Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.105860
Revised: March 26, 2025
Accepted: May 26, 2025
Published online: July 27, 2025
Processing time: 165 Days and 5.3 Hours
There is an ongoing debate regarding the relationship between intraoperative blood transfusions and patient outcomes. Unifying the results is difficult because of differences in surgery type, target population and postoperative observation indicators.
To evaluate the risk factors for intraoperative blood transfusion and its impact on postoperative outcomes in elderly gastrointestinal cancer patients.
This was a retrospective single-center study of elderly patients (≥ 65 years old) who underwent elective abdominal surgery for gastrointestinal cancer with general anesthesia. Patients with chronic kidney disease and missing related data were excluded. The primary outcomes included acute kidney injury (AKI), myo
A total of 967 patients were included in this study. A lower preoperative hema
These results demonstrate that intraoperative blood transfusion increases the risk of poorer outcomes in elderly patients receiving gastrointestinal cancer surgery. These findings provide new ideas for improving the prognosis of elderly cancer patients.
Core Tip: This study retrospectively analyzes large - sample clinical data of elderly gastrointestinal tumor patients. By comprehensively analyzing incidences of postoperative complications, it evaluates the impact of intraoperative blood transfusion on the postoperative outcomes of radical resection of major gastrointestinal tumors (surgery duration > 2 hours). The research fills the void of lacking relevant clinical data support for elderly patients in this field. It offers clinical guidance, and provides evidence for transfusion and prognosis management in elderly gastrointestinal tumor patients.
- Citation: Guo MM, Ji CY, Gu RR, Nan K, Miao CH, Wu QC. Risk factors and outcomes of intraoperative blood transfusion in elderly patients undergoing gastrointestinal cancer surgery. World J Gastrointest Surg 2025; 17(7): 105860
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/105860.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.105860
With the rapid growth of the aging population in China, the need for surgery among elderly patients has increased dramatically[1]. Gastrointestinal cancer surgery, which is the most common operation performed in elderly patients, has attracted substantial attention because of its association with high amounts of blood loss, high rates of blood transfusion during surgery and a high incidence of adverse outcomes after surgery[2]. The relationship between intraoperative blood transfusions and patient outcomes is still not clear[3,4]. Although many related studies have been conducted in the past 10 years, it is difficult to draw consistent conclusions because of differences in surgery type, target population and post
Elderly patients have more complications, lower circulatory capacity and poorer tolerance[8]. Some studies have suggested that blood transfusion can lead to transfusion-related immune regulation through various mechanisms, such as immune suppression or immune disorders, and the degree of its impact depends on the underlying condition of the patient[9,10]. The results of animal models have revealed that mice of different ages have different immunosuppressive reactivities[11]. These findings indicate that the effect of blood transfusion on the outcome of surgery depends on the age distribution[12]. Given the group’s ability to respond to the environment differently, elderly patients should be treated as a special group to evaluate the effect of intraoperative blood transfusion on the outcomes of this group[7]. Intraoperative blood transfusion has always been a popular topic in clinical research, but few studies have been conducted in elderly populations or have observed multisystem recovery.
This study was carried out in a large tertiary general hospital. After the effects of patient demographics and preoperative clinical status were excluded, the relationship between intraoperative blood transfusion and postoperative outcomes was explored in elderly patients who received gastrointestinal cancer surgery. The risk factors for intraoperative blood transfusion were further explored.
The Institutional Review Board for Clinical Investigations at Nanjing Drum Tower Hospital (Drum Tower Hospital, Medical School of Nanjing University, No. 321 Zhongshan Road, Nanjing 210008, Jiangsu Province, China) approved the study and agreed to waive the need for informed consent (approval No. 2017-049-01). All patient data were anonymized to ensure that personal and electronic databases were encrypted to restrict access permissions. No additional interventions were performed on the patients during the research process, and data extraction and analysis were based on existing clinical records; therefore, there are no additional ethical risks.
We conducted a retrospective study of elderly patients (≥ 65 years) who received major elective abdominal surgery between 2018 and 2020. The inclusion criteria were patients who received elective gastrointestinal surgery under general anesthesia, stayed for at least 1 day, had a preoperative creatinine concentration measurement and had at least one postoperative creatinine measurement. The exclusion criteria were patients with chronic kidney disease (estimated preoperative glomerular filtration rate less than 60 mL/minute/1.73 m2, nephrectomy or renal transplantation) and without cancer. Patients who did not successfully complete surgery or whose related data were missing were also excluded.
The electronic medical record system was used to collect perioperative data, which included age, sex, comorbidities, American Society of Anesthesiologists (ASA) physical status score (classified as 1 or 2, 3, and 4 or 5), preoperative laboratory values and pathological tumor stage. Heart diseases include coronary heart disease and moderate to severe valve disease. Pulmonary diseases include chronic obstructive pulmonary disease, asthma, bronchiectasis and interstitial lung disease.
Intraoperative hemodynamic data were recorded by our electronic anesthesia information management system. The collected data included the operative site, duration of surgery, amount of intraoperative blood loss and whether blood transfusion is required. None of the data could be modified manually. The type of operation was divided into 3 categories according to the operative site (stomach, colon, or rectum). Intraoperative blood loss was standardized by body weight (mL/kg). Intraoperative red blood cell transfusion was initiated when hemoglobin fell below < 7 g/dL. Transfusion decisions for patients with hemoglobin levels between 7-10 g/dL were made through comprehensive clinical evaluation by physicians.
Consistent with the Acute Kidney Injury Network threshold, patients were considered to have acute kidney injury (AKI) if the postoperative creatinine concentration was either more than 1.5-fold greater or 0.3 mg/dL greater than the preoperative concentration[13]. The preoperative creatinine value was defined as the last measured value prior to surgery. The postoperative creatinine value that was used was the highest concentration measured within 7 days after surgery[14]. Myocardial injury after noncardiac surgery (MINS) was defined as an increase in either fourth-generation troponin T or creatinine kinase-myocardial band above the upper limit value 7 days after the operation. The upper limit was defined as 0.03 ng/mL for troponin T and 8.8 ng/mL for creatinine kinase-myocardial band[15,16]. Postoperative respiratory complications were defined as new respiratory diseases after surgery, including hypoxemia[17], pneumonia[18], respiratory failure[19], and pulmonary embolism[20]. Hypoxemia was defined as SpO2 < 94% when inhaling air[21]. Postoperative pneumonia was diagnosed through a combination of clinical symptoms and chest computed tomography examination[22]. Postoperative respiratory failure was defined as PaO2 < 60 mmHg when inhaling air[23]. Pulmonary embolism was diagnosed by computed tomography pulmonary angiography[24].
Continuous variables are presented as the means ± SDs or medians (25th and 75th percentiles) and were analyzed via Student’s t test or the Mann-Whitney U test, as appropriate. Categorical variables were analyzed via the χ2 test or Fisher’s exact test and are presented as n (%). Statistically significant continuous variables were transformed to categorical variables by their cutoff points, which were defined by receiving operating characteristic curve analysis. Patients were classified into 4 categories according to their preoperative hematocrit levels: > 30.0%, 27.0% to 30.0%, 24.0% to 26.9%, and 15.0% to 23.9%[18]. Patients with a hematocrit level > 30.0% were considered the reference group.
Primary variables were selected based on previous researches and clinical relevance. Univariate and multivariate logistic regression models were applied to evaluate the associations between intraoperative blood transfusion and postoperative outcomes. Univariable predictors with P < 0.10 were initially included, then selected via multiple stepwise regression analysis (final model P < 0.05). Baseline characteristics were compared between patients who did and did not receive intraoperative blood transfusions. Univariate associations with P < 0.10 were included in further multivariate logistic regression analysis to determine the risk factors for intraoperative red blood cell transfusion. All retained variables had variance inflation factor < 10, indicating no severe collinearity. Finally, we quantified the discriminatory power and calibration ability of the final multivariate models by the c-index and Hosmer-Lemeshow statistic, respectively[25]. All of the analyses were conducted using the software Statistical Package for Social Sciences (SPSS version 23.0, Chicago, IL, United States).
The sample size was calculated on the basis of our previous investigation and the authoritative literature. The incidence rates of MINS and AKI after noncardiac surgery (P) are 7.4% and 2.3%, respectively[26]. Approximately 400 eligible surgeries (N) were performed at this medical center. According to the formula n = 1.962 × P × (1 - P)/0.012; nc = n/[1 + (n - 1)/N], through screening, the sample size available for the primary analysis was 967, which was sufficient to meet the statistical needs of formula calculation. There is no prior publication of the data in this manuscript.
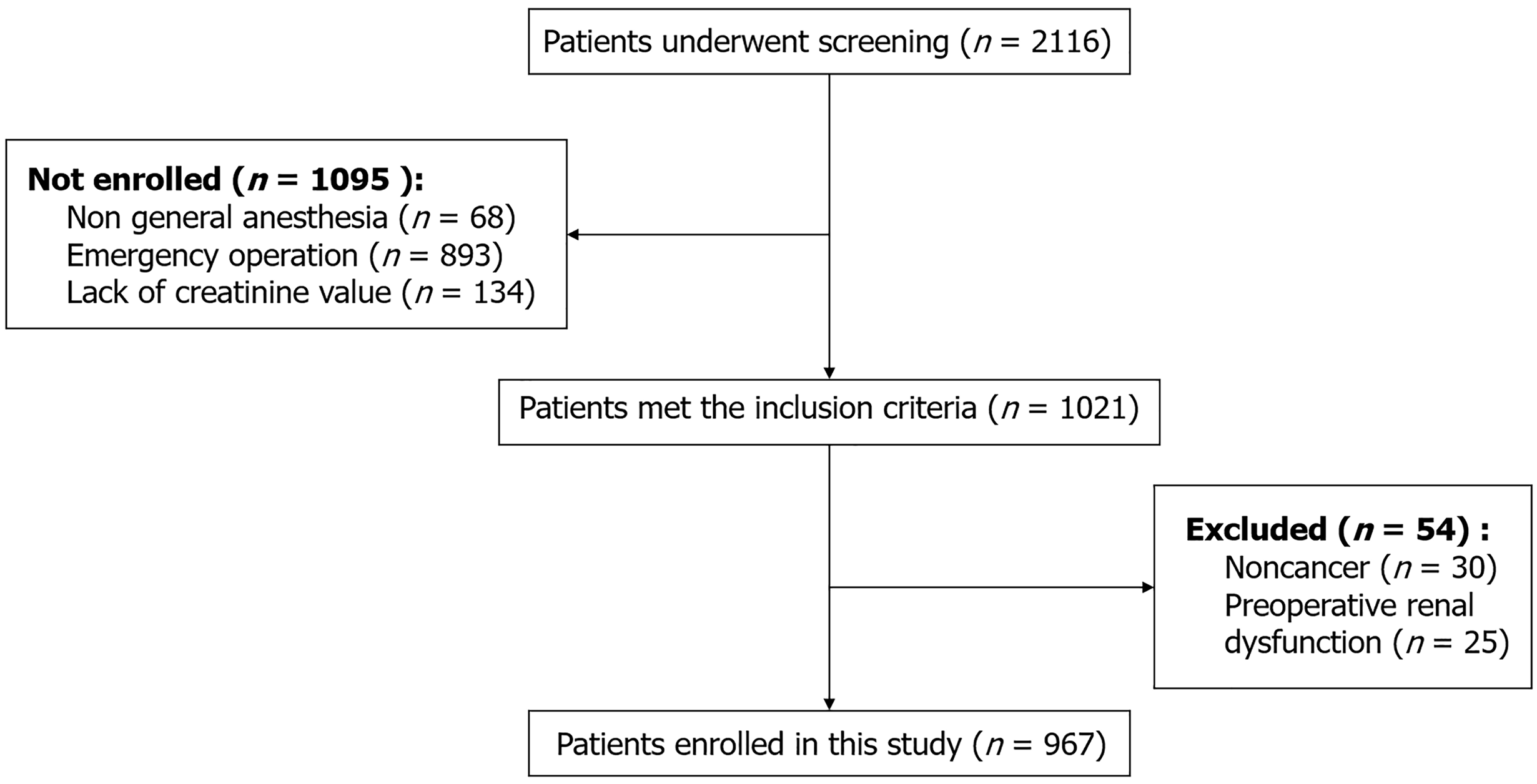
This study included 1021 patients, 54 of whom were noncancer patients or had preoperative renal function impairment. After exclusion, 967 patients were ultimately analyzed (Figure 1). In this study, the incidence rates of AKI (8.3% vs 2.7%, P = 0.001), MINS (5.5% vs 1.2%, P = 0.001) and respiratory complications (15.9% vs 7.5%, P = 0.001) significantly increased after intraoperative transfusion (Table 1). Supplementary Table 1 lists the results of the univariate analysis of post
| Transfusion | Non-transfusion | P value | |
| AKI | 12 (8.3) | 22 (2.7) | 0.001 |
| MINS | 8 (5.5) | 10 (1.2) | 0.001 |
| Respiratory complications | 23 (15.9) | 62 (7.5) | 0.001 |
| Risk factor | OR (95%CI) | P value | aOR (95%CI) | P value |
| AKI | ||||
| Blood transfusion (%) | 3.277 (1.584-6.779) | 0.001 | 2.975 (1.416-6.252) | 0.004 |
| Age, year | 1.110 (1.055-1.168) | < 0.0005 | 1.101 (1.045-1.159) | < 0.0005 |
| Preoperative albumin (g/dL) | 0.924 (0.847-1.008) | 0.075 | Reference | 0.514 |
| MINS | ||||
| Blood transfusion (%) | 4.742 (1.839-12.226) | 0.001 | 5.109 (1.943-13.438) | 0.001 |
| Age, year | 1.086 (1.013-1.164) | 0.020 | Reference | 0.063 |
| Heart disease (%) | 3.059 (1.069-8.750) | 0.037 | Reference | 0.075 |
| Site of procedure (%) | 0.041 | 0.026 | ||
| Stomach | Reference | Reference | ||
| Colon | 2.912 (1.096-7.738) | 0.032 | 3.437 (1.270-9.301) | 0.015 |
| Rectum | 0.466 (0.057-3.812) | 0.476 | 0.625 (0.075-5.211) | 0.664 |
| ASA score (%) | Reference | 0.076 | Reference | 0.096 |
| Respiratory complications | ||||
| Blood transfusion (%) | 2.311 (1.380-3.869) | 0.001 | 2.221 (1.225-4.025) | 0.009 |
| Age, year | 1.039 (1.002-1.077) | 0.040 | 1.033 (0.996-1.072) | 0.081 |
| Preoperative hematocrit level (%) | 0.037 | 0.265 | ||
| > 30.0% | - | Reference | ||
| 27.0%-30.0% | 0.952 (0.399-2.273) | 0.912 | 0.623 (0.251-1.594) | 0.331 |
| 24.0%-26.9% | 2.965 (1.415-6.216) | 0.004 | 1.815 (0.799-4.122) | 0.154 |
| 15.0%-23.9% | 1.252 (0.284-5.512) | 0.766 | 0.729 (0.155-3.430) | 0.689 |
| Incidence (%) | aOR (95%CI) | P value | c-index | H-L statistic | |
| AKI | 8.3 | 2.975 (1.416-6.252) | 0.004 | 0.711 | 5.360 |
| MINS | 5.5 | 5.109 (1.943-13.438) | 0.001 | 0.757 | 2.829 |
| Respiratory | 15.9 | 2.221 (1.225-4.025) | 0.009 | 0.972 | 6.189 |
The baseline characteristics of the excluded patients were not significantly different from those of the patients who were included (data not displayed). The basic status of the elderly patients in the group was poor. A total of 80.67% (n = 780) of the patients had an ASA grade greater than II. Overall, 145 (15%) patients received intraoperative blood transfusions (Table 4).
| Characteristics | Transfusion (n = 145) | Non-transfusion (n = 822) | P value |
| Age, year | 72 (68, 78) | 71 (68, 76) | 0.175 |
| Males | 101 (69.7) | 595 (72.4) | 0.500 |
| Blood loss (mL/kg) | 6.91 ± 6.40 | 3.26 ± 3.01 | < 0.0005 |
| Weight (kg) | 61.75 ± 11.11 | 63.32 ± 10.13 | 0.089 |
| Preoperative albumin (g/dL) | 36.27 ± 3.42 | 38.22 ± 4.03 | < 0.0005 |
| Duration of surgery > 300 minutes | 59 (40.7) | 171 (20.8) | < 0.0005 |
| Heart disease | 18 (12.4) | 93 (11.3) | 0.702 |
| Hypertension | 66 (45.5) | 353 (43.1) | 0.583 |
| Stroke | 17 (11.7) | 65 (7.9) | 0.128 |
| Pulmonary disease | 6 (4.1) | 60 (7.3) | 0.164 |
| Obesity | 21 (14.5) | 113 (13.7) | 0.813 |
| Cancer stage | 0.016 | ||
| I | 14 (9.6) | 170 (20.7) | |
| II | 48 (33.1) | 233 (28.3) | |
| III | 72 (49.7) | 350 (42.6) | |
| IV | 11 (7.6) | 69 (8.4) | |
| Preoperative hematocrit level | < 0.0005 | ||
| > 30.0% | 70 (48.3) | 752 (91.5) | |
| 27.0%-30.0% | 34 (23.4) | 43 (5.2) | |
| 24.0%-26.9% | 26 (17.9) | 22 (2.7) | |
| 15.0%-23.9% | 15 (10.3) | 5 (0.6) | |
| ASA score | 0.325 | ||
| 2 | 32 (22.1) | 155 (18.9) | |
| 3 | 107 (73.8) | 646 (78.6) | |
| 4 | 6 (4.1) | 21 (2.6) | |
| Site of procedure | 0.001 | ||
| Stomach | 436 (53.0) | 99 (68.3) | |
| Colon | 235 (28.6) | 34 (23.4) | |
| Rectum | 151 (18.4) | 12 (8.3) |
Intraoperative blood transfusions were more common among patients who had greater blood loss (6.91 vs 3.26, P < 0.0005), lower preoperative albumin values (36.27 vs 38.22, P < 0.0005) and an operative length > 300 minutes (40.7% vs 20.8%, P < 0.0005). Moreover, patients who received blood transfusions typically had lower preoperative hematocrit levels and more advanced cancer stages (all P < 0.05) (Table 4). The predictive model of intraoperative blood transfusion is shown in Table 5. After excluding the effects of preoperative nutritional status, disease stage and surgical site, a longer duration of operation (> 300 minutes), greater blood loss and preoperative hematocrit level < 30.0% were still independent risk factors for receiving an intraoperative blood transfusion (P < 0.0005). On the basis of the Hosmer-Lemeshow statistic (P > 0.05), the discriminatory ability (c statistic = 0.883) and calibrating ability of the model were good.
| Risk factor | aOR (95%CI) | P value |
| Duration of surgery > 300 minutes (%) | 2.738 (1.641-4.568) | < 0.0005 |
| Blood loss (mL/kg) | 1.230 (1.161-1.303) | < 0.0005 |
| Preoperative hematocrit level (%) | < 0.0005 | |
| > 30.0% | Reference | |
| 27.0%-30.0% | 12.040 (6.540-22.166) | < 0.0005 |
| 24.0%-26.9% | 16.408 (8.115-33.177) | < 0.0005 |
| 15.0%-23.9% | 36.584 (11.003-121.638) | < 0.0005 |
A total of 967 elderly patients who received elective gastrointestinal cancer surgery were enrolled in this study. The incidence of intraoperative blood transfusion was 15.0% (n = 145). We found that the duration of surgery, intraoperative blood loss and preoperative hematocrit level were all risk factors for receiving an intraoperative blood transfusion. Therefore, correction of preoperative anemia is necessary to reduce the need for intraoperative blood transfusions. Targeted interventions for preoperative anemia are carried out on the basis of the etiology and laboratory indicators, such as hemoglobin and the serum iron concentration[27]. Common intervention strategies include the use of iron supplements to treat iron deficiency anemia and folic acid to treat megaloblastic anemia[28]. Although there is no consistent conclusion regarding the correlation between cancer stage and intraoperative blood transfusion, most studies have shown that there is a positive correlation. However, in our multivariate analysis, advanced cancer stage was not found to be an independent risk factor for intraoperative blood transfusion.
Hypoalbuminemia (< 3.5 g/dL) reduces the stability of red blood cell membranes and shortens the lifespan of red blood cells[29]. Low nutritional status increases the need for blood transfusion, leading to a decrease in the hemoglobin threshold used to determine whether a patient requires a blood transfusion[30]. However, in this study, after the influence of confounding factors was removed, sex and preoperative nutritional status did not independently increase the risk of intraoperative blood transfusion.
Consistent with previous studies, our multivariate model revealed that age and blood transfusion were independently associated with AKI[31,32]. However, the results could not explain the relative importance due to the possibility of synergy between them. In this study, intraoperative blood transfusion increased the risk of AKI by 2.975-fold. Blood transfusion can cause ischemia-reperfusion injury, leading to microcirculatory disorders and inflammatory storms and increasing the risk of AKI[33]. Older age may further increase the correlation between intraoperative blood transfusion and AKI.
It has been reported that blood transfusions can prevent tachycardia caused by anemia to reduce the risk of myocardial injury[34]. In contrast, we were surprised to find that intraoperative blood transfusion increased MINS. Blood transfusion increases the risk of MINS by 5.109-fold. This result may be because blood transfusion activates the systemic inflammatory response by releasing proinflammatory mediators, leading to coronary endothelial dysfunction[35]. Moreover, the levels of complement C3a/C5a increase after blood transfusion, inducing the aggregation of neutrophils and platelets and promoting the formation of microthrombi[36]. In addition, after blood transfusion, the hemorheological properties of patients change, which affects the metabolism of myocardial cells and exacerbates myocardial oxidative stress[37]. Elderly patients have insufficient vascular endothelial function reserve, and they are more likely to suffer from coronary artery spasm induced by metabolic disorders associated with nitric oxide after blood transfusion, thus triggering myocardial injury with MINS[38].
There is controversy in the literature regarding the relationship between blood transfusion and respiratory complications. Some studies suggest that blood transfusion may increase the risk of acute respiratory distress syndrome through inflammatory reactions, whereas other studies support the benefits of correcting anemia and improving the supply of oxygen. This study suggests that intraoperative blood transfusion increases the risk to the respiratory system by 2.221-fold. During processing and storage, erythrocytes undergo ATP and 2,3-diphosphoglycerate depletion, resulting in a reduction in plastic deformation capacity[39-42]. These changes reduce their oxygen-carrying capacity[31]. The oxygen dissociation curve shifts to the left, and oxygen utilization decreases, exacerbating tissue hypoxia injury and damaging microvascular oxygenation. In addition, injecting red blood cells to remove NO (a vasodilator) results in the contraction of small vessels[43]. All of these factors ultimately lead to tissue ischemia[44,45]. Moreover, the inflammatory response increases capillary permeability, on the basis of which plasma colloid osmotic pressure and blood volume increase, followed by an increase in transfusion-associated circulatory overload[46,47]. Elderly patients seem to have a greater risk of circulatory overload, which is accompanied by more complications, less circulatory capacity and poorer tolerance[8].
The effect of intraoperative blood transfusions on postoperative outcomes may be directly attributable to red blood cells or an indirect manifestation of intraoperative hypoperfusion or acute ischemia and hypoxia[48]. Our multivariate model indicated that blood loss, length of operation and preoperative anemia were risk factors for intraoperative blood transfusion, so we cannot completely exclude the potential impact of these factors on postoperative outcomes. In the present study, intraoperative blood transfusion was an independent risk factor for AKI, MINS and respiratory complications. The incidence of complications related to blood transfusion during hospitalization is low, and one of the main reasons for this low incidence is a lag in diagnosis[46]. As a retrospective study, none of our patients were diagnosed with transfusion-related lung or kidney injury, while our results were consistent with those of previous studies.
Because the preoperative conditions of patients are difficult to change, doctors could reduce the probability of intraoperative blood transfusion by using some of the factors under comprehensive consideration. On the basis of our findings, we suggest a series of measures to reduce the need for intraoperative blood transfusion, including strengthening clinical techniques, improving operative procedures, shortening the operative time and controlling bleeding. It was also necessary to actively correct preoperative anemia. Preoperative use of erythropoietin has been reported to ameliorate preoperative anemia and thus reduce the risk of intraoperative blood transfusion. However, it is still unclear whether this drug should be used for preoperative pathological anemia in cancer patients.
If there is a need to improve anemia rapidly when the cancer has progressed to a very serious stage, in addition to transfusion support, pharmacological hemoglobin-elevating therapies can be considered, such as erythropoiesis-stimulating agents and intravenous iron, or symptomatic supportive treatments, such as oxygen therapy and glucocorticoids, for immediate intervention[49]. Recent studies have highlighted the therapeutic potential of hemoglobin stabilizers (e.g., hypoxia-inducible factor stabilizers) and alternative strategies to transfusion (e.g., artificial oxygen carriers) in cancer-related anemia. However, further research is needed to explore their value in emergency settings[5].
The results of this study provide new directions for future research on transfusion strategies for elderly patients. Further research can explore the mechanisms underlying the observed associations, such as the role of transfusion-related inflammatory responses or microvascular changes. In addition, prospective studies or randomized controlled trials can help establish causal relationships and provide information for transfusion guidelines suitable for elderly patients. Regarding academic positioning, this study is a continuation and deepening of previous work. Previous studies focused on the postoperative outcomes of elderly patients undergoing major abdominal surgeries but did not conduct in-depth analyses of this special subgroup of elderly cancer patients, who should be studied independently as a special group owing to their pathological and physiological characteristics.
There are several inherent limitations of retrospective studies, including differences in anesthesia management practices, lack of uniform blood transfusion standards and failure to explain all potential confounding variables. First, we collected the baseline characteristics of the patients as much as possible. Second, compared with the results of other studies, the homogeneity of the patient group was reduced, resulting in deviations from individual differences; thus, the sample size was limited. Third, this was a single-center study. The bias in selecting patients and surgical types limits our results from being extended to other groups and medical centers. Furthermore, we cannot completely exclude the potential impact of intraoperative and postoperative circulatory changes (including the use of vasoactive drugs). Moreover, the transfusion units used in this study were not recorded. Although the lack of data on transfusion units may affect the analysis of dose-response relationships, the core conclusion of this study (the association between transfusion and outcomes) remains reliable. Subsequent studies will prospectively record blood transfusion volumes (accurate to units) and use standardized transfusion report templates to address this limitation. Finally, the causal relationship between intraoperative blood transfusion and postoperative outcomes cannot be determined. Prospective clinical trials are needed to further prove whether restricting intraoperative blood transfusion actually reduces the incidence of complications during hospitalization. The goal is to develop better individualized intraoperative blood transfusion protocols[50]. Meanwhile, future prospective studies would benefit from adopting the Clavien-Dindo classification to enable more granular and internationally comparable analysis of complication severity. Incorporating time-to-event (survival) analysis in prospective designs will also provide critical insights into the temporal dynamics of postoperative complications.
In conclusion, in the present study, with many patients, we found that intraoperative blood transfusion increased the risk of poorer outcomes in elderly patients (aged ≥ 65 years) who underwent gastrointestinal cancer surgery. Improvements in preoperative anemia, improvements in clinical techniques and the development of accepted guidelines are necessary to reduce the need for intraoperative blood transfusions.
| 1. | McIsaac DI, MacDonald DB, Aucoin SD. Frailty for Perioperative Clinicians: A Narrative Review. Anesth Analg. 2020;130:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 2. | Shibutani M, Maeda K, Kashiwagi S, Hirakawa K, Ohira M. The Impact of Intraoperative Blood Loss on the Survival After Laparoscopic Surgery for Colorectal Cancer. Anticancer Res. 2021;41:4529-4534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Mendez E, Puig G, Barquero M, Leon A, Bellafont J, Colomina MJ. Enhanced recovery after surgery: a narrative review on patient blood management recommendations. Minerva Anestesiol. 2023;89:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Duclos A, Frits ML, Iannaccone C, Lipsitz SR, Cooper Z, Weissman JS, Bates DW. Safety of inpatient care in surgical settings: cohort study. BMJ. 2024;387:e080480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Prescott LS, Taylor JS, Lopez-Olivo MA, Munsell MF, VonVille HM, Lairson DR, Bodurka DC. How low should we go: A systematic review and meta-analysis of the impact of restrictive red blood cell transfusion strategies in oncology. Cancer Treat Rev. 2016;46:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Zhou PY, Tang Z, Liu WR, Tian MX, Jin L, Jiang XF, Wang H, Tao CY, Ding ZB, Peng YF, Qiu SJ, Dai Z, Zhou J, Fan J, Shi YH. Perioperative blood transfusion does not affect recurrence-free and overall survivals after curative resection for intrahepatic cholangiocarcinoma: a propensity score matching analysis. BMC Cancer. 2017;17:762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Henke PK, Park YJ, Hans S, Bove P, Cuff R, Kazmers A, Schreiber T, Gurm HS, Grossman PM. The Association of Peri-Procedural Blood Transfusion with Morbidity and Mortality in Patients Undergoing Percutaneous Lower Extremity Vascular Interventions: Insights from BMC2 VIC. PLoS One. 2016;11:e0165796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Monteiro L, Maricoto T, Solha IS, Monteiro-Soares M, Martins C. Computerised decision to reduce inappropriate medication in the elderly: a systematic review with meta-analysis protocol. BMJ Open. 2018;8:e018988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 510] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 10. | Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1003] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 11. | Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2015;13:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Book BK, Volz MA, Ward EK, Eckert GJ, Pescovitz MD, Wiebke EA. Differences in alloimmune response between elderly and young mice. Transplant Proc. 2013;45:1838-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Koza Y. Acute kidney injury: current concepts and new insights. J Inj Violence Res. 2016;8:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (4)] |
| 14. | Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 544] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 15. | Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, Berwanger O, Pearse RM, Biccard BM, Abraham V, Malaga G, Hillis GS, Rodseth RN, Cook D, Polanczyk CA, Szczeklik W, Sessler DI, Sheth T, Ackland GL, Leuwer M, Garg AX, Lemanach Y, Pettit S, Heels-Ansdell D, Luratibuse G, Walsh M, Sapsford R, Schünemann HJ, Kurz A, Thomas S, Mrkobrada M, Thabane L, Gerstein H, Paniagua P, Nagele P, Raina P, Yusuf S, Devereaux PJ, Devereaux PJ, Sessler DI, Walsh M, Guyatt G, McQueen MJ, Bhandari M, Cook D, Bosch J, Buckley N, Yusuf S, Chow CK, Hillis GS, Halliwell R, Li S, Lee VW, Mooney J, Polanczyk CA, Furtado MV, Berwanger O, Suzumura E, Santucci E, Leite K, Santo JA, Jardim CA, Cavalcanti AB, Guimaraes HP, Jacka MJ, Graham M, McAlister F, McMurtry S, Townsend D, Pannu N, Bagshaw S, Bessissow A, Bhandari M, Duceppe E, Eikelboom J, Ganame J, Hankinson J, Hill S, Jolly S, Lamy A, Ling E, Magloire P, Pare G, Reddy D, Szalay D, Tittley J, Weitz J, Whitlock R, Darvish-Kazim S, Debeer J, Kavsak P, Kearon C, Mizera R, O'Donnell M, McQueen M, Pinthus J, Ribas S, Simunovic M, Tandon V, Vanhelder T, Winemaker M, Gerstein H, McDonald S, O'Bryne P, Patel A, Paul J, Punthakee Z, Raymer K, Salehian O, Spencer F, Walter S, Worster A, Adili A, Clase C, Cook D, Crowther M, Douketis J, Gangji A, Jackson P, Lim W, Lovrics P, Mazzadi S, Orovan W, Rudkowski J, Soth M, Tiboni M, Acedillo R, Garg A, Hildebrand A, Lam N, Macneil D, Mrkobrada M, Roshanov PS, Srinathan SK, Ramsey C, John PS, Thorlacius L, Siddiqui FS, Grocott HP, McKay A, Lee TW, Amadeo R, Funk D, McDonald H, Zacharias J, Villar JC, Cortés OL, Chaparro MS, Vásquez S, Castañeda A, Ferreira S, Coriat P, Monneret D, Goarin JP, Esteve CI, Royer C, Daas G, Chan MT, Choi GY, Gin T, Lit LC, Xavier D, Sigamani A, Faruqui A, Dhanpal R, Almeida S, Cherian J, Furruqh S, Abraham V, Afzal L, George P, Mala S, Schünemann H, Muti P, Vizza E, Wang CY, Ong GS, Mansor M, Tan AS, Shariffuddin II, Vasanthan V, Hashim NH, Undok AW, Ki U, Lai HY, Ahmad WA, Razack AH, Malaga G, Valderrama-Victoria V, Loza-Herrera JD, De Los Angeles Lazo M, Rotta-Rotta A, Szczeklik W, Sokolowska B, Musial J, Gorka J, Iwaszczuk P, Kozka M, Chwala M, Raczek M, Mrowiecki T, Kaczmarek B, Biccard B, Cassimjee H, Gopalan D, Kisten T, Mugabi A, Naidoo P, Naidoo R, Rodseth R, Skinner D, Torborg A, Paniagua P, Urrutia G, Maestre ML, Santaló M, Gonzalez R, Font A, Martínez C, Pelaez X, De Antonio M, Villamor JM, García JA, Ferré MJ, Popova E, Alonso-Coello P, Garutti I, Cruz P, Fernández C, Palencia M, Díaz S, Del Castillo T, Varela A, de Miguel A, Muñoz M, Piñeiro P, Cusati G, Del Barrio M, Membrillo MJ, Orozco D, Reyes F, Sapsford RJ, Barth J, Scott J, Hall A, Howell S, Lobley M, Woods J, Howard S, Fletcher J, Dewhirst N, Williams C, Rushton A, Welters I, Leuwer M, Pearse R, Ackland G, Khan A, Niebrzegowska E, Benton S, Wragg A, Archbold A, Smith A, McAlees E, Ramballi C, Macdonald N, Januszewska M, Stephens R, Reyes A, Paredes LG, Sultan P, Cain D, Whittle J, Del Arroyo AG, Sessler DI, Kurz A, Sun Z, Finnegan PS, Egan C, Honar H, Shahinyan A, Panjasawatwong K, Fu AY, Wang S, Reineks E, Nagele P, Blood J, Kalin M, Gibson D, Wildes T; Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group, on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators; Appendix 1. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Study Investigators Writing Group; Appendix 2. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN Operations Committee; Vascular events In noncardiac Surgery patIents cOhort evaluatioN VISION Study Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 16. | Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, Kurz A. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology. 2017;126:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 798] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 17. | Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1197] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 18. | Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, Salloum R, Meredith UW, Osler TM. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 19. | Silliman CC, Curtis BR, Kopko PM, Khan SY, Kelher MR, Schuller RM, Sannoh B, Ambruso DR. Donor antibodies to HNA-3a implicated in TRALI reactions prime neutrophils and cause PMN-mediated damage to human pulmonary microvascular endothelial cells in a two-event in vitro model. Blood. 2007;109:1752-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Hashemi Tayer A, Amirizadeh N, Ahmadinejad M, Nikougoftar M, Deyhim MR, Zolfaghari S. Procoagulant Activity of Red Blood Cell-Derived Microvesicles during Red Cell Storage. Transfus Med Hemother. 2019;46:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Liu K, Scott JB, Jing G, Li J. Management of Postoperative Hypoxemia. Respir Care. 2021;66:1136-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Kamiya A, Hayashi T, Sakon R, Ishizu K, Wada T, Otsuki S, Yamagata Y, Katai H, Yoshikawa T. Long-term postoperative pneumonia in elderly patients with early gastric cancer. BMC Surg. 2022;22:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 23. | Thompson SL, Lisco SJ. Postoperative Respiratory Failure. Int Anesthesiol Clin. 2018;56:147-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Essien EO, Rali P, Mathai SC. Pulmonary Embolism. Med Clin North Am. 2019;103:549-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 25. | Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 854] [Article Influence: 213.5] [Reference Citation Analysis (0)] |
| 26. | Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 1068] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 27. | Rozental O, Cushing MM, Shander A, Isbister JP, Lasocki S, Meybohm P, Muñoz M, Spahn DR, Weiniger CF, Trentino KM, Girardi NI. Penny-wise and pound-foolish: the challenges of preoperative anaemia management. Br J Anaesth. 2023;131:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Lynch KT, Hassinger TE. Preoperative Identification and Management of Anemia in the Colorectal Surgery Patient. Clin Colon Rectal Surg. 2023;36:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Fajgenbaum DC, Uldrick TS, Bagg A, Frank D, Wu D, Srkalovic G, Simpson D, Liu AY, Menke D, Chandrakasan S, Lechowicz MJ, Wong RS, Pierson S, Paessler M, Rossi JF, Ide M, Ruth J, Croglio M, Suarez A, Krymskaya V, Chadburn A, Colleoni G, Nasta S, Jayanthan R, Nabel CS, Casper C, Dispenzieri A, Fosså A, Kelleher D, Kurzrock R, Voorhees P, Dogan A, Yoshizaki K, van Rhee F, Oksenhendler E, Jaffe ES, Elenitoba-Johnson KS, Lim MS. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129:1646-1657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 30. | Liu B, Wang ZJ, Huang XL. The geriatric nutritional risk index predicts blood transfusion risk in elderly patients undergoing posterior lumbar interbody fusion: a retrospective study. J Orthop Surg Res. 2024;19:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Khan UA, Coca SG, Hong K, Koyner JL, Garg AX, Passik CS, Swaminathan M, Garwood S, Patel UD, Hashim S, Quantz MA, Parikh CR. Blood transfusions are associated with urinary biomarkers of kidney injury in cardiac surgery. J Thorac Cardiovasc Surg. 2014;148:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Thongprayoon C, Cheungpasitporn W, Gillaspie EA, Greason KL, Kashani KB. Association of blood transfusion with acute kidney injury after transcatheter aortic valve replacement: A meta-analysis. World J Nephrol. 2016;5:482-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Lasorsa F, Rutigliano M, Milella M, d'Amati A, Crocetto F, Pandolfo SD, Barone B, Ferro M, Spilotros M, Battaglia M, Ditonno P, Lucarelli G. Ischemia-Reperfusion Injury in Kidney Transplantation: Mechanisms and Potential Therapeutic Targets. Int J Mol Sci. 2024;25:4332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 34. | Carson JL, Stanworth SJ, Guyatt G, Valentine S, Dennis J, Bakhtary S, Cohn CS, Dubon A, Grossman BJ, Gupta GK, Hess AS, Jacobson JL, Kaplan LJ, Lin Y, Metcalf RA, Murphy CH, Pavenski K, Prochaska MT, Raval JS, Salazar E, Saifee NH, Tobian AAR, So-Osman C, Waters J, Wood EM, Zantek ND, Pagano MB. Red Blood Cell Transfusion: 2023 AABB International Guidelines. JAMA. 2023;330:1892-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 249] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 35. | Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133:1840-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 36. | Torres Fuentes CE, Rodríguez Mantilla IE, Cáceres DNG, Camargo Gonzalez DF. Red Blood Cell Transfusion and its Relationship with Pedicle Thrombosis in Microvascular Free Flaps. J Reconstr Microsurg. 2022;38:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Lorente JA, Landín L, De Pablo R, Renes E, Rodríguez-Díaz R, Liste D. Effects of blood transfusion on oxygen transport variables in severe sepsis. Crit Care Med. 1993;21:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 92] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 797] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 39. | Längst E, Crettaz D, Delobel J, Renella R, Bardyn M, Turcatti G, Tissot JD, Prudent M. In vitro-transfusional model for red-blood-cell study: the advantage of lowering hematocrit. Blood Transfus. 2023;21:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Barshtein G, Pajic-Lijakovic I, Gural A. Deformability of Stored Red Blood Cells. Front Physiol. 2021;12:722896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Yoshida T, Prudent M, D'alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17:27-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 193] [Reference Citation Analysis (0)] |
| 42. | Orlov D, Karkouti K. The pathophysiology and consequences of red blood cell storage. Anaesthesia. 2015;70 Suppl 1:29-37, e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Almac E, Bezemer R, Hilarius-Stokman PM, Goedhart P, de Korte D, Verhoeven AJ, Ince C. Red blood cell storage increases hypoxia-induced nitric oxide bioavailability and methemoglobin formation in vitro and in vivo. Transfusion. 2014;54:3178-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Wang H, Wei HW, Shen HC, Li ZZ, Cheng Y, Duan LS, Yin L, Yu J, Guo JR. To study the effect of oxygen carrying capacity on expressed changes of erythrocyte membrane protein in different storage times. Biosci Rep. 2020;40:BSR20200799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 45. | Engoren M, Brown RR, Dubovoy A. A retrospective analysis of the effect of blood transfusion on cerebral oximetry entropy and acute kidney injury. Perfusion. 2017;32:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Bosboom JJ, Klanderman RB, Zijp M, Hollmann MW, Veelo DP, Binnekade JM, Geerts BF, Vlaar APJ. Incidence, risk factors, and outcome of transfusion-associated circulatory overload in a mixed intensive care unit population: a nested case-control study. Transfusion. 2018;58:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Bosboom JJ, Klanderman RB, Migdady Y, Bolhuis B, Veelo DP, Geerts BF, Murphy MF, Vlaar APJ. Transfusion-Associated Circulatory Overload: A Clinical Perspective. Transfus Med Rev. 2019;33:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol). 2006;18:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Fu Z, Geng X, Chi K, Song C, Wu D, Liu C, Hong Q. Efficacy and Safety of Daprodustat Vs rhEPO for Anemia in Patients With Chronic Kidney Disease: A Meta-Analysis and Trial Sequential Analysis. Front Pharmacol. 2022;13:746265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Grüßer L, Keszei A, Coburn M, Rossaint R, Ziemann S, Kowark A; ETPOS Study Group. Intraoperative transfusion practices and perioperative outcome in the European elderly: A secondary analysis of the observational ETPOS study. PLoS One. 2022;17:e0262110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/