Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.106487
Revised: March 25, 2025
Accepted: April 18, 2025
Published online: July 27, 2025
Processing time: 140 Days and 4.2 Hours
Neoadjuvant chemotherapy improves the resection rate and reduces postope
To evaluate the safety and efficacy of Baohe Pingwei powder combined with neoadjuvant chemotherapy in postoperative patients with GC and to provide evidence-based medical evidence for the treatment of postoperative patients with GC with integrated traditional Chinese and Western medicine.
A retrospective analysis was conducted on 80 postoperative patients with GC admitted to the Department of Gastroenterology of our hospital and treated between May 2024 and November 2024. According to different treatment methods, they were divided into a control group (54 patients received S-1 + oxaliplatin chemotherapy 4 weeks after surgery) and a study group (26 cases were combined with Baohe Pingwei powder combined with S-1 + oxaliplatin). Clinical data were collected to compare the differences in objective response rate (ORR), disease control rate (DCR), progression-free survival, overall survival, and adverse reactions of patients with GC after surgery under different treatment methods. Further based on the control of GC, patients were divided into an effective group (62 cases) and an ineffective group (18 cases). The relationship between Baohe Pingwei powder and clinical efficacy was analyzed through univariate and multivariate logistic regression analysis as well as a multivariate Cox risk model.
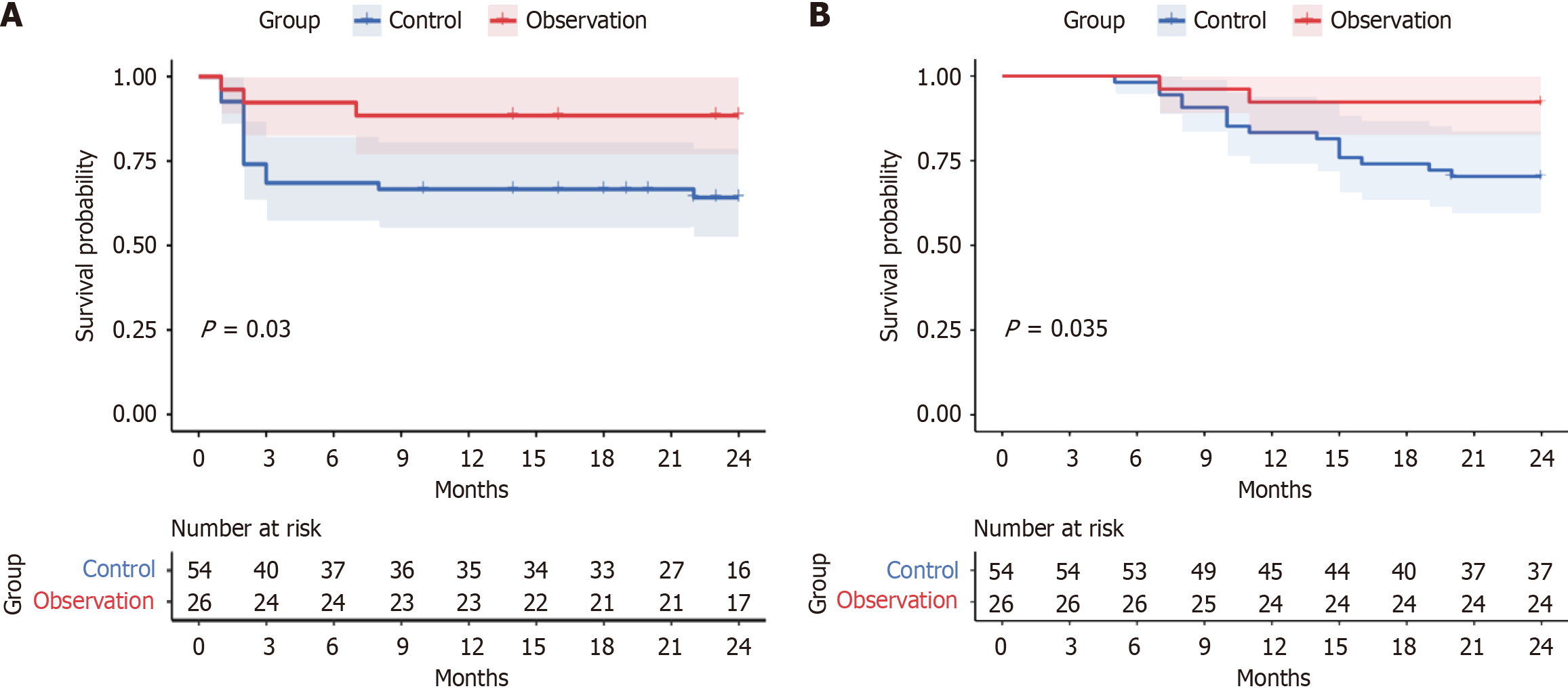
The baseline characteristics including age, gender, and other demographic factors showed no significant differences between the control and observation groups (P > 0.05). In the observation group, there were 24 cases of effective treatment and 2 cases of ineffective treatment, with an ORR of 84.62% and a DCR of 92.31%. In the control group, there were 38 cases of effective treatment and 16 cases of ineffective treatment, with an ORR of 46.30% and a DCR of 70.37%. The treatment effect of the observation group was significantly higher than that of the control group (P < 0.05). The Kaplan Meier curve showed that the risk of tumor recurrence and death in the observation group was significantly reduced compared to the control group (log rank P = 0.030 and P = 0.035, respectively). Subsequent stratification based on treatment response identified 62 patients in the effective group and 18 in the ineffective group. Intergroup comparison showed that the effective group had a higher proportion of Baohe Pingwei powder (P = 0.000), and there were statistically significant differences in tumor size, differentiation degree, and post-treatment levels of CD3+, CD4+, CA19-9, CA242, IL-6, IL-10, and TNF-α between the groups (P < 0.05). Further univariate and multivariate logistic analysis revealed that CD3+ and CD4+ T cell levels were significantly associated with treatment efficacy. The use of Baohe Pingwei powder was a protective factor for effective treatment, while CA19-9 and IL-6 levels were independent risk factors for ineffective treatment (P < 0.05). Multivariate Cox proportional hazards model analysis found that without adjusting the model, the risk of ine
The combination therapy of Baohe Pingwei powder with neoadjuvant chemotherapy demonstrated significant clinical benefits in postoperative patients with GC, including improved the ORR, DCR, extended progression-free survival, and overall survival as well as a reduced incidence of treatment-related adverse events. Furthermore, our finding indicated that decreased CD3+ and CD4+ levels along with evaluated CA199 and IL-6 levels served as important biomarkers predicting increased risk of treatment failure in this patient population.
Core Tip: Baohe Pingwei powder when combined with neoadjuvant chemotherapy exhibited significant efficacy in postoperative patients with gastric cancer. This study revealed that the addition of Baohe Pingwei powder enhanced treatment outcomes, improved immune function, and reduced adverse reactions compared with chemotherapy alone. The most innovative finding was the ability of the powder to prolong recurrence-free survival, highlighting its potential as an adjuvant therapy for patients with gastric cancer undergoing surgery.
- Citation: Ma HY, Liu X, Chen YW, Liang J, Wang J, Zhang MM, Yang Q. Baohe Pingwei power plus neoadjuvant chemotherapy for gastric cancer. World J Gastrointest Surg 2025; 17(7): 106487
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/106487.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.106487
Gastric cancer (GC) is a common malignant tumor of the digestive system, ranking fifth in global incidence and fourth in cancer-related mortality, seriously threatening patients’ quality of life[1]. Radical gastrectomy is the primary treatment for GC. This procedure involves surgical removal of gastric lesions and clearance of surrounding lymph nodes to control tumor progression[2]. However, due to the aggressive nature of tumor cells, surgery cannot clear some of the cancer tissue that is difficult to see with the naked eye, and there are disadvantages such as recurrence after surgery. Therefore, further combination adjuvant chemotherapy is needed to prolong the patient survival time[3].
The SOX chemotherapy regimen, consisting of oxaliplatin and tegafur, is commonly used in clinical practice for GC. It can effectively reduce tumor volume and improve clinical staging[4] while also lowering the risk of disease recurrence and metastasis. However, some patients may experience a series of toxic reactions such as gastrointestinal discomfort, decreased immune function, and bone marrow suppression after applying chemotherapy regimens[5], and those side effects affect the treatment effect. In recent years, with the development of traditional Chinese medicine, the application of traditional Chinese medicine-assisted therapy in the treatment of digestive system tumors has become increasingly widespread. Baohe Pingwei powder is a traditional formula composed of Atractylodes macrocephala, Magnolia officinalis, Glycyrrhiza uralensis, and Citri Reticulatae Pericarpium. Previous studies found that Pingwei powder has significant effects in drying dampness, strengthening the spleen, and regulating qi and the stomach, but its application value in the treatment of GC is relatively limited[6].
This study aimed to investigate the therapeutic effects of combining Baohe Pingwei powder with neoadjuvant chemotherapy in postoperative patients with GC. The findings may provide new treatment approaches and theoretical foundations for improving the prognosis of these patients in clinical practice.
Eighty postoperative patients with GC treated in our digestive surgery department from May 2024 to November 2024 were selected as the research subjects. According to different treatment methods, they were divided into two groups: A control group (54 cases) and a study group (26 cases). This study was approved by the hospital ethics committee.
Inclusion criteria: (1) All patients met the diagnostic criteria for GC[7]; (2) According to the indications for radical gastrectomy for GC, radical gastrectomy for GC was performed; (3) The clinical stage was stage II to III; (4) Complete clinical data; and (5) No allergic reaction to Baohe Pingwei powder.
Exclusion criteria: (1) Abnormal liver and kidney function; (2) The patient developed serious complications such as pyloric obstruction, acute gastric perforation, and gastrointestinal bleeding; (3) Comorbid with other tumor diseases; (4) Previously suffered from infectious diseases; and (5) Mental disorders present.
Control group: All patients included in this study received SOX chemotherapy intervention 4 to 6 weeks after radical gastrectomy for GC. On the first day of treatment, oxaliplatin (Shandong Luoxin Pharmaceutical Group Co., Ltd., national drug approval number H20123346, specification 10 mL: 50 mg) was intravenously administered at a dose of 130 mg/m2 with a drip duration ranging from 2-6 h. Starting the next day and continuing until the 14th day, the patient took Tegio (Jiangsu Hengrui Pharmaceutical Co., Ltd., National Medical Products Approval No. H20140137, specification 20 mg). The dosage of medication was determined based on the patient’s body surface area. When the patient’s body surface area exceeded 1.50 m2, 60 mg orally was recommended each time. When the body surface area ranged from 1.25 m2 to 1.50 m2, 50 mg taken orally was recommended each time. When the body surface area was less than 1.25 m2, 40 mg orally was recommended each time. The above doses needed to be taken twice a day, with a treatment cycle of 21 days, for a total of three cycles of treatment.
Observation group: In addition to the treatment plan of the control group, Baohe Pingwei powder was introduced as an adjuvant therapy. The formula for Baohe Pingwei powder includes 25 g, 15 g, 10 g, and 6 g of Atractylodes macrocephala, Magnolia officinalis, Citri Reticulatae Pericarpium, and Licorice, respectively. After boiling the medicinal herbs with water, 400 mg of the juice was extracted. The patient needed to take it once a day in the morning and once in the evening for a complete dose. This treatment lasted for three cycles of chemotherapy.
General information: Gender, age, degree of differentiation, TNM staging, tumor size, tumor location, surgical scope, etc was collected.
Therapeutic efficacy: Efficacy, progression-free survival (PFS, from treatment initiation to disease onset or last follow-up), overall survival (OS, from treatment initiation to patient death), and adverse reactions were collected.
Clinical efficacy: The efficacy was evaluated based on RECIST 1.1[8]. Specifically, if the target lesion completely disappeared and no new lesions appeared, it was judged as complete remission (CR). If the total diameter of the lesion shrank by at least 30% and lasted for ≥ 4 weeks, it was judged as partial remission (PR). If the therapeutic effect did not meet the above two criteria, it was considered stable disease (SD). If the total diameter of the lesion increased by ≥ 20% or new lesions appeared, it was judged as progressive disease. Objective response rate (ORR) is the percentage of the sum of CR and PR cases to the total number of cases. The disease control rate (DCR) is the percentage of the sum of CR, PR, and SD cases to the total number of cases.
Adverse reactions: The incidence of anemia, gastrointestinal discomfort, and bone marrow suppression between the two groups of patients was compared.
Inflammation level: Fasting venous blood samples (4 mL) were collected from all patients before and after treatment, and the supernatant was obtained by centrifugation. The levels of TNF-α, IL-6, and IL-10 in the samples were detected by ELISA.
Immune function: After collection of 4 mL of venous blood from patients, the immune function of the two groups of patients was measured before treatment and after three chemotherapy cycles. Specifically, the levels of CD3+ and CD4+ in the blood samples were measured using an ABcyte type Abbott flow cytometer for accurate measurement.
Tumor markers: In the morning 4 mL of fasting venous blood was collected from the patient. The samples were centrifuged at a speed of 3000 r/min for 10 min, and the upper serum was collected. ELISA was used to detect the levels of CA19-9 and CA242 in the sample, following the instructions of the corresponding reagent kit.
Follow-up: Starting from the day the third course of treatment was completed, the patient was followed up for a period of 2 years through outpatient follow-up and telephone calls. The follow-up content included the patient’s survival and tumor recurrence, and the PFS and OS time was calculated. The follow-up endpoint was set as the patient’s all-cause death or the end of the follow-up time.
The endpoint of this study was the clinical treatment and incidence of adverse reactions in postoperative patients with GC after 3 courses of treatment. Patients who received SOX chemotherapy were included in the control group (54 cases), and patients who received Baohe Pingwei powder combined with SOX chemotherapy were included in the study group (26 cases). Then, based on the disease control status of patients after clinical treatment, patients with disease control (CR, PR, SD) were included in the effective group (62 cases), and patients with progressive disease were included in the ineffective group (18 cases).
The data involved in this study were analyzed using SPSS 26.0 and Stata 16.0 software, and a two-sided test was used. Normality tests on the collected measurement data were performed. For data that conformed to a normal distribution, it was presented as mean ± SD, and independent t-test for intergroup comparison was used. For count data, it was expressed as n (%) and compared between groups using the χ2 test. The PFS and OS curves of postoperative patients with GC in the control group and observation group were plotted using the Kaplan-Meier method, and the differences between the groups were compared using the log rank method. The Cox proportional hazards regression model was used to analyze the relationship between clinical efficacy and Baohe Pingwei powder. Three models were gradually adjusted for confounding factors: Model 1. Unadjusted; Model 2. Adjusting CD3+ and CD4+; and Model 3. Adjusting CD3+, CD4+, CA19-9, and IL-6. The results were represented by hazard ratios (HR) and 95% confidence intervals (CI). The screening of confounding factors was determined based on the results of univariate logistic analysis
Of the 80 patients with GC, 54 cases (67.50%) were included in the control group and 26 cases (32.50%) in the observation group. There were no significant differences in age, gender, tumor location, clinical stage, and other baseline data between the two groups (P > 0.05) (Table 1).
| Characteristic | Control group (n = 54) | Observation group (n = 26) | χ2/t | P value | |
| Age (mean ± SD, years) | 68.85 ± 1.26 | 68.33 ± 1.12 | 1.790 | 0.077 | |
| Gender | Male | 41 (75.93) | 22 (84.62) | 0.792 | 0.374 |
| Female | 13 (24.07) | 4 (15.38) | |||
| Tumor location | Gastric antrum or pylorus | 39 (72.22) | 18 (69.23) | 0.315 | 0.753 |
| Stomach body | 8 (14.81) | 3 (11.54) | |||
| Gastric fundus or cardia | 7 (12.97) | 5 (19.23) | |||
| Clinical staging | Phase II | 23 (42.59) | 16 (61.54) | 2.521 | 0.112 |
| Phase III | 31 (57.41) | 10 (38.46) | |||
| Surgical scope | Partial excision | 25 (46.30) | 17 (65.38) | 2.564 | 0.109 |
| Fully cut | 29 (53.70) | 9 (24.62) | |||
| Tumor size | < 2 cm | 8 (14.81) | 7 (26.92) | 0.672 | 0.502 |
| 2-5 cm | 36 (66.67) | 14 (53.85) | |||
| > 5 cm | 10 (18.52) | 5 (19.23) | |||
| Degree of differentiation | High | 2 (3.70) | 2 (7.69) | 0.455 | 0.649 |
| In | 16 (29.63) | 8 (30.77) | |||
| Low | 36 (66.67) | 16 (61.54) | |||
| Smoke | Have | 23 (42.59) | 10 (38.46) | 0.124 | 0.725 |
| Without | 31 (57.41) | 16 (61.54) | |||
| Drink | Have | 36 (66.67) | 19 (73.08) | 0.336 | 0.562 |
| Without | 18 (33.33) | 7 (26.92) | |||
| Hypertension | Have | 22 (40.74) | 14 (53.85) | 1.218 | 0.270 |
| Without | 32 (59.26) | 12 (46.15) | |||
| Diabetes mellitus | Have | 17 (31.48) | 13 (50.00) | 2.568 | 0.109 |
| Without | 37 (68.52) | 13 (50.00) | |||
In the observation group, there were 24 cases of effective treatment and 2 cases of ineffective treatment, with an ORR of 84.62% and a DCR of 92.31%. In the control group, there were 38 cases of effective treatment and 16 cases of ineffective treatment, with an ORR of 46.30% and a DCR of 70.37%. The treatment effect of the observation group was significantly higher than that of the control group (P < 0.05) (Table 2). The Kaplan-Meier curves (Figure 1) indicated that the risk of tumor recurrence and death in the observation group was significantly reduced compared with the control group (log rank P = 0.030, 0.035). Further comparison was made on the incidence of adverse reactions in postoperative patients with GC. Among the patients in the observation group, 7 cases (26.92%) experienced adverse reactions, while in the control group, 28 cases (51.85%) experienced adverse reactions. The incidence of adverse reactions in the observation group was significantly lower than that in the control group (χ2 = 4.432, P = 0.035).
| Group | CR | PR | SD | PD | ORR | DCR |
| Control group (n = 54) | 11 (20.37) | 14 (25.93) | 13 (24.07) | 16 (29.63) | 25 (46.30) | 38 (70.37) |
| Observation group (n = 26) | 13 (50.00) | 9 (34.62) | 2 (7.69) | 2 (7.69) | 22 (84.62) | 24 (92.31) |
| χ2/t | - | - | - | - | 10.634 | 4.844 |
| P value | - | - | - | - | 0.001 | 0.028 |
According to the patient’s disease control status, they were divided into an effective group (n = 62) and an ineffective group (n = 18). Among them, Baohe Pingwei powder was used in 24 cases (38 cases did not use Baohe Pigwei powder). Two cases in the ineffective group used Baohe Pingwei powder, while 16 cases did not use it. The proportion of the effective group using Baohe Pingwei powder was higher (P = 0.000). Intergroup comparison showed that there were statistically significant differences (P < 0.05) in the use of Baohe Pingwei powder, tumor size, differentiation degree, and post-treatment levels of CD3+, CD4+, CA19-9, CA242, IL-6, IL-10, and TNF-α between groups, while other indicators showed no significant differences (P > 0.05), as shown in Table 3.
| Characteristic | Total number of cases (n = 80) | Effective (n = 62) | Invalid (n = 18) | χ2/t | P value | |
| Use Baohe Pingwei powder | 26 (32.50) | 24 (38.71) | 2 (11.11) | 4.843 | 0.029 | |
| Age (mean ± SD, years) | 68.51 ± 1.94 | 68.67 ± 2.13 | 68.02 ± 2.04 | 1.150 | 0.254 | |
| Gender | Male | 63 (78.75) | 50 (80.65) | 13 (72.22) | 0.195 | 0.659 |
| Female | 17 (21.25) | 12 (19.35) | 5 (27.78) | |||
| Tumor location | Gastric antrum or pylorus | 57 (71.25) | 45 (72.58) | 12 (66.67) | 0.456 | 0.648 |
| Stomach body | 11 (13.75) | 8 (12.90) | 3 (16.67) | |||
| Gastric fundus or cardia | 12 (15.00) | 9 (14.52) | 3 (16.67) | |||
| Clinical staging | Phase II | 39 (48.75) | 31 (50.00) | 8 (44.44) | 0.172 | 0.678 |
| Phase III | 41 (51.25) | 31 (50.00) | 10 (55.56) | |||
| Surgical scope | Partial excision | 42 (52.50) | 33 (53.23) | 9 (50.00) | 0.058 | 0.809 |
| Fully cut | 38 (47.50) | 29 (46.77) | 9 (50.00) | |||
| Tumor size | < 2 cm | 15 (18.75) | 13 (20.97) | 2 (11.11) | 2.172 | 0.030 |
| 2-5 cm | 50 (62.50) | 41 (66.13) | 9 (50.00) | |||
| > 5 cm | 15 (18.75) | 8 (12.90) | 7 (38.89) | |||
| Degree of differentiation | High | 4 (5.00) | 2 (3.23) | 2 (11.11) | 2.675 | 0.008 |
| In | 24 (30.00) | 15 (24.19) | 9 (50.00) | |||
| Low | 52 (65.00) | 45 (72.58) | 7 (38.89) | |||
| Smoke | Have | 33 (41.25) | 27 (43.55) | 6 (33.33) | 0.601 | 0.438 |
| Without | 47 (58.75) | 35 (56.45) | 12 (66.67) | |||
| Drink | Have | 55 (68.75) | 44 (70.97) | 11 (61.11) | 0.601 | 0.427 |
| Without | 25 (31.25) | 18 (29.03) | 7 (38.89) | |||
| Hypertension | Have | 36 (45.00) | 26 (41.94) | 10 (55.56) | 1.667 | 0.197 |
| Without | 44 (55.00) | 37 (59.68) | 7 (38.89) | |||
| Diabetes mellitus | Have | 30 (37.50) | 24 (38.71) | 6 (33.33) | 0.172 | 0.678 |
| Without | 50 (62.50) | 38 (61.29) | 12 (66.67) | |||
| CD3+ (mean ± SD, %) | Before treatment | 56.59 ± 1.67 | 57.36 ± 2.34 | 56.84 ± 2.01 | 0.0855 | 0.395 |
| After treatment | 37.34 ± 1.58 | 45.67 ± 2.03 | 32.16 ± 1.35 | 26.521 | 0.000 | |
| CD4+ (mean ± SD, %) | Before treatment | 39.46 ± 1.26 | 39.15 ± 2.06 | 40.12 ± 2.37 | 1.700 | 0.082 |
| After treatment | 25.64 ± 1.03 | 30.58 ± 1.64 | 21.34 ± 1.42 | 21.642 | 0.000 | |
| CA19-9 (mean ± SD, %) | Before treatment | 71.69 ± 2.45 | 71.64 ± 2.43 | 72.87 ± 2.16 | 1.935 | 0.057 |
| After treatment | 36.98 ± 2.06 | 35.69 ± 1.26 | 38.59 ± 2.16 | 7.207 | 0.000 | |
| CA242 (U/mL) | Before treatment | 44.27 ± 0.28 | 44.17 ± 0.25 | 44.15 ± 0.03 | 0.337 | 0.737 |
| After treatment | 26.31 ± 1.34 | 37.12 ± 2.13 | 12.24 ± 0.15 | 49.299 | 0.000 | |
| IL-6 (mean ± SD, ng/mL) | Before treatment | 428.94 ± 2.69 | 429.67 ± 2.16 | 429.32 ± 2.15 | 0.606 | 0.546 |
| After treatment | 317.65 ± 2.36 | 310.84 ± 2.67 | 328.12 ± 2.37 | 24.751 | 0.000 | |
| IL-10 (mean ± SD, ng/mL) | Before treatment | 92.84± 1.52 | 92.98 ± 1.76 | 92.48 ± 1.38 | 1.109 | 0.271 |
| After treatment | 234.18 ± 2.68 | 61.08 ± 1.26 | 86.41 ± 1.28 | 74.824 | 0.000 | |
| TNF-α (mean ± SD, ng/mL) | Before treatment | 321.23 ± 2.03 | 321.08 ± 0.99 | 321.54 ± 2.31 | 1.237 | 0.220 |
| After treatment | 261.33 ± 2.07 | 220.09 ± 1.28 | 319.26 ± 2.17 | 243.829 | 0.000 |
Taking the effectiveness of patient treatment as the dependent variable (assigned values: Effective = 0, ineffective = 1), the indicators with significant differences (P < 0.05) in the clinical data of patients with different therapeutic effects were included, including count data on the use of Baohe Pingwei powder (assigned values: Use = 0, not use = 1), tumor size (assigned values: < 2 cm = 1, 2-5 cm = 2, > 5 cm = 3), and differentiation degree (assigned values: Low = 1, medium = 2, high = 3). After treatment, the measurement data of CD3+, CD4+, CA199, CA242, IL-6, IL-10, and TNF-α were substituted with the original values.
Univariate logistic regression found that the degree of differentiation by CD3+, CD4+ levels were closely related to the occurrence of ineffective treatment (P < 0.05). The use of Baohe Pingwei powder, CA19-9, and IL-6 levels were also closely related to the occurrence of ineffective treatment (P < 0.05). When included in the multivariate logistic regression analysis, it was found that CD3+, CD4+ levels and the use of Baohe Pingwei powder were protective factors for effective treatment, while CA19-9 and IL-6 levels were independent risk factors for ineffective treatment (P < 0.05). The use of Baohe Pingwei powder was a protective factor for effective treatment, while CA19-9 and IL-6 levels were independent risk factors for ineffective treatment (P < 0.05) (Table 4).
| Influence factor | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Tumor size | 1.354 (1.264-1.469) | 0.071 | - | - |
| Degree of differentiation | 1.352 (1.028-1.574) | 0.028 | 1.282 (1.019-1.408) | 0.135 |
| CD3+ | 0.726 (0.628-0.881) | 0.036 | 0.072 (0.011-0.145) | 0.026 |
| CD4+ | 0.883 (0.708-0.934) | 0.024 | 0.424 (0.285-0.662) | 0.033 |
| Baohe Pingwei powder | 0.528 (0.482-0.711) | 0.037 | 0.233 (0.162-0.378) | 0.019 |
| CA19-9 | 1.269 (1.112-1.420) | 0.044 | 1.523 (1.012-1.963) | 0.007 |
| CA242 | 1.046 (0.823-1.349) | 0.258 | - | - |
| IL-6 | 1.226 (1.022-1.406) | 0.016 | 2.026 (1.929-2.244) | 0.016 |
| IL-10 | 1.134 (0.698-1.342) | 0.082 | - | - |
| TNF-α | 1.308 (0.896-1.653) | 0.108 | - | - |
The Cox proportional hazards model was used to further analyze the relationship between CD3+, CD4+, CA19-9, IL-6, and treatment failure. Without adjusting the Cox regression analysis model, it was found that with the increase of CD3+ and CD4+ and the decrease of CA19-9 and IL-6, the risk of treatment failure in patients significantly decreased (group 1 as a reference; group 2 HR: 0.624, 95%CI: 0.437-0.986, P = 0.019). After further adjusting for tumor size, CA242, IL-10, and TNF-α, the Cox regression results still showed that with the increase of CD3+ and CD4+ and the decrease of CA19-9 and IL-6, the risk of ineffective treatment in patients was significantly reduced (group 1 as a reference; group 2 HR: 0.727, 95%CI: 0.421-0.833, P = 0.008). After further adjusting for confounding factors such as Baohe Pingwei powder and differentiation degree, Cox regression results showed an increased risk of treatment failure. With the decrease of CD3+ and CD4+ and the increase of CA19-9 and IL-6, the risk of treatment failure in patients significantly increased (group 1 as a reference; group 2 HR: 1.439; 95%CI: 1.208-1.614, P = 0.006) (Table 5).
| Model 1 | Model 2 | Model 3 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Ineffective group | Ref | Ref | Ref | |||
| Effective group | 0.624 (0.437-0.986) | 0.019 | 0.727 (0.421-0.833) | 0.008 | 1.439 (1.208-1.614) | 0.006 |
| CD3+ | 0.422 (0.309-0.528) | 0.026 | 0.608 (0.417-0.839) | 0.000 | 0.619 (0.455-0.808) | 0.000 |
| CD4+ | 0.122 (0.019-0.321) | 0.021 | 0.218 (0.107-0.474) | 0.044 | 0.344 (0.274-0.541) | 0.042 |
| CA199 | 1.242 (1.024-1.842) | 0.005 | 1.789 (1.424-1.872) | 0.028 | 1.678 (1.564-1.971) | 0.004 |
| IL-6 | 1.341 (1.276-1.452) | 0.038 | 1.541 (1.442-1.772) | 0.001 | 1.841 (1.652-1.938) | 0.000 |
GC is a common digestive tract malignant tumor disease induced by uncontrolled proliferation of gastric mucosal epithelial cells, and its survival period is closely related to tumor staging. The prognosis of GC is closely associated with tumor stage, with early-stage cases exhibiting a 5-year survival rate of up to 90%. However, when the cancer infiltrates the submucosal or serosal layer, the 5-year survival rate drops significantly to 20%[9]. The combination of radical gastrectomy and neoadjuvant chemotherapy is currently an important treatment for GC, especially advanced GC. However, in clinical practice, the recurrence rate of GC is relatively high, and the survival time of patients is not ideal.
This study demonstrated that the integration of Baohe Pingwei powder with neoadjuvant chemotherapy significantly improved clinical efficacy, prolonging both OS and PFS in patients. In addition, the study also grouped 80 postoperative patients with GC based on their treatment efficacy and found that the proportion of patients in the ineffective group who used Baohe Pingwei powder after GC surgery was significantly lower. Baohe Pingwei powder was an independent protective factor for effective treatment. The reason for the analysis was that Magnolia officinalis is present in the combination of Baohe Pingwei powder, and its main active ingredient Magnolol has an anti-GC function[10].
When combined with cisplatin, it can reduce the proliferation activity of MKN-45 GC cells, upregulate Bax expression levels, inhibit matrix metalloproteinase-9 expression, and reduce the resistance of MKN-45 GC cells to cisplatin[11]. Additionally, magnolol activates the c-Jun N-terminal kinase signaling pathway, upregulates caspase-3, and induces apoptosis in SGC-7901 GC cells[12]. In addition, the effective extract of Atractylodes lancea (Thumb.), a component of its formula, can inhibit the phosphorylation of the Akt and IκB-α pathway proteins, thereby reducing the expression levels of iNOS and TNF-α mRNA and inhibiting the NF-κB signaling pathway[13]. These mechanisms may alleviate postoperative gastric inflammation, modulate the tumor microenvironment, and ultimately enhance treatment efficacy.
CA19-9 is a common clinical biomarker for gastrointestinal tumors, and its level is closely related to tumor cell activity and malignancy. In this study, further univariate and multivariate logistic regression analysis was conducted on patients in the effective and ineffective groups. The results showed that, in addition to Baohe Pingwei powder, CD3+ and CD4+ were also protective factors for effective treatment, while CA19-9 and IL-6 were risk factors for ineffective treatment. This suggests that Baohe Pingwei powder may enhance immune function, suppress tumor markers, and mitigate inflammation, thereby impeding tumor progression.
The components in Baohe Pingwei powder have multiple effective ingredients that regulate the body’s immune and inflammatory responses. Glycyrrhetinic acid can regulate the balance of Th1/Th2 cells, promote regulatory T cell transformation, upregulate CD4+CD25+Foxp3+cell levels, and regulate the body’s immune balance[14]. The flavonoids in Citri Reticulatae Pericarpium can stimulate the expression of CD3+T and CD4+T in mice, thereby increasing the number of CD4+ T/CD8+ T cells, enhancing the body’s immunity, increasing CD3+ and CD4+ expression, hindering tumor cell immune escape, and thus improving the therapeutic effect[15]. At the same time, Hesperidin can downregulate the expression of matrix metalloproteinase-9 by inhibiting the IL-6-mediated STAT3 signaling pathway, thereby inhibiting epithelial mesenchymal transition[16], weakening the invasion of tumor cells, reducing the release of CA19-9 on the cell surface caused by tumor cell invasion, downregulating CA19-9 levels, and reducing tumor invasion ability[17], ultimately leading to better treatment outcomes for postoperative patients with GC in the observation group. In GC cells, tumor cells with low differentiation levels exhibit high levels of CA19-9 and stronger invasiveness. Previous studies have also shown[18] that patients with high histological differentiation have a better prognosis than those with low differentiation. However, this study did not find that differentiation level is an independent risk factor affecting the efficacy of GC treatment, and this result may be related to the small sample size included.
In addition, this study also found that the incidence of adverse reactions in the observation group was significantly lower than that in the control group, indicating that Baohe Pingwei powder can alleviate SOX chemotherapy-induced toxicity while demonstrating higher safety. The reason for this is that Atractylodes lancea (Thumb.) can reduce the expression of MLCK and inhibit p-MLC2 by inhibiting the SCF/c-kit pathway, resulting in a decrease in the release of downstream TNF-α, IL-6, and VIP. At the same time, it upregulates the expression of ZO-1 and occludin, alleviates gastrointestinal mucosal inflammatory damage, and repairs the intestinal mucosal barrier[19], which to some extent alleviates the gastrointestinal reactions caused by SOX chemotherapy and reduces the incidence of adverse reactions. In addition, 18 beta glycyrrhetinic acid in Glycyrrhiza uralensi can stimulate the production of regulatory T cells, restore the balance of CD4+ T cell subsets, and reduce high mobility group box-1 and antiplatelet antibody levels, hindering the occurrence of autoimmune thrombocytopenia caused by bone marrow suppression[20].
Although this study confirmed the combination of Baohe Pingwei powder combined with SOX chemotherapy to improve the clinical efficacy of treating postoperative GC patients and to reduce the occurrence of adverse reactions, there were still shortcomings in this study: (1) This study was a single center retrospective study with a small sample size that may affect the generalizability and extrapolation of the research results; (2) This study had a short follow-up period and was unable to determine the long-term prognosis of postoperative patients with GC treated with Baohe Pingwei powder combined with SOX chemotherapy; and (3) This study only explored the observation of various clinical indicators before and after chemotherapy in postoperative patients with GC and aimed to explore the specific mechanism of Baohe Pingwei powder in the treatment of postoperative patients with GC. Therefore, in future research, further multicenter and multi-indicator large sample observations can be conducted, and long-term follow-up can be carried out to improve the reliability and authenticity of the research results.
Our findings demonstrated that the adjunctive use of Baohe Pingwei powder with SOX chemotherapy significantly enhanced clinical outcomes in postoperative patients with GC. This therapy not only prolonged both FPS and OS but also reduced treatment-related adverse events. Importantly, Baohe Pingwei powder emerged as an independent protective factor in the clinical treatment of postoperative GC. These compelling SOX chemotherapy data represent a promising therapeutic strategy worthy of broader clinical adoption for postoperative patients with GC.
We are deeply indebted to all the patients who participated in this study as well as the nurses and medical staff for their dedication to patient care and data collection. Special thanks to our colleagues in the Department of Spleen and Stomach Diseases and the Clinical Medicine Research Center for their invaluable insights and collaborative efforts.
| 1. | Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 488] [Reference Citation Analysis (2)] |
| 2. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1194] [Article Influence: 238.8] [Reference Citation Analysis (9)] |
| 3. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 599] [Reference Citation Analysis (5)] |
| 4. | Wang X, Li S, Sun Y, Li K, Shen X, Xue Y, Liang P, Li G, Chen L, Zhao Q, Li G, Fu W, Liang H, Xin H, Suo J, Fang X, Zheng Z, Xu Z, Chen H, Zhou Y, He Y, Huang H, Zhu L, Yang K, Ji J, Ye Y, Zhang Z, Li F, Wang X, Tian Y, Park S, Chen L. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer. 2021;21:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Yu P, Zhu S, Pu Y, Cai B, Ma X, Zhang C. Efficacy and safety evaluation of PSOX, DOF and SOX regimens as neoadjuvant chemotherapy for advanced gastric cancer. Future Oncol. 2022;18:4483-4492. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Li G, Wang X, Luo L, Zhang H, Song X, Zhang J, Liu D. Identification of chemical constituents of Qingjin Yiqi granules and comparative study on pharmacokinetics of 23 main bioactive components in normal and Lung-Qi deficiency rats by UPLC-MS/MS method. J Chromatogr B Analyt Technol Biomed Life Sci. 2023;1226:123802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 1174] [Article Influence: 293.5] [Reference Citation Analysis (0)] |
| 8. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22855] [Article Influence: 1344.4] [Reference Citation Analysis (1)] |
| 9. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 239] [Reference Citation Analysis (3)] |
| 10. | Wang X, Liu Q, Fu Y, Ding RB, Qi X, Zhou X, Sun Z, Bao J. Magnolol as a Potential Anticancer Agent: A Proposed Mechanistic Insight. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 11. | Naghashpour M, Dayer D, Karami H, Naghashpour M, Moghadam MT, Haeri SMJ, Suzuki K. Evaluating the Magnolol Anticancer Potential in MKN-45 Gastric Cancer Cells. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Chen Y, He X, Feng D, Li S. Compare the Effects of Magnolol on Gastric Cancer Cells Through c-Jun N-Terminal Kinase Signaling Pathway and Gold Magnetic. J Nanosci Nanotechnol. 2021;21:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Hossen MJ, Amin A, Fu XQ, Chou JY, Wu JY, Wang XQ, Chen YJ, Wu Y, Li J, Yin CL, Liang C, Chou GX, Yu ZL. The anti-inflammatory effects of an ethanolic extract of the rhizome of Atractylodes lancea, involves Akt/NF-κB signaling pathway inhibition. J Ethnopharmacol. 2021;277:114183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Yamamoto Y, Uchiyama M, Iguchi K, Kawai K, Imazuru T, Kawamura M, Shimokawa T. Effects of Glycyrrhizic Acid in Licorice on Prolongation of Murine Cardiac Allograft Survival. Transplant Proc. 2022;54:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Lyu SW, Li Y, Yu X, Guo YY, Yang DY, Sun S, Shang EY. [Contributions of flavonoids from citri reticulatae pericarpium to gastric hormones, CD3~+ and TFF3 mRNA expression in rats with spleen deficiency intervened by Liujunzi Decoction]. Zhongguo Zhong Yao Za Zhi. 2022;47:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Han D, Gong H, Wei Y, Xu Y, Zhou X, Wang Z, Feng F. Hesperidin inhibits lung fibroblast senescence via IL-6/STAT3 signaling pathway to suppress pulmonary fibrosis. Phytomedicine. 2023;112:154680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 17. | Liu C, Deng S, Jin K, Gong Y, Cheng H, Fan Z, Qian Y, Huang Q, Ni Q, Luo G, Yu X. Lewis antigen-negative pancreatic cancer: An aggressive subgroup. Int J Oncol. 2020;56:900-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Yu X, Hu F, Li C, Yao Q, Zhang H, Xue Y. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. Onco Targets Ther. 2018;11:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 19. | Xie Y, Zhan X, Tu J, Xu K, Sun X, Liu C, Ke C, Cao G, Zhou Z, Liu Y. Atractylodes oil alleviates diarrhea-predominant irritable bowel syndrome by regulating intestinal inflammation and intestinal barrier via SCF/c-kit and MLCK/MLC2 pathways. J Ethnopharmacol. 2021;272:113925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Wang H, Yu T, An N, Sun Y, Xu P, Han P, Zhao Y, Wang L, Ni X, Li Y, Li G, Liu Y, Peng J, Hou M, Hou Y. Enhancing regulatory T-cell function via inhibition of high mobility group box 1 protein signaling in immune thrombocytopenia. Haematologica. 2023;108:843-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/