INTRODUCTION
Constipation represents a prevalent clinical condition with significant epidemiological variation. According to epidemiologic studies employing the Rome IV criteria, the global prevalence of constipation is 10.1% [95% confidence interval (CI): 8.7-11.6] in the general population[1], indicating a significant sex disparity, with a greater prevalence among females. Notably, prevalence rates differ substantially across age groups: 14.4% (95%CI: 11.2-17.6) in pediatric populations[2] vs 18.9% (95%CI: 14.7-23.9) among older adults[3]. Of clinical relevance, obstructed defecation syndrome affects approximately 60% of individuals with constipation[4,5], whereas dyssynergic defecation constitutes 50% of chronic constipation cases referred to tertiary care centers[6,7]. Furthermore, according to current literature, up to 44% of patients with constipation have pelvic floor dysfunction[8]. Primary care settings and resource-limited regions lack specialized diagnostic modalities, including defecography and pelvic floor magnetic resonance imaging, and personnel with technical expertise, thus digital rectal examination (DRE) is not part of routine clinical practice. Notably, in even tertiary medical centers, specific imaging procedures are difficult to perform on pregnant patients or those with claustrophobia.
Functional defecation disorders (FDDs) encompass two predominant subtypes: Dyssynergic defecation and inadequate defecatory propulsion. Current evidence has revealed the remarkable diagnostic validity of DRE for detecting dyssynergic defecation, with a pooled sensitivity of 75.0%-93.2%, specificity of 58.7%-87.0%, and positive predictive value of 91%-97%[9-11]. Despite these robust metrics, DRE remains underutilized in both medical education and clinical workflows. Although major gastroenterological societies consistently recommend DRE in constipation evaluation, many clinicians omit this examination during patient assessments[9]. Alarmingly, 17% of medical school graduates report never having performed DRE during training[12], whereas only 31% of practicing clinicians express full confidence in their ability to perform DRE[13]. As with most clinical skills, proficiency is strongly correlated with operator experience, which enhances both clinician competence and patient compliance[13]. Critically, experienced practitioners achieve clinically meaningful diagnostic yield through DRE[14], reinforcing its status as an essential component of comprehensive physical examination[15-19]. These findings underscore the urgent need for systematic DRE training integration into medical curricula and primary care competency programs.
In this review, we interrogate the clinical validity of DRE in detecting pathognomonic features of pelvic floor dyssynergia and structural defects underlying FDDs. We also advocate for protocol standardization to enhance diagnostic reproducibility. Concurrently, we delineate tactile assessment limitations and explore pragmatic synergies between DRE and emerging point-of-care technologies to refine diagnostic workflows.
DIAGNOSTIC CRITERIA FOR FDDS
The diagnostic criteria for FDDs (encompassing dyssynergic defecation and inadequate defecatory propulsion) are as follows: (1) Functional constipation with symptoms persisting for ≥ 6 months and/or irritable bowel syndrome with constipation for the past 3 months according to the Rome IV criteria; and (2) Objective documentation of defecatory dysfunction manifesting as at least two of the following pathophysiological findings[20-22]: Abnormal balloon expulsion test indicating impaired rectal evacuation; Anorectal manometry or pelvic floor electromyography revealing paradoxical pelvic floor muscle contraction; Dynamic imaging evidence (e.g., defecography) of inadequate rectal propulsive forces or incomplete rectal emptying.
CLASSIFICATION OF FDDS
FDD classification is rooted in the Rome IV criteria for functional constipation, wherein dyssynergic defecation is subclassified into two pathophysiological subtypes: Structural pelvic floor dysfunction (e.g., rectocele, internal rectal prolapse, and descending perineum syndrome), characterized by impaired rectal motility, and nonrelaxing pelvic floor dysfunction (e.g., puborectalis muscle dyssynergia and spastic pelvic floor syndrome), resulting from paradoxical contraction of the pelvic floor muscles during defecation[23]. A third distinct entity, inadequate defecatory propulsion, refers to insufficient rectal contractile forces during evacuation, which constitutes the focus of this review regarding DRE applications. Notably, overlap between these subtypes is frequently observed in clinical practice[24,25].
DRE FOR FDDS
Preprocedural preparation
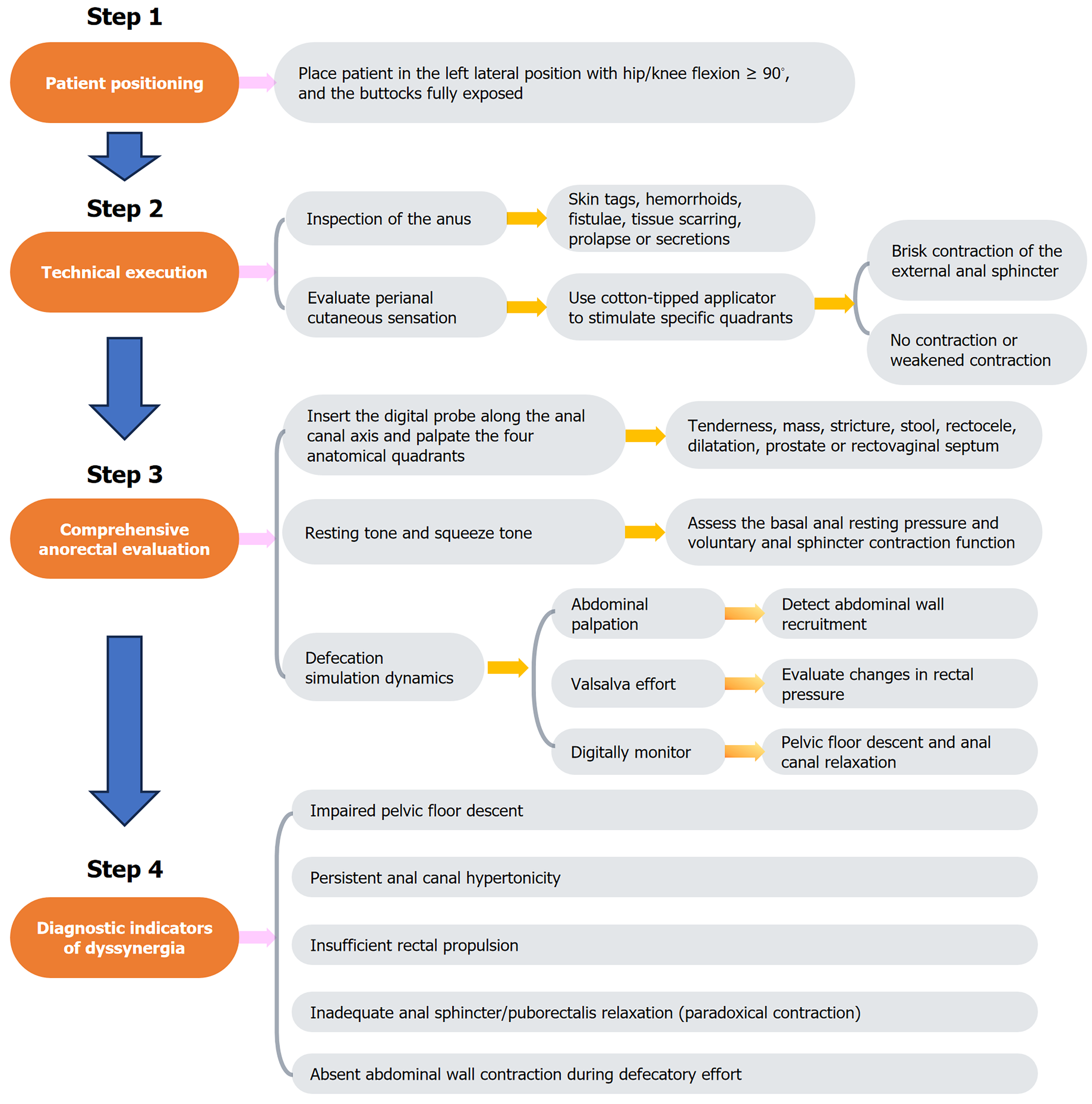
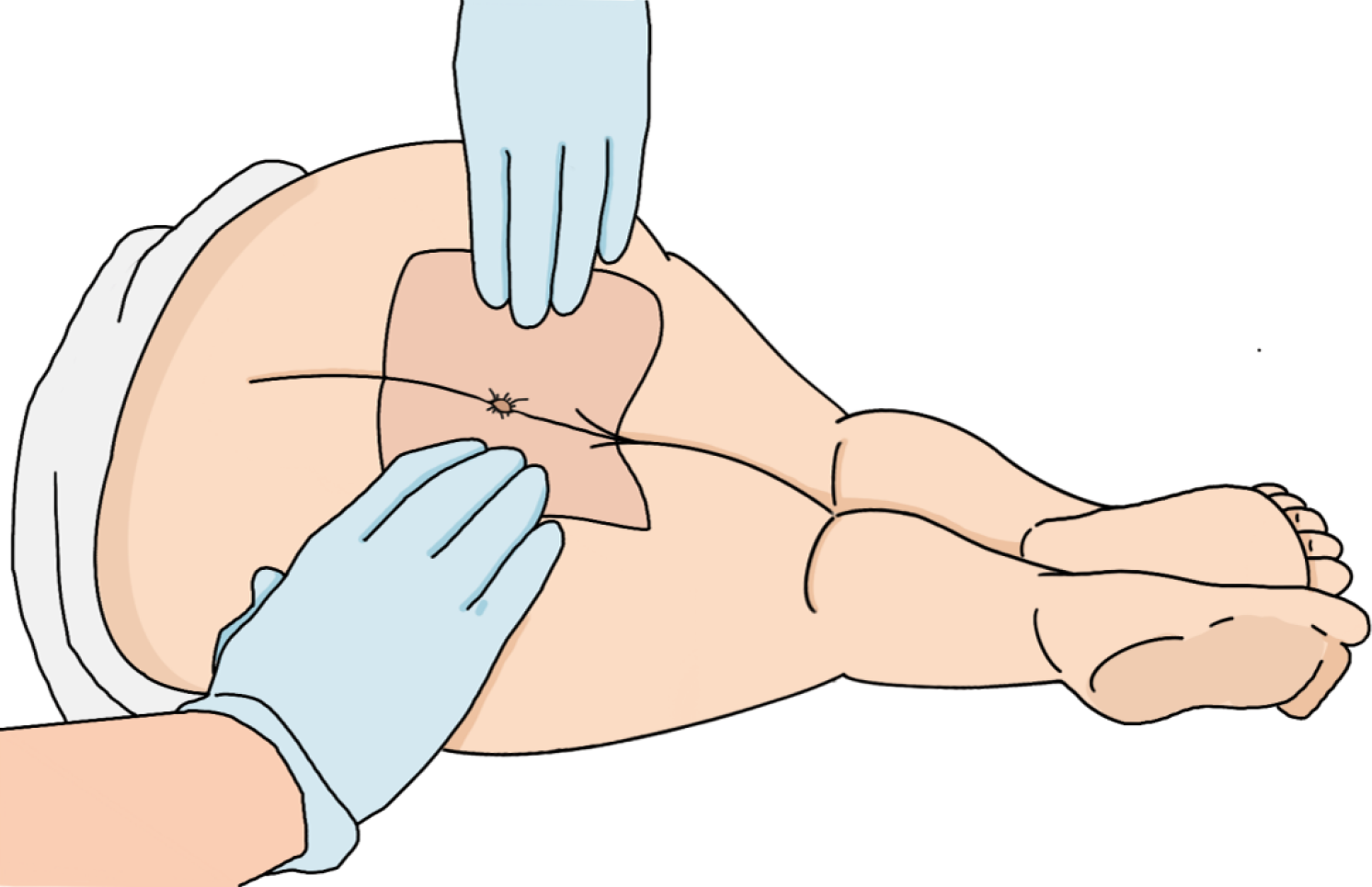
The key steps involved in DRE are illustrated in Figure 1. Prior to DRE, clinicians must establish clear communication with the patient, detailing the procedure’s clinical rationale and obtaining informed consent. A chaperone is strongly recommended to increase patient comfort and mitigate anxiety. The examination should be performed in private under adequate illumination and thermoregulation (20 °C-24 °C). Patients were placed in the left lateral decubitus position with hip/knee flexion ≥ 90° and the buttocks fully exposed via patient-assisted retraction (Figure 2).
Figure 1 Steps of digital rectal examination.
Figure 2 Patient positioning for digital rectal examination.
Technical execution
Using sterile gloves, the examiner systematically inspects the perianal region for pathological markers, including hemorrhoids, fistulae, tissue scarring, or neoplastic lesions. A standardized neurological assessment is performed using a cotton-tipped applicator to evaluate perianal cutaneous sensation via quadrant-specific stimulation (3:00, 6:00, 9:00, 12:00 positions) while observing transient external anal sphincter contractions.
Comprehensive anorectal evaluation
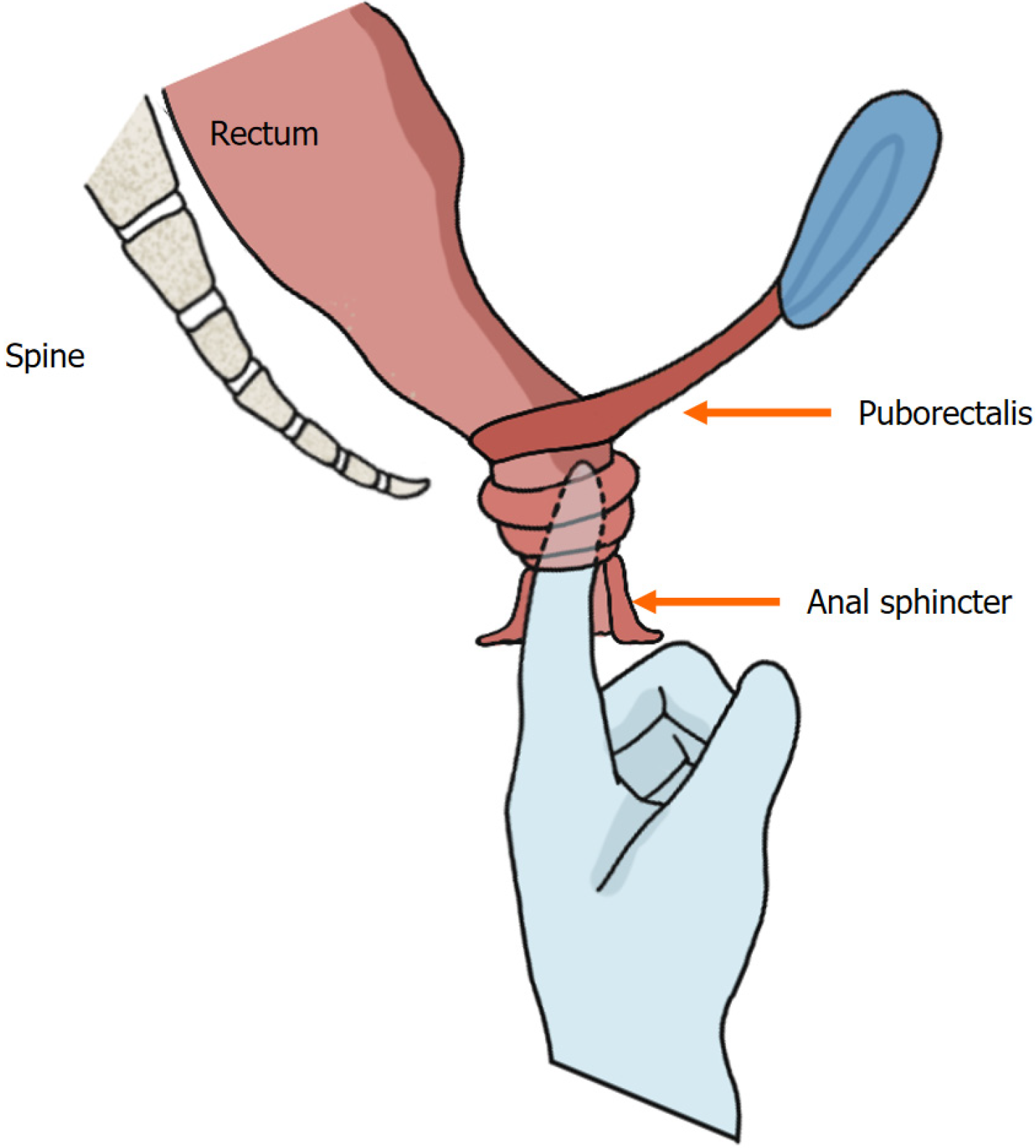
The perianal region is gently massaged with a lubricated gloved index finger to facilitate sphincter relaxation. The digital probe is inserted along the anal canal axis, and then the four anatomical quadrants are palpated (Figure 3). Basal anal resting pressure (measured during patient relaxation) and voluntary sphincter contraction (strength/duration) are then assessed. Defecation simulation dynamics: Simultaneous abdominal palpation (left hand supraumbilical) to detect abdominal wall recruitment; Rectal pressure elevation via Valsalva effort; Pelvic floor descent and anal canal relaxation monitored digitally.
Figure 3 Digital insertion and anorectal palpation.
Diagnostic indicators of dyssynergia
Pathognomonic findings include ≥ 1 of the following[26,27]: (1) Absent abdominal wall contraction during defecatory effort; (2) Inadequate anal sphincter/puborectalis relaxation (paradoxical contraction); (3) Failure to generate adequate rectal propulsive forces; (4) Impaired pelvic floor descent; and (5) Persistent anal canal hypertonicity.
CHARACTERISTICS OF DRE
Rectocele
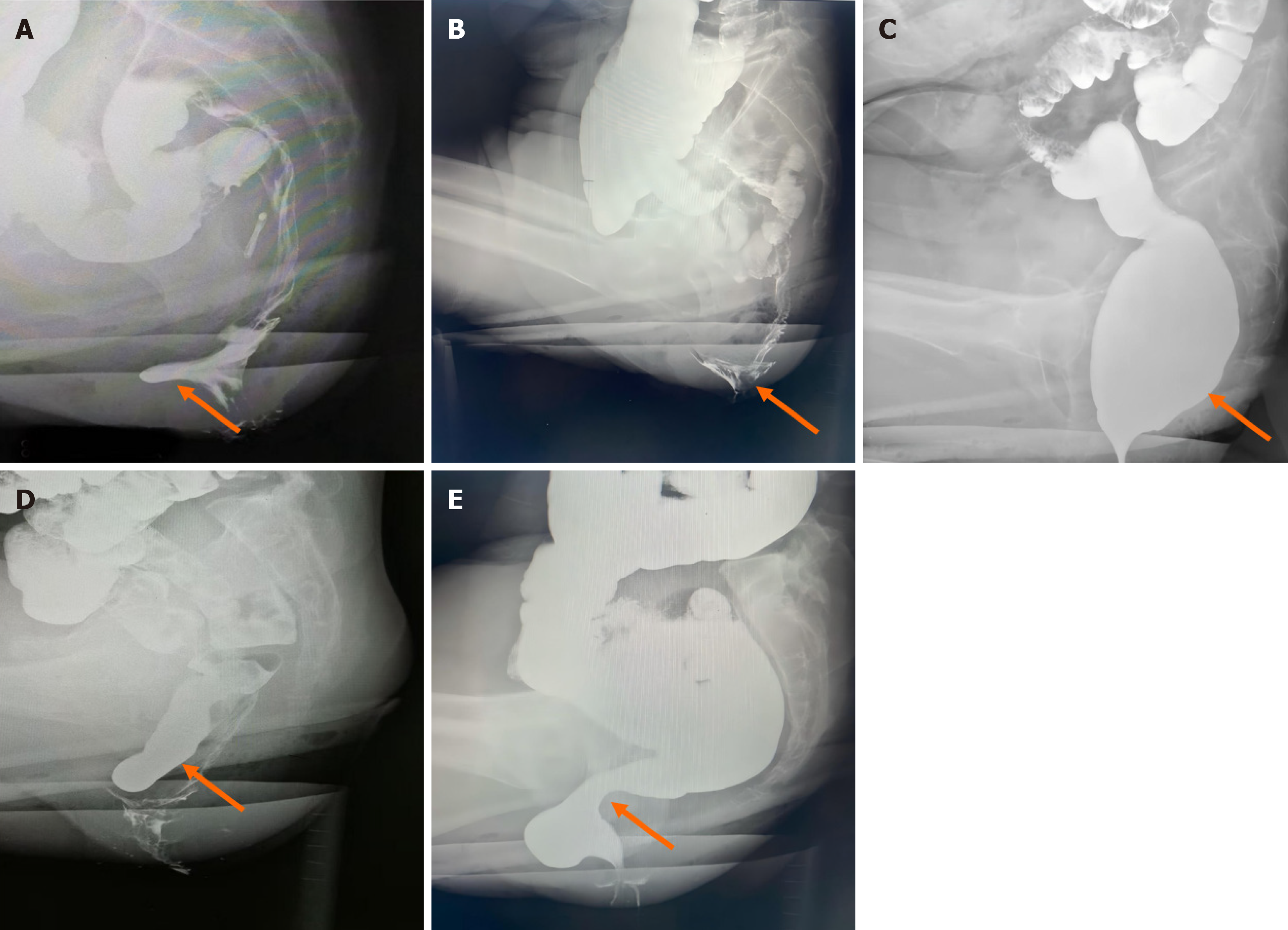
Patients manifest defecatory dysfunction characterized by digital vaginal pressure during evacuation to mechanically redirect fecal flow. Digital palpation at the proximal anal canal revealed an anterior rectal wall defect with reducible herniation into the vaginal lumen (Figure 4A), demonstrating diminished rectovaginal septum integrity and palpable posterior vaginal wall displacement. Provocative maneuvers (Valsalva) exacerbate the magnitude of the defect, with delayed/incomplete tissue recoil after examination. Anatomic severity is stratified by hernia depth: Mild (< 2 cm), moderate (2 cm-4 cm), or severe (> 4 cm)[28]. A clinically significant interpretation requires symptom correlation, given the high prevalence of asymptomatic rectoceles (detected in 80% of nulliparous women)[29].
Figure 4 Morphological changes demonstrated by defecography (indicated by the arrow).
A: Sac-like protrusion of the anterior rectal wall; B: Internal rectal prolapse in a funnel-shaped configuration; C: Rectal luminal distension; D: The sigmoid colon descends below the pubococcygeal line; E: Evident pressure trace of the puborectalis muscle.
Internal rectal prolapse
Internal rectal prolapse represents intraluminal invagination of the rectal wall without external anal protrusion[30], which is distinct from external rectal prolapse characterized by complete rectal extrusion through the anal canal[31]. Digital examination revealed redundant rectal mucosa with a folded configuration and preserved mobility, smooth mucosal surface texture, and palpable fecal residue within the rectal lumen. During simulated defecation, the examiner perceives the circumferential mucosal encirclement of the digit, corresponding to “rectal intussusception” when thickened mucosa forms a complete annular obstruction. Positional changes (the squatting maneuver) elicit cervix-like palpation of the distal rectal component. Defecographic correlation demonstrated funnel-shaped mucosal descent (Figure 4B).
Rectal dilatation
Digital examination reveals a spacious rectal lumen with no mucosal wrapping of the examining digit, often requiring maximal digital flexion to achieve bowel wall contact. This finding correlates with radiographic evidence of colonic dilation on barium enema (Figure 4C). Affected patients exhibit diminished rectal contractility, resulting in ineffective fecal expulsion and frequent detection of high-consistency fecal impaction. Critical clinical observations include the following: Fecal consistency documentation; Patient awareness assessment of rectal content and impaired fecal perception suggesting compromised rectal sensitivity[27].
Insufficient rectal propulsion
Patients typically report symptoms of ineffective straining, incomplete evacuation, and rectal outlet obstruction. This condition stems from compromised rectal contractility, resulting in insufficient propulsive forces for effective fecal expulsion. Digital assessment during simulated defecation fails to detect appreciable expulsion forces against the examining digit. Current diagnostic paradigms lack standardized criteria, necessitating a multimodal approach that combines symptomatology, DRE findings, and objective functional testing[10].
Descending perineum syndrome
Descending perineum syndrome is defined as excessive descent of the pelvic organs below the pubococcygeal line[32] (Figure 4D), which constitutes approximately 10% of tertiary referrals for constipation[33]. This syndrome manifests a wide range of symptoms, including sensations of anal obstruction during defecation and incomplete evacuation, as well as urinary or fecal incontinence. On DRE, the pelvic floor muscles are palpated as abnormally flaccid, with reduced anal canal contraction tone. During simulated defecation maneuvers, perineal dilatation manifests as a hemispheric bulge. In female patients, pregnancy and vaginal delivery are considered the primary etiological factors for abnormal pelvic floor muscle relaxation[34,35].
Puborectalis muscle dyssynergia
During normal defecation, the puborectalis muscle relaxes, leading to an increased anorectal angle and dilatation of the anal canal, thereby facilitating fecal expulsion. At the termination of defecation, the puborectalis muscle recontracts, restoring anal canal closure[36]. In puborectalis syndrome, DRE reveals elevated anal canal pressure, progressive elongation of the anal canal, and a direct correlation between canal depth and pressure intensity. The hypertrophied puborectalis muscle exhibits palpable tenderness and firm consistency, with sharply defined margins observed in select cases. During simulated defecation maneuvers, paradoxical contraction of the puborectalis muscle is palpated (i.e., absence of relaxation), which exacerbates fecal obstruction despite increased straining efforts. Defecographic evaluation revealed characteristic puborectalis muscle compression patterns, as illustrated in Figure 4E.
Spastic pelvic floor syndrome
This syndrome is characterized by progressively worsening symptoms of strain during defecation, incomplete fecal evacuation, an anal blockage sensation, and persistent urges without successful evacuation, significantly impairing quality of life. It represents neuromuscular dysfunction of the pelvic floor muscles (external anal sphincter and puborectalis). DRE reveals sustained anal constriction with elevated resting sphincter tone, often preventing painless insertion of the examining finger. During simulated defecation, paradoxical contraction of the anal canal replaces the normal relaxation response.
OTHER ETIOLOGIES OF DEFECATION DISORDERS DETECTABLE THROUGH DRE
Anorectal stenosis
Patients in this group commonly present with symptoms such as straining during defecation. The condition is most frequently attributed to congenital malformations, chronic inflammation, trauma, or surgical interventions involving the anal or rectal region[37-39]. On clinical examination, the sites stenosis are typically located closer to the anal orifice, where DRE often reveals an inability to pass the index finger due to palpable scar tissue hyperplasia and compromised anal canal elasticity. In cases of postoperative anastomotic stenosis in rectal cancer patients, the stenotic segment is generally situated proximal to the surgical anal canal, presenting as a ring-like narrowing that is readily detectable through DRE.
Solitary rectal ulcer syndrome
Solitary rectal ulcer syndrome (SRUS) represents a rare inflammatory disorder characterized by chronic rectal ulceration with concomitant evacuation impairment. Compared with internal rectal prolapse and rectocele, SRUS is associated with a higher prevalence of constipation and elevated anal sphincter tone[40,41]. Digital examination detects irregular mucosal thickening with indurated mobility, often eliciting palpation-induced tenderness that necessitates differentiation from rectal malignancies. Although pathognomonic DRE features are lacking, SRUS findings serve as critical screening indicators that prompt definitive diagnostic investigations.
Rectal carcinoma
DRE serves as a valuable tool for the initial screening of rectal carcinoma. During DRE, irregular mucosal hyperplasia is typically detected, demonstrating poor mobility and varying degrees of luminal stenosis within the rectal cavity. Following withdrawal of the examining finger, residual pus, blood, mucus, and necrotic tissue are commonly identified on the glove. Particular attention should be given to thorough evaluation of the posterior rectal wall to prevent oversight of highly positioned or posteriorly located lesions. When a suspicious mass is palpated, its anatomical location, dimensions, morphological features, consistency, and involvement of the intestinal lumen must be documented in detail, as this information is critical for guiding subsequent diagnostic and therapeutic strategies[42].
LIMITATIONS AND FUTURE DIRECTIONS
While indispensable in routine practice, DRE is not without constraints that merit deliberate scrutiny. Chief among these is the need for operator expertise when performing this technique, where inter clinician discrepancies in discerning subtle anomalies-such as gradations in internal anal sphincter tone or the presence of non-relaxing puborectalis syndrome-remain a persistent challenge. These inconsistencies, rooted in the inherently subjective interpretation of tactile feedback and variable training exposure, highlight a critical need for standardized proficiency benchmarks. Moreover, the qualitative nature of DRE limits its capacity to provide serialized, objective metrics essential for monitoring disease trajectories or quantifying therapeutic responses in conditions like pelvic floor dyssynergia.
To transcend these limitations, a dual-pronged strategy integrating pedagogical innovation and technological augmentation is imperative. First, the development of accredited, simulation-based certification programs-incorporating validated tactile discrimination modules and competency assessments-would ensure uniform skill acquisition across training cohorts. Parallel to this, multicenter validation studies comparing structured DRE protocols against gold-standard functional imaging modalities (e.g., three dimensional high-resolution anorectal manometry, dynamic defecography) are crucial to establish population-adjusted diagnostic thresholds. On the technological frontier, the advent of haptic feedback gloves offers a promising avenue for real-time sphincter tone quantification, potentially reducing diagnostic subjectivity[43]. Concurrently, deep learning-assisted pattern recognition algorithms trained on multisite DRE datasets can standardize the interpretation of palpatory findings such as rectocele reducibility or mucosal prolapse severity. In resource-constrained environments, coupling DRE with portable transperineal ultrasound systems may deliver cost-effective, point-of-care triage without necessitating advanced infrastructure.
The aim of these synergistic approaches is not to supplant DRE’s clinical primacy but to refine its precision, ensuring its continued relevance in an era increasingly driven by quantitative biomarkers and personalized therapeutic algorithms.
CONCLUSION
DRE effectively identifies functional defecatory pathologies, including rectocele, internal rectal prolapse, and puborectalis muscle dyssynergia. While its diagnostic sensitivity exhibits operator-dependent variability inherent to manual palpation techniques, its clinical utility can be optimized through protocol-driven training and competency-based skill acquisition. As a cost-effective, noninvasive, and immediately deployable screening tool, DRE holds value in resource-constrained settings lacking advanced anorectal functional testing. Even in tertiary care centers, proficient DRE execution enables stratified diagnostic algorithms, guiding targeted therapeutic interventions to enhance constipation management efficacy. These attributes underscore DRE’s imperative for global clinical practice integration.
ACKNOWLEDGEMENTS
We extend our sincere appreciation to Dr. Zhen XB from the Department of Constipation at Chengdu Anorectal Hospital for providing select defecography images that greatly enriched this article. We are truly grateful for his valuable contribution.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade A
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Yang WY S-Editor: Fan M L-Editor: A P-Editor: Xu ZH