is important to consider that the recruitment of 67% of the patients was terminated because the discontinuation criteria for RCT on TTB were met. Therefore, there was insufficient statistical power to compare PFS with the other endpoints. Conversely, a retrospective comparative study conducted on several cases of definitive CRT reported that both OS and PFS were significantly better in the PBT group[28]. However, this report should be interpreted cautiously, as the median follow-up period was significantly lower in the PBT group (44.8 months vs 65.1 months). The meta-analysis also requires caution in interpretation as it includes many retrospective studies and cases of preoperative treatment and the use of data taken from graphs not specified in the text; however, similar to the retrospective comparison report mentioned above, the meta-analysis concluded that OS and PFS were significantly improved in the PBT group[31].
| Ref. | Modality | Definitive settings (%) | Number of patients | Median follow up (months) | OS (%) | PFS (%) | |||||||
| 1 year | 1.5 years | 2 years | 3 years | 5 years | 1 years | 2 years | 3 years | 5 years | |||||
| Choi et al[25] | 3DCRT | 0 | 16 | 17.0 | - | - | 67.8 | - | - | - | 33.3 | - | - |
| PBT | 0 | 15 | 17.0 | - | - | 68.6 | - | - | - | 34.5 | - | - | |
| Xi et al[28] | 3DCRT | 100 | 41 | 65.1 | - | - | - | 31.6 | - | - | - | 20.4 | |
| PBT | 100 | 132 | 44.8 | - | - | - | 41.6 | - | - | - | 34.9 | ||
| Bhangoo et al[27] | IMRT | 28 | 32 | 14 | 71 | - | - | - | - | 45 | - | - | - |
| PBT | 19 | 32 | 10 | 74 | - | - | - | - | 71 | - | - | - | |
| Lin et al[30] | IMRT | 50.8 | 61 | 44.1 | - | - | - | 50.8 | - | - | - | 44.8 | - |
| PBT | 54.3 | 46 | 44.1 | - | - | - | 51.2 | - | - | - | 44.5 | - | |
| Sumiya et al[36] | 3DCRT (all) | 100 | 15 | - | - | - | 47.7 | - | - | - | - | - | - |
| PBT (all) | 100 | 54 | - | - | - | 77.2 | - | - | - | - | - | ||
| 3DCRT (≥ stage 2) | 100 | 14 | - | - | - | 41.1 | - | - | - | - | - | - | |
| PBT (≥ stage 2) | 100 | 31 | - | - | - | 68.9 | - | - | - | - | - | ||
| DeCesaris et al[40] | IMRT | 0 | 36 | - | - | 59 | - | - | - | - | - | - | - |
| PBT | 0 | 18 | - | - | 83 | - | - | - | - | - | - | - | |
Conversely, while the numerical survival rate is important, survival with a maintained QOL is also important. There are few reports on long-term QOL after CRT, even after treatment with X-ray therapy[45]. The phase 2 RCT reported no significant difference in QOL between IMRT and PBT cases; however, the comparison was limited to 3 months or more at most, there was no annual comparison, and only a few people were evaluated. As mentioned in the Discussion section of this report, in addition to the lack of detection power for QOL[30], the inability to evaluate during the period when late toxicity occurs may have influenced this finding. Although it is difficult to properly evaluate long-term QOL, it is desirable to report on QOL evaluations annually. However, this phase 2 RCT showed a significant improvement in TTB considering toxicity in PBT cases, and that late toxicity may affect QOL, it is possible that performing PBT may improve QOL in the long term. If toxicity can be reduced by PBT, it may be possible to maintain survival while maintaining QOL compared with X-ray therapy.
Moreover, it has been suggested that lymphopenia may contribute to worsening prognosis not only in EC but also in other cancers[46-48]. Therefore, PBT may play a significant role in reducing the occurrence rate of severe lymphopenia.
A direct comparison of a reasonable number of cases treated with preoperative chemotherapy followed by surgery and PBT with chemotherapy was recently reported in Japan[40]. In this study, the 3-year OS rates were 74.8% and 72.7% in the PBT and surgery groups, respectively, although the 3-year PFS was worse in the PBT group than in the surgery group (51.4% vs 59.6%, respectively). This result may be related to the significantly poorer prognosis after recurrence in patients who underwent surgery than those who underwent PBT (hazard ratio, 0.58). In particular, the results were better in the group that underwent salvage surgery or endoscopic treatment for local recurrence, which occurred more frequently than surgery. Although recurrence is common with PBT, careful follow-up after treatment may improve the prognosis significantly because 61% of the recurrences were local-only progressions in this report. Only one-third of the cases in this study underwent surgery after triplet chemotherapy, which is the current standard treatment in Japan. Although there is room for improvement in the treatment outcomes of the surgery group using triplet chemotherapy, this report suggests that it is possible to achieve treatment outcomes of PBT comparable to those of preoperative chemotherapy followed by surgery if appropriate treatment for recurrence after PBT is administered. In contrast, a similar report, although fewer cases, reported a worse prognosis in the PBT group for cT3-4 cases[49]. This result was thought to be influenced by the greater likelihood of local recurrence in patients undergoing PBT; therefore, we believe it is important to conduct follow-up observations assuming that therapeutic intervention will be performed for post-radiation recurrence. An RCT would be desirable to draw clear conclusions regarding the comparison between PBT and surgery. However, it is hoped that more rigorous prospective trials will be conducted, even though the treatments are completely different and difficult to perform.
Although no specific indicators were used in this study, Ogawa et al[41] speculated that preoperative chemotherapy followed by surgery might have a greater impact on the patient's general condition after treatment than PBT combined with chemotherapy. Although minimally invasive surgery improves QOL after surgery[50], extensive resection is unavoidable; therefore, surgery may result in a lower QOL, as was the case in a previous RCT[9] or meta-analysis[45] that compared X-ray therapy with surgery alone, which assessed QOL. In addition, 41.4% of the PBT group in this study underwent X-ray therapy during ENI; therefore, the difference in the impact on daily life may be even greater if treatment was performed with PBT alone. PBT has less toxicity than X-ray therapy; therefore, QOL may be maintained. Further investigation is needed in the future, with a particular focus on the differences in long-term QOL.
In the EC radiotherapy field, clinical trials of immune checkpoint inhibitor combination therapies are being conducted, as shown in Keynote 975[51]. It has been suggested that the efficacy of immune checkpoint inhibitors may be adversely affected by lymphopenia[52,53]. Colomb et al[52] reported that patients who undergo maintenance therapy with durvalumab after CRT for lung cancer may have a better prognosis if their lymphocyte counts are maintained. They concluded that maintaining lymphocyte counts is important. In addition, a meta-analysis examining lymphocyte count reduction due to all treatments and the effect of immune checkpoint inhibitors on lung cancer cases reported that lymphocyte count reduction contributed to a worsening prognosis[53]. This was a report on lung cancer, and since there are no current reports on EC, it is not known whether the same applies to EC; most of the reports were retrospective, but a similar effect may occur in EC. Therefore, the role of PBT in suppressing lymphopenia may become important if the Keynote 975 trial produces positive results.
In addition, future results, including a phase 3 RCT of PBT and IMRT with OS as the primary endpoint (NCT03801876) and a phase 1 clinical trial to confirm the effect of dose escalation with PBT in neoadjuvant CRT (NCT02213497), will likely clarify the role of PBT in EC compared to other treatment methods.
Although there is no conclusion regarding the OS improvement effect of PBT for EC compared with X-ray therapy, PBT reduces toxicity, such as cardiac, pulmonary, and bone marrow suppression. Therefore, even if OS does not improve, long-term QOL can be maintained in PBT cases. In addition, compared to radical surgery, if combined with appropriate recurrence treatment, good results can be obtained while suppressing the disadvantage of an increase in local recurrence.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12775] [Article Influence: 6387.5] [Reference Citation Analysis (8)] |
| 2. | Teng Y, Xia C, Cao M, Yang F, Yan X, He S, Cao M, Zhang S, Li Q, Tan N, Wang J, Chen W. Esophageal cancer global burden profiles, trends, and contributors. Cancer Biol Med. 2024;21:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 3. | He H, Chen N, Hou Y, Wang Z, Zhang Y, Zhang G, Fu J. Trends in the incidence and survival of patients with esophageal cancer: A SEER database analysis. Thorac Cancer. 2020;11:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Keshava HB, Rosen JE, DeLuzio MR, Kim AW, Detterbeck FC, Boffa DJ. "What if I do nothing?" The natural history of operable cancer of the alimentary tract. Eur J Surg Oncol. 2017;43:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, van Grieken NCT, Vogel A, Smyth EC; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 437] [Article Influence: 109.3] [Reference Citation Analysis (1)] |
| 6. | Porschen R, Fischbach W, Gockel I, Hollerbach S, Hölscher A, Jansen PL, Miehlke S, Pech O, Stahl M, Vanhoefer U, Ebert MPA. Updated German guideline on diagnosis and treatment of squamous cell carcinoma and adenocarcinoma of the esophagus. United European Gastroenterol J. 2024;12:399-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, Kuribayashi S, Kono K, Kojima T, Takeuchi H, Tsushima T, Toh Y, Nemoto K, Booka E, Makino T, Matsuda S, Matsubara H, Mano M, Minashi K, Miyazaki T, Muto M, Yamaji T, Yamatsuji T, Yoshida M. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20:343-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 283] [Reference Citation Analysis (0)] |
| 8. | Kato K, Machida R, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, Kojima T, Abe T, Bamba T, Watanabe M, Kawakubo H, Shibuya Y, Tsubosa Y, Takegawa N, Kajiwara T, Baba H, Ueno M, Takeuchi H, Nakamura K, Kitagawa Y; JCOG1109 investigators. Doublet chemotherapy, triplet chemotherapy, or doublet chemotherapy combined with radiotherapy as neoadjuvant treatment for locally advanced oesophageal cancer (JCOG1109 NExT): a randomised, controlled, open-label, phase 3 trial. Lancet. 2024;404:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 146] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 9. | Ariga H, Nemoto K, Miyazaki S, Yoshioka T, Ogawa Y, Sakayauchi T, Jingu K, Miyata G, Onodera K, Ichikawa H, Kamei T, Kato S, Ishioka C, Satomi S, Yamada S. Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2009;75:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Kato K, Ito Y, Nozaki I, Daiko H, Kojima T, Yano M, Ueno M, Nakagawa S, Takagi M, Tsunoda S, Abe T, Nakamura T, Okada M, Toh Y, Shibuya Y, Yamamoto S, Katayama H, Nakamura K, Kitagawa Y; Japan Esophageal Oncology Group of the Japan Clinical Oncology Group. Parallel-Group Controlled Trial of Surgery Versus Chemoradiotherapy in Patients With Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology. 2021;161:1878-1886.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Yano T, Muto M, Hattori S, Minashi K, Onozawa M, Nihei K, Ishikura S, Ohtsu A, Yoshida S. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008;40:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Hatogai K, Yano T, Kojima T, Onozawa M, Daiko H, Nomura S, Yoda Y, Doi T, Kaneko K, Ohtsu A. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest Endosc. 2016;83:1130-1139.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Takeuchi H, Ito Y, Machida R, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Okuno T, Hironaka S, Nozaki I, Ura T, Chin K, Kojima T, Seki S, Sakanaka K, Fukuda H, Kitagawa Y; Japan Esophageal Oncology Group of the Japan Clinical Oncology Group. A Single-Arm Confirmatory Study of Definitive Chemoradiation Therapy Including Salvage Treatment for Clinical Stage II/III Esophageal Squamous Cell Carcinoma (JCOG0909 Study). Int J Radiat Oncol Biol Phys. 2022;114:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 14. | Kumagai K, Mariosa D, Tsai JA, Nilsson M, Ye W, Lundell L, Rouvelas I. Systematic review and meta-analysis on the significance of salvage esophagectomy for persistent or recurrent esophageal squamous cell carcinoma after definitive chemoradiotherapy. Dis Esophagus. 2016;29:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | Sasaki M, Tamamura H, Tameshige Y, Azuma Y, Maeda Y, Matsushita K, Sato Y, Takamatsu S, Inoue K, Tabata Y, Yoshimura H, Yamamoto K. Dose Evaluation in 2-Phase Method for Advanced Esophageal Cancer by Hybrid Irradiation Techniques. Int J Part Ther. 2024;11:100010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Makishima H, Ishikawa H, Terunuma T, Hashimoto T, Yamanashi K, Sekiguchi T, Mizumoto M, Okumura T, Sakae T, Sakurai H. Comparison of adverse effects of proton and X-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J Radiat Res. 2015;56:568-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Hirano Y, Onozawa M, Hojo H, Motegi A, Zenda S, Hotta K, Moriya S, Tachibana H, Nakamura N, Kojima T, Akimoto T. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2018;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Shiraishi Y, Xu C, Yang J, Komaki R, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol. 2017;125:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Cui Y, Pan Y, Li Z, Wu Q, Zou J, Han D, Yin Y, Ma C. Dosimetric analysis and biological evaluation between proton radiotherapy and photon radiotherapy for the long target of total esophageal squamous cell carcinoma. Front Oncol. 2022;12:954187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Oonsiri S, Kitpanit S, Kannarunimit D, Chakkabat C, Lertbutsayanukul C, Prayongrat A. Comparison of intensity modulated proton therapy beam configurations for treating thoracic esophageal cancer. Phys Imaging Radiat Oncol. 2022;22:51-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Barsky AR, George J, Wroe AJ, Mittauer KE, Kaiser A, Herrera R, Yu J, Gutierrez AN, Alvarez D, McCulloch J, Kasper ME, Mehta MP, Chuong MD. Dosimetric comparison of magnetic resonance-guided radiation therapy, intensity-modulated proton therapy and volumetric-modulated arc therapy for distal esophageal cancer. Med Dosim. 2024;49:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Wang X, Palaskas NL, Yusuf SW, Abe JI, Lopez-Mattei J, Banchs J, Gladish GW, Lee P, Liao Z, Deswal A, Lin SH. Incidence and Onset of Severe Cardiac Events After Radiotherapy for Esophageal Cancer. J Thorac Oncol. 2020;15:1682-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Macomber MW, Bowen SR, Gopan O, Yeung R, Apisarnthanarax S, Zeng J, Patel S. Heart Dose and Outcomes in Radiation Treatment for Esophageal Cancer. Cureus. 2018;10:e2378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Choi JH, Lee JM, Kim MS, Lee Y, Suh YG, Lee SU, Lee DY, Oh ES, Kim TH, Moon SH. A Comparative Analysis of Photon versus Proton Beam Therapy in Neoadjuvant Concurrent Chemoradiotherapy for Intrathoracic Squamous Cell Carcinoma of the Esophagus at a Single Institute. Cancers (Basel). 2022;14:2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Suh YG, Bayasgalan U, Kim HT, Lee JM, Kim MS, Lee Y, Lee DY, Lee SU, Kim TH, Moon SH. Photon Versus Proton Beam Therapy for T1-3 Squamous Cell Carcinoma of the Thoracic Esophagus Without Lymph Node Metastasis. Front Oncol. 2021;11:699172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Bhangoo RS, DeWees TA, Yu NY, Ding JX, Liu C, Golafshar MA, Rule WG, Vora SA, Ross HJ, Ahn DH, Beamer SE, Jaroszewski DE, Hallemeier CL, Liu W, Ashman JB, Sio TT. Acute Toxicities and Short-Term Patient Outcomes After Intensity-Modulated Proton Beam Radiation Therapy or Intensity-Modulated Photon Radiation Therapy for Esophageal Carcinoma: A Mayo Clinic Experience. Adv Radiat Oncol. 2020;5:871-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Xi M, Xu C, Liao Z, Chang JY, Gomez DR, Jeter M, Cox JD, Komaki R, Mehran R, Blum MA, Hofstetter WL, Maru DM, Bhutani MS, Lee JH, Weston B, Ajani JA, Lin SH. Comparative Outcomes After Definitive Chemoradiotherapy Using Proton Beam Therapy Versus Intensity Modulated Radiation Therapy for Esophageal Cancer: A Retrospective, Single-Institutional Analysis. Int J Radiat Oncol Biol Phys. 2017;99:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Warren S, Hurt CN, Crosby T, Partridge M, Hawkins MA. Potential of Proton Therapy to Reduce Acute Hematologic Toxicity in Concurrent Chemoradiation Therapy for Esophageal Cancer. Int J Radiat Oncol Biol Phys. 2017;99:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Lin SH, Hobbs BP, Verma V, Tidwell RS, Smith GL, Lei X, Corsini EM, Mok I, Wei X, Yao L, Wang X, Komaki RU, Chang JY, Chun SG, Jeter MD, Swisher SG, Ajani JA, Blum-Murphy M, Vaporciyan AA, Mehran RJ, Koong AC, Gandhi SJ, Hofstetter WL, Hong TS, Delaney TF, Liao Z, Mohan R. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J Clin Oncol. 2020;38:1569-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 31. | Zhou P, Du Y, Zhang Y, Zhu M, Li T, Tian W, Wu T, Xiao Z. Efficacy and Safety in Proton Therapy and Photon Therapy for Patients With Esophageal Cancer: A Meta-Analysis. JAMA Netw Open. 2023;6:e2328136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 32. | Wang X, van Rossum PSN, Chu Y, Hobbs BP, Grassberger C, Hong TS, Liao Z, Yang J, Zhang X, Netherton T, Mohan R, Lin SH. Severe Lymphopenia During Chemoradiation Therapy for Esophageal Cancer: Comprehensive Analysis of Randomized Phase 2B Trial of Proton Beam Therapy Versus Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2024;118:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 33. | Fang P, Shiraishi Y, Verma V, Jiang W, Song J, Hobbs BP, Lin SH. Lymphocyte-Sparing Effect of Proton Therapy in Patients with Esophageal Cancer Treated with Definitive Chemoradiation. Int J Part Ther. 2018;4:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, Mehran RJ, Hofstetter WL, Blum-Murphy M, Ajani JA, Komaki R, Minsky B, Mohan R, Hsu CC, Hobbs BP, Lin SH. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 35. | Ebrahimi S, Lim G, Liu A, Lin SH, Ellsworth SG, Grassberger C, Mohan R, Cao W. Radiation-Induced Lymphopenia Risks of Photon Versus Proton Therapy for Esophageal Cancer Patients. Int J Part Ther. 2021;8:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Sumiya T, Ishikawa H, Hiroshima Y, Nakamura M, Murakami M, Mizumoto M, Okumura T, Sakurai H. The impact of lymphopenia during chemoradiotherapy using photons or protons on the clinical outcomes of esophageal cancer patients. J Radiat Res. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Routman DM, Garant A, Lester SC, Day CN, Harmsen WS, Sanheuza CT, Yoon HH, Neben-Wittich MA, Martenson JA, Haddock MG, Hallemeier CL, Merrell KW. A Comparison of Grade 4 Lymphopenia With Proton Versus Photon Radiation Therapy for Esophageal Cancer. Adv Radiat Oncol. 2019;4:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Zhu C, Mohan R, Lin SH, Jun G, Yaseen A, Jiang X, Wang Q, Cao W, Hobbs BP. Identifying Individualized Risk Profiles for Radiotherapy-Induced Lymphopenia Among Patients With Esophageal Cancer Using Machine Learning. JCO Clin Cancer Inform. 2021;5:1044-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Dai D, Tian Q, Yu G, Shui Y, Jiang H, Wei Q. Severe Radiation-Induced Lymphopenia Affects the Outcomes of Esophageal Cancer: A Comprehensive Systematic Review and Meta-Analysis. Cancers (Basel). 2022;14:3024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | DeCesaris CM, Berger M, Choi JI, Carr SR, Burrows WM, Regine WF, Simone CB 2nd, Molitoris JK. Pathologic complete response (pCR) rates and outcomes after neoadjuvant chemoradiotherapy with proton or photon radiation for adenocarcinomas of the esophagus and gastroesophageal junction. J Gastrointest Oncol. 2020;11:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Ogawa K, Ishikawa H, Toyozumi T, Noma K, Kono K, Hojo H, Tamamura H, Azami Y, Ishida T, Nabeya Y, Iwata H, Araya M, Tokumaru S, Maruo K, Oda T, Matsubara H. Comparison of proton-based definitive chemoradiotherapy and surgery-based therapy for esophageal squamous cell carcinoma: a multi-center retrospective Japanese cohort study. Esophagus. 2024;21:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Isozaki T, Ishikawa H, Yamada S, Nabeya Y, Minashi K, Murakami K, Matsubara H. Outcomes of definitive carbon-ion radiotherapy for cT1bN0M0 esophageal squamous cell carcinoma. Esophagus. 2024;21:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Okonogi N, Hashimoto T, Ishida M, Ohno T, Terunuma T, Okumura T, Sakae T, Sakurai H. Designed-seamless irradiation technique for extended whole mediastinal proton-beam irradiation for esophageal cancer. Radiat Oncol. 2012;7:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | McKenzie E, Zhang S, Zakariaee R, Guthier CV, Hakimian B, Mirhadi A, Kamrava M, Padda SK, Lewis JH, Nikolova A, Mak RH, Atkins KM. Left Anterior Descending Coronary Artery Radiation Dose Association With All-Cause Mortality in NRG Oncology Trial RTOG 0617. Int J Radiat Oncol Biol Phys. 2023;115:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 45. | van den Boorn HG, Stroes CI, Zwinderman AH, Eshuis WJ, Hulshof MCCM, van Etten-Jamaludin FS, Sprangers MAG, van Laarhoven HWM. Health-related quality of life in curatively-treated patients with esophageal or gastric cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;154:103069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 46. | Dai D, Tian Q, Shui Y, Li J, Wei Q. The impact of radiation induced lymphopenia in the prognosis of head and neck cancer: A systematic review and meta-analysis. Radiother Oncol. 2022;168:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Venkatesulu BP, Chan DP, Giridhar P, Upadhyay R, Sharma A, Elghazawy H, Elumalai T, V P, Mallick S, Hsieh CE. A systematic review and meta-analysis of the impact of radiation-related lymphopenia on outcomes in pancreatic cancer. Future Oncol. 2022;18:1885-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Cao H, Yan H, Bai S, Gu B. Radiation-induced lymphopenia and the survival of women with cervical cancer: a meta-analysis. J Obstet Gynaecol. 2023;43:2194991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Ogawa K, Ishikawa H, Hisakura K, Hiroshima Y, Moriwaki T, Yamada T, Yamamoto Y, Akashi Y, Owada Y, Ohara Y, Enomoto T, Furuya K, Doi M, Shimomura O, Takahashi K, Hashimoto S, Sakurai H, Oda T. Retrospective analysis of neoadjuvant chemotherapy followed by surgery versus definitive chemoradiotherapy with proton beam for locally advanced esophageal squamous cell carcinoma. Int J Clin Oncol. 2021;26:1856-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Taioli E, Schwartz RM, Lieberman-Cribbin W, Moskowitz G, van Gerwen M, Flores R. Quality of Life after Open or Minimally Invasive Esophagectomy in Patients With Esophageal Cancer-A Systematic Review. Semin Thorac Cardiovasc Surg. 2017;29:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, Mamon HJ, Moehler M, Fu X, Cho BC, Bordia S, Bhagia P, Shih CS, Desai A, Enzinger P. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol. 2021;17:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 52. | Colomb A, Allignet B, Lamkhioued M, Swalduz A, Falchero L, Kienlen A, Duruisseaux M, Moncharmont C. Prognostic value of neutrophil to lymphocyte ratio and lymphocyte counts before durvalumab consolidation after radio-chemotherapy in locally advanced non-small cell lung cancer. Radiat Oncol. 2024;19:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | Zhang Y, Huang C, Li S. Influence of treatment-related lymphopenia on the efficacy of immune checkpoint inhibitors in lung cancer: a meta-analysis. Front Oncol. 2023;13:1287555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/