Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.105833
Revised: April 9, 2025
Accepted: May 30, 2025
Published online: July 27, 2025
Processing time: 165 Days and 23 Hours
Pheochromocytoma and paraganglioma (PGL) are a rare group of neuroendocrine neoplasms with characteristic genetic diversity and catecholamine secretion patterns. They arise from non-neuronal and non-epithelial neuroendocrine cells of the paraganglia, and have the highest rate of heritability among all tumors.
A 76-year-old woman presented with the complaint of dizziness that had persisted for one week. She had a 30-year history of hypertension. Despite long-term use of antihypertensive drugs, her blood pressure was not effectively controlled. A tumor was subsequently found in the head of the pancreas by computed tomography and magnetic resonance imaging and she was initially diagnosed with an aneurysm. On December 21, 2021, she underwent resection of the retroperitoneal tumor and pancreatic repair surgery. However, after postoperative pathological analysis and immunohistochemistry, the diagnosis was revised to PGL. After two years and eight months of follow-up, the tumor did not recur or metastasize, and her blood pressure returned to normal without taking antihypertensive drugs.
The possibility of PGL should be considered when a tumor is identified and patients have catecholamine secretion related symptoms that are difficult to control with medications.
Core Tip: We report a rare case of paraganglioma (PGL) of the pancreatic head in a patient with a history of persistent hypertension. Due to atypical clinical presentation and imaging features, it is difficult to differentiate PGL from peripancreatic tumor. PGL may induce persistent clinical symptoms related to hypertension without causing other clinical manifestations that draw the attention of from patients over a long period. In order to improve the diagnostic accuracy of pancreatic PGL, the combination of tests for specific biomarkers (metanephrines/normetanephrines is recommended) and imaging examinations based on individual circumstances is highly recommended. Surgery is still recommended as the mainstay of treatment.
- Citation: Luo SZ, Liu JR, Liu TQ, Chen Q. Functional paraganglioma of the pancreatic head: A case report and review of literature. World J Gastrointest Surg 2025; 17(7): 105833
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/105833.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.105833
Pheochromocytoma (PCC) originates from the adrenal medulla, whereas paraganglioma (PGL) arise from extra-adrenal paraganglia[1]. PGLs are a rare group of vascular neuroendocrine tumors of paraganglionic origin. According to the fourth edition of the World Health Organization classification of endocrine tumors, instead of benign or malignant, PGLs are now categorized as either metastatic or nonmetastatic[2]. The overall incidence of PCC/PGL varies from 0.04/100000 to 0.95/100000 per year, showing an increasing trend over time[3]. In this context, PGLs comprise 15% of PCCs and PGL[4]. The most common site of occurrence is the carotid body, retroperitoneum and base of the skull; therefore, pancreatic PGL (PPGL) is rare[5]. In this study, we report a case of a PGL in the head of the pancreas with dizziness as the only chief complaint and hypertension as the only known disease.
A 76-year-old female presented with dizziness for one week.
Thirty years ago, the patient was diagnosed with hypertension, and she took valsartan tablets (80 mg once daily) to lower her blood pressure. Despite the medication, her blood pressure did not decrease.
The patient was previously in good health.
She denied any family history of malignant tumors.
On admission to the hospital, the patient underwent a comprehensive physical examination. The results indicated that, apart from a blood pressure measurement of 151/81 mmHg, the patient presented with a soft and non-tender abdomen, devoid of any malformations or palpable masses. No other abnormal findings were detected during the examination.
A battery of laboratory examinations encompassing routine blood work, urinalysis, assessments of liver and kidney function, electrolyte analyses, and tumor marker screenings was conducted, all of which returned normal results.
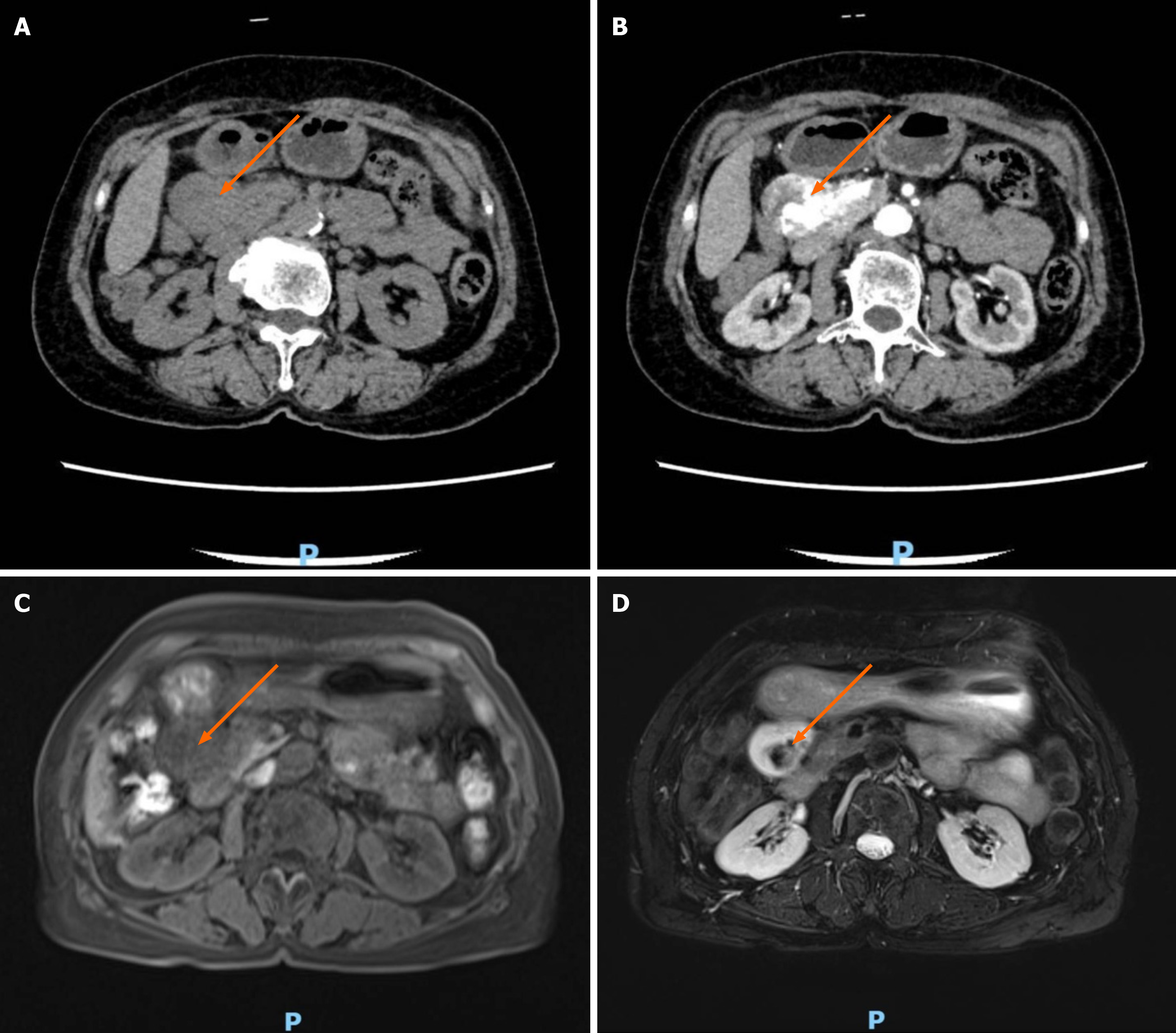
Given that neither the patient’s physical examination nor the laboratory examination results were sufficient to elucidate the disease etiology, we proceeded to incorporate imaging examinations into the diagnostic workup. Ultrasound revealed a swollen mass in the head of the pancreas. A contrast computed tomography (CT) scan of the upper abdomen revealed a mass in the head of the pancreas. An enhanced scan revealed blood vessel penetration. Dilated blood vessels were observed in the center of the mass (Figure 1A and B). A contrast magnetic resonance imaging (MRI) scan of the right abdominal area (pancreatic head) revealed a tumor approximately 3.2 cm × 4.0 cm × 5.2 cm in size. The border was clear, the T1 weighted imaging signal was slightly low, and the T2 weighted imaging signal was mainly high. Within the small patchy low signal, the diffusion weighted imaging signal was high. The apparent diffusion coefficient tumor scan signal was an equal signal, the enhanced arterial scan showed patchy enhancement in the center of the lesion, and the delayed scan showed nonenhancement within the strip of patchy area (Figure 1C and D). In combination with CT findings, we suspected that the mass was an aneurysm.
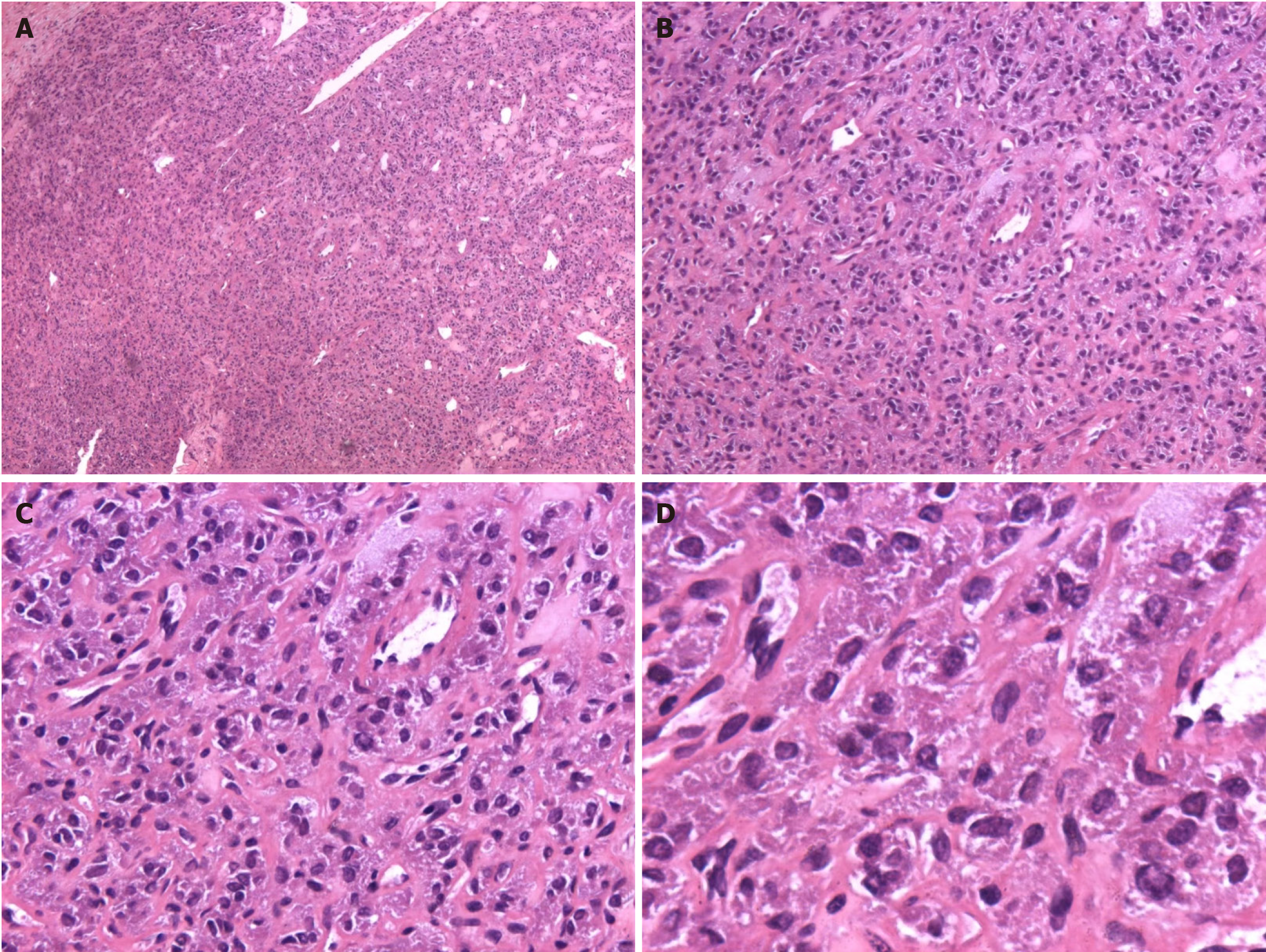
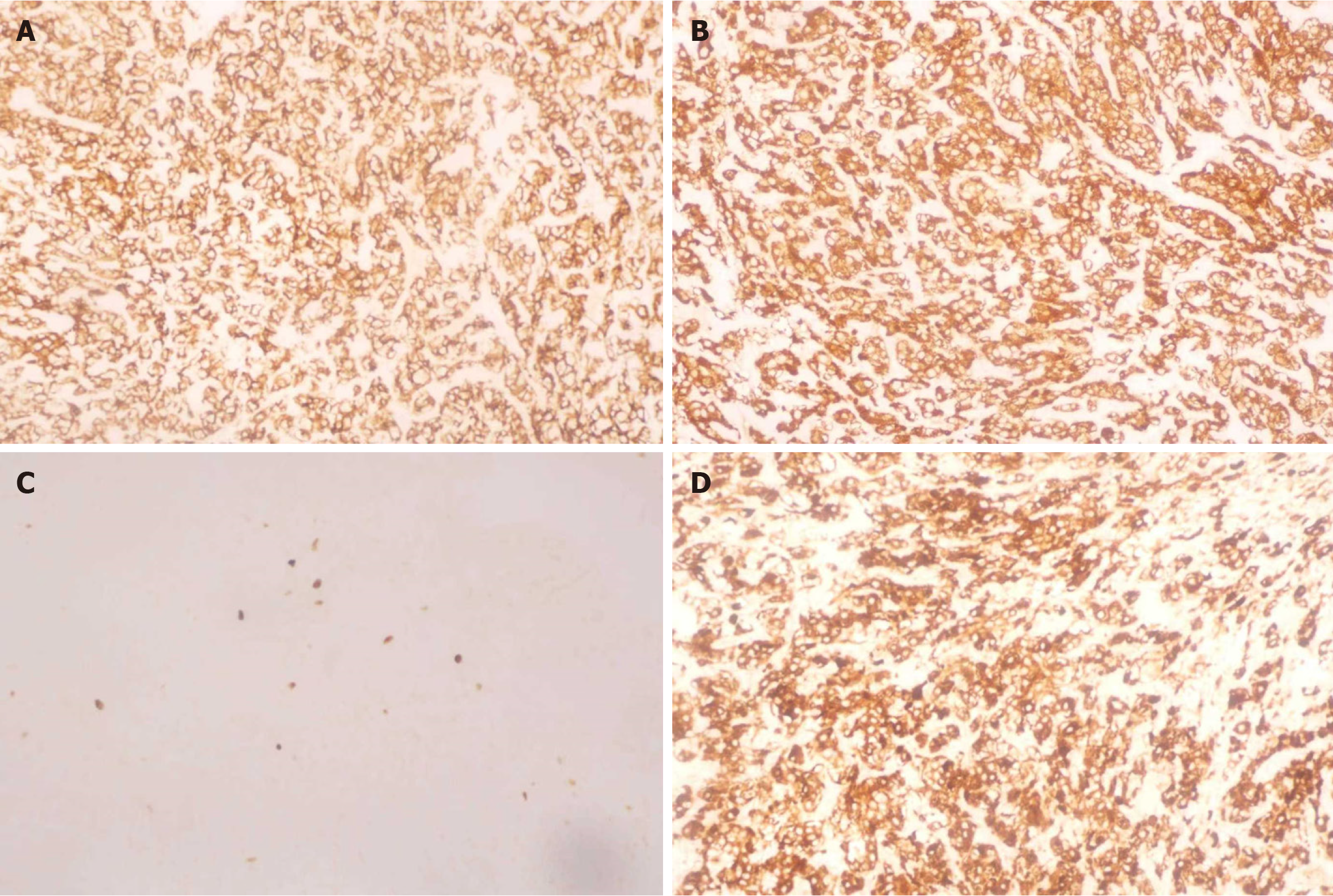
Following postoperative pathological analysis and immunohistochemistry, the diagnosis was changed to PGL (Figures 2 and 3).
We subsequently informed the patient of the suspected aneurysm. Upon obtaining her informed consent, we made a decision to carry out resection of the mass. Initial blood pressure was controlled by administering oral antihypertensive drugs, namely valsartan capsules (80 mg once daily) and amlodipine (5 mg once daily). Valsartan capsules and amlodipine were discontinued immediately once the blood pressure dropped to an operable range, that is, when the blood pressure was below 140/90 mmHg. During the resection procedure, a round mass in the pancreatic head was discovered. The surrounding connective tissue was subsequently freed, the vascular branches entering the tumor were ligated, and the tumor was completely removed. Tumor removal and an intraoperative rapid histological examination were performed. Rapid intraoperative pathology revealed that the tumor was to be treated as benign. Subsequent, examination confirmed that there was no pancreatic duct loss, and the pancreas was repaired. The surgical incision was gradually closed layer by layer.
Two years and eight months after surgery, the tumor did not recur or metastasize, and the patient’s blood pressure returned to normal without additional antihypertensive medication.
The prevalence of PCC and PGL ranges between 0.2% and 0.6% among hypertensive outpatients[6-9]. PPGL is even rarer among PGL occurring outside the adrenal gland, as there are only 6 cases of functional PPGL (12%) in 50 clinical cases of PPGL reported globally in the English-based literature. Elderly females are predominant with a male-to-female ratio of 8:17 among all patients, and the mean age at first diagnosis in PPGL patients was 52 years (range from 19 to 85 years, Supplementary Table 1)[10-47]. PPGL often presents as a solid mass in the head of the pancreas, which is rarely cystic and occasionally occurs in the tail and body. Non-functional PGL is clinically silent and usually found based on imaging diagnosis, while high-level catecholamines (CAs) secretion, persistent hypertension, palpitations, and paroxysmal headaches are frequently observed in cases of functional PGL[48]. Abdominal pain, lumbar pain, constipation or dyspepsia are the most common onset symptoms as they were observed in 32 cases, while 16 patients presented no symptoms and were diagnosed after physical examinations. In addition, CA secretion induced symptoms such as hypertension, palpitations, headache or malaise were major symptoms in 2 cases.
In the case reported in this paper, the patient was diagnosed with hypertension 30 years previously and regularly took antihypertensive drugs. Therefore, it is difficult to trace the relevant information and clarify the sequence between hypertension and tumor occurrence. PGL has been reported to have a relatively higher overall survival rate and disease-specific survival rate (73.3% and 80.5% respectively) compared to PCC (54.0% and 73.0% respectively), which makes long-term survival with the tumor possible[49]. Combined with the data in the table, it is evident that the size of the tumor did not invariably correlate with either the manifestation or absence of symptoms, or with the severity. Primary tumor diagnosis at an older age (P = 0.0011), male sex (P = 0.014), major adverse cardiovascular events, synchronous metastases (P < 0.0001), larger tumor size (P = 0.0039), increased dopamine levels (P = 0.0195), and failure to remove the primary tumor, have been highlighted as risk factors correlated with PGL progression[50,51].
The development of functional PGL commonly accompanies overproduction of CAs, therefore, accurate measurement of CAs and their metabolites is of great significance for clinical decision-making. Measuring metanephrine (MN) in blood and urine is considered the gold standard for functional PGL diagnosis, with a sensitivity of 99% for incidental and hereditary PGL and a specificity of 95% for heritability (89% for incidentals), which is better than any test combination. If the concentration of MN is only 2-4 times above normal values, the evaluation should be performed at baseline and 3 hours after oral administration of 0.3 mg clonidine (clonidine test), and suppression lower than 40% indicates PGL. In addition, the diagnostic sensitivity and specificity of chromogranin A are 83%-86% and 76%-98%, respectively, which could also be an alternative or complement[52-56].
In our case, the examination of MN and normetanephrine was overlooked initially, but CT and MRI scanning were performed instead for localization and diagnosis. Also, endoscopic ultrasound-guided fine-needle aspiration was not conducted due to the risks of mass metastasis. Homogeneity or heterogeneity can be observed in masses on contrast enhanced CT in PPGL patients, together with necrosis, hemorrhage or calcification; however, the appearance may be similar to an aneurysm. On contrast enhanced MRI, the typical appearance of salt-and-pepper can be found in cases of PGL, which is formed by the rapid flow cavity in the enlarged tumor blood vessels and hypersignal areas secondary to slow flow and internal hemorrhage of the tumor[57]. CT is still regarded as the optimal modality for localization and diagnosis of PPGL due to its cost-effectiveness, remarkable sensitivity, relatively short scanning duration, as well as excellent spatial resolution capabilities in thoracic, abdominal, and pelvic regions. If MN biochemical test results are positive, enhanced CT scanning is recommended[58,59]. Single photon emission CT and positron emission tomography can also be considered due to their high sensitivity and specificity for specific molecular targets, such as norepinephrine transporter and L-amino acid transporter 1, but are costly and radioactive[58]. Genetic testing in routine practice is highly recommended for any patients diagnosed with PGL by the working group on endocrine hypertension of the European Society of Hypertension, as genetic alterations explain 80% of all cases[60]. New genes or new presentations of known genes associated with PGL development include DNA methyltransferase 3 alpha, dihydrolipoamide S-succinyltransferase, SUCLG2a, mastermind like transcriptional coactivator 3 fusions, RET fusions, and patients may benefit from related genetic counseling and clinical surveillance[61].
The therapy of choice is surgery whenever possible for PGL. As the major potential postoperative complications consist of hypertension, hypotension and rebound hypoglycemia, multidisciplinary efforts from the surgeon, anesthetist and cardiologist during the perioperative period are demanded. A crucial factor for the treatment lies in making sufficient preoperative preparations, including control of hypertension and blood volume improvement[62]. The use of α-blockers (prazosin, doxazosin, and terazosin, etc.) is mandatory for 7 to 30 days in the preoperative management of PGL patients undergoing tumor resection in order to prevent hypertensive crises and cardiac arrhythmias[63]. However, α-blockers can induce dilation of constricted blood vessels, and blood volume deficiency. Therefore, blood volume should be routinely expanded preoperatively with crystalloid and colloid fluids to avoid severe postoperative hypotensive events. It is generally accepted that good preoperative fluid circulation is manifested by a stable blood pressure below 130/80 mmHg, heart rate between 60 and 80 beats per minute, and the absence of paroxysmal headache, dizziness, palpitations, excessive sweating, warm extremity ends and red nail beds[64]. During the operation, the key points include minimizing tumor compression, ensuring the integrity of the tumor envelope, monitoring the patient’s vital signs and maintaining the patient’s internal environmental homeostasis[65]. On the basis of the above treatment, all patients who have been diagnosed with PGL require life-long and personalized follow-up in accordance with their underlying germline or somatic mutations, together with disease characteristics[66].
PGL can be diagnosed according to a high secretion level of CA via serum MN/normetanephrine and special imaging characteristics, including homogeneous masses and salt-and-pepper appearance, before surgery and/or postoperative pathology with or without typical clinical features. The recommended management of PGL should be multidisciplinary, involving surgeons, cardiologists and anesthetists.
| 1. | Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol. 2017;28:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 316] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 2. | Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, Tischler A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr Pathol. 2022;33:90-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 262] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 3. | Al Subhi AR, Boyle V, Elston MS. Systematic Review: Incidence of Pheochromocytoma and Paraganglioma Over 70 Years. J Endocr Soc. 2022;6:bvac105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 4. | Disick GI, Palese MA. Extra-adrenal pheochromocytoma: diagnosis and management. Curr Urol Rep. 2007;8:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Hamidi O. Metastatic pheochromocytoma and paraganglioma: recent advances in prognosis and management. Curr Opin Endocrinol Diabetes Obes. 2019;26:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000;6:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 259] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 369] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Sinclair AM, Isles CG, Brown I, Cameron H, Murray GD, Robertson JW. Secondary hypertension in a blood pressure clinic. Arch Intern Med. 1987;147:1289-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Cope C, Greenberg SH, Vidal JJ, Cohen EA. Nonfunctioning nonchromaffin paraganglioma of the pancreas. Arch Surg. 1974;109:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Fujino Y, Nagata Y, Ogino K, Watahiki H, Ogawa H, Saitoh Y. Nonfunctional paraganglioma of the pancreas: report of a case. Surg Today. 1998;28:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Parithivel VS, Niazi M, Malhotra AK, Swaminathan K, Kaul A, Shah AK. Paraganglioma of the pancreas: literature review and case report. Dig Dis Sci. 2000;45:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ohkawara T, Naruse H, Takeda H, Asaka M. Primary paraganglioma of the head of pancreas: contribution of combinatorial image analyses to the diagnosis of disease. Intern Med. 2005;44:1195-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Kim SY, Byun JH, Choi G, Yu E, Choi EK, Park SH, Lee MG. A case of primary paraganglioma that arose in the pancreas: the Color Doppler ultrasonography and dynamic CT features. Korean J Radiol. 2008;9 Suppl:S18-S21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Tsukada A, Ishizaki Y, Nobukawa B, Kawasaki S. Paraganglioma of the pancreas: a case report and review of the literature. Pancreas. 2008;36:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Sangster G, Do D, Previgliano C, Li B, LaFrance D, Heldmann M. Primary retroperitoneal paraganglioma simulating a pancreatic mass: a case report and review of the literature. HPB Surg. 2010;2010:645728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | He J, Zhao F, Li H, Zhou K, Zhu B. Pancreatic paraganglioma: A case report of CT manifestations and literature review. Quant Imaging Med Surg. 2011;1:41-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Lightfoot N, Santos P, Nikfarjam M. Paraganglioma mimicking a pancreatic neoplasm. JOP. 2011;12:259-261. [PubMed] |
| 19. | Singhi AD, Hruban RH, Fabre M, Imura J, Schulick R, Wolfgang C, Ali SZ. Peripancreatic paraganglioma: a potential diagnostic challenge in cytopathology and surgical pathology. Am J Surg Pathol. 2011;35:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Higa B, Kapur U. Malignant paraganglioma of the pancreas. Pathology. 2012;44:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 21. | Ganc RL, Castro AC, Colaiacovo R, Vigil R, Rossini LG, Altenfelder R. Endoscopic ultrasound-guided fine needle aspiration for the diagnosis of nonfunctional paragangliomas: a case report and review of the literature. Endosc Ultrasound. 2012;1:108-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Al-Jiffry BO, Alnemary Y, Khayat SH, Haiba M, Hatem M. Malignant extra-adrenal pancreatic paraganglioma: case report and literature review. BMC Cancer. 2013;13:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Borgohain M, Gogoi G, Das D, Biswas M. Pancreatic paraganglioma: An extremely rare entity and crucial role of immunohistochemistry for diagnosis. Indian J Endocrinol Metab. 2013;17:917-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Straka M, Soumarova R, Migrova M, Vojtek C. Pancreatic paraganglioma - a rare and dangerous entity. Vascular anatomy and impact on management. J Surg Case Rep. 2014;2014:rju074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Liao Q, Hu Y, Zhao Y. Paraganglioma of the pancreas: a potentially functional and malignant tumor. World J Surg Oncol. 2014;12:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Meng L, Wang J, Fang SH. Primary pancreatic paraganglioma: a report of two cases and literature review. World J Gastroenterol. 2015;21:1036-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 27. | Misumi Y, Fujisawa T, Hashimoto H, Kagawa K, Noie T, Chiba H, Horiuchi H, Harihara Y, Matsuhashi N. Pancreatic paraganglioma with draining vessels. World J Gastroenterol. 2015;21:9442-9447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Ünver M, Öztürk Ş, Erol V, Cartı EB, Bozbıyık O, Kebapçı E, Ölmez M, Akbulut G. Retroperitoneal paraganglioma presenting with pancytopenia: A rare case with rare manifestation. Int J Surg Case Rep. 2015;14:77-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Ginesu GC, Barmina M, Paliogiannis P, Trombetta M, Cossu ML, Feo CF, Addis F, Porcu A. Nonfunctional paraganglioma of the head of the pancreas: A rare case report. Int J Surg Case Rep. 2016;28:81-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Tumuluru S, Mellnick V, Doyle M, Goyal B. Pancreatic Paraganglioma: A Case Report. Case Rep Pancreat Cancer. 2016;2:79-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Lin S, Peng L, Huang S, Li Y, Xiao W. Primary pancreatic paraganglioma: a case report and literature review. World J Surg Oncol. 2016;14:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 32. | Liang W, Xu S. CT and MR Imaging Findings of Pancreatic Paragangliomas: A Case Report. Medicine (Baltimore). 2016;95:e2959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Furcea L, Mois E, Al Hajjar N, Seicean A, Badea R, Graur F. Pancreatic gangliocytic paraganglioma - CEUS appearance. J Gastrointestin Liver Dis. 2017;26:336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Nonaka K, Matsuda Y, Okaniwa A, Kasajima A, Sasano H, Arai T. Pancreatic gangliocytic paraganglioma harboring lymph node metastasis: a case report and literature review. Diagn Pathol. 2017;12:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Zeng J, Simsir A, Oweity T, Hajdu C, Cohen S, Shi Y. Peripancreatic paraganglioma mimics pancreatic/gastrointestinal neuroendocrine tumor on fine needle aspiration: Report of two cases and review of the literature. Diagn Cytopathol. 2017;45:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Nguyen E, Nakasaki M, Lee TK, Lu D. Diagnosis of paraganglioma as a pancreatic mass: A case report. Diagn Cytopathol. 2018;46:804-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Fite JJ, Maleki Z. Paraganglioma: Cytomorphologic features, radiologic and clinical findings in 12 cases. Diagn Cytopathol. 2018;46:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Chattoraj AK, Rao UM, Sarkar N, Jakka S. Non-functional retroperitoneal paraganglioma: A case report. J Family Med Prim Care. 2019;8:1497-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Zongo N, Koama A, Kambou/Tiemtoré BMA, Nde/Ouédraogo NA, Zida M, Ouédraogo MNL, Ouangré E, Sanou A, Lompo OM, Diallo O, Lougué/Sorgho C, Cissé R. Ectopic pheochromocytoma or paraganglioma of the ZUCKERKANDL organ: A case report and review of the literature. Int J Surg Case Rep. 2019;60:120-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Abbasi A, Wakeman KM, Pillarisetty VG. Pancreatic paraganglioma mimicking pancreatic neuroendocrine tumor. Rare Tumors. 2020;12:2036361320982799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Jiang CN, Cheng X, Shan J, Yang M, Xiao YQ. Primary pancreatic paraganglioma harboring lymph node metastasis: A case report. World J Clin Cases. 2021;9:8071-8081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Lanke G, Stewart JM, Lee JH. Pancreatic paraganglioma diagnosed by endoscopic ultrasound-guided fine needle aspiration: A case report and review of literature. World J Gastroenterol. 2021;27:6322-6331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Park JS, Min SJ, Min SK, Choi J. Pancreatic Paraganglioma: a Case Report and Literature Review. Investig Magn Reson Imaging. 2021;25:47. [DOI] [Full Text] |
| 44. | Wang W, Qin Y, Zhang H, Chen K, Liu Z, Zheng S. A rare case of retroperitoneal paraganglioma located in the neck of the pancreas: a case report and literature review. Gland Surg. 2021;10:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Kim M, Aploks K, Vargas-Pinto S, Dong X. RET T244I Germline Variant Mutation in a Patient with Pancreatic Paraganglioma and Primary Hyperparathyroidism. Int J Endocrinol Metab. 2022;20:e121056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Li T, Yi RQ, Xie G, Wang DN, Ren YT, Li K. Pancreatic paraganglioma with multiple lymph node metastases found by spectral computed tomography: A case report and review of the literature. World J Clin Cases. 2022;10:11638-11645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Zhao Z, Guo Y, Liu L, Zhang L, Li S, Yang J. Primary non-functional pancreatic paraganglioma: A case report and review of the literature. J Int Med Res. 2022;50:3000605221143023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Sherwani P, Anand R, Narula MK, Siddiqui AA, Aggarwal S. Concurrent nonfunctional paraganglioma of the retroperitoneum and urinary bladder: A case report with literature review. Indian J Radiol Imaging. 2015;25:198-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Goffredo P, Sosa JA, Roman SA. Malignant pheochromocytoma and paraganglioma: a population level analysis of long-term survival over two decades. J Surg Oncol. 2013;107:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Hamidi O, Young WF Jr, Iñiguez-Ariza NM, Kittah NE, Gruber L, Bancos C, Tamhane S, Bancos I. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J Clin Endocrinol Metab. 2017;102:3296-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 51. | Raber W, Schendl R, Arikan M, Scheuba A, Mazal P, Stadlmann V, Lehner R, Zeitlhofer P, Baumgartner-Parzer S, Gabler C, Esterbauer H. Metastatic disease and major adverse cardiovascular events preceding diagnosis are the main determinants of disease-specific survival of pheochromocytoma/paraganglioma: long-term follow-up of 303 patients. Front Endocrinol (Lausanne). 2024;15:1419028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 52. | Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1592] [Cited by in RCA: 1857] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 53. | Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev. 2003;24:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 264] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 54. | Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88:2656-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 55. | Garcia-Carbonero R, Matute Teresa F, Mercader-Cidoncha E, Mitjavila-Casanovas M, Robledo M, Tena I, Alvarez-Escola C, Arístegui M, Bella-Cueto MR, Ferrer-Albiach C, Hanzu FA. Multidisciplinary practice guidelines for the diagnosis, genetic counseling and treatment of pheochromocytomas and paragangliomas. Clin Transl Oncol. 2021;23:1995-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 56. | Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 703] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 57. | Et-Tahir Y, Merzem A, Belgadir H, Amriss O, Moussali N, El Benna N. Salt and pepper appearance: A characteristic feature of paragangliomas. J Clin Neurosci. 2023;114:144-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 58. | Timmers HJLM, Taïeb D, Pacak K, Lenders JWM. Imaging of Pheochromocytomas and Paragangliomas. Endocr Rev. 2024;45:414-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 59. | Mohammed MF, ElBanna KY, Ferguson D, Harris A, Khosa F. Pheochromocytomas Versus Adenoma: Role of Venous Phase CT Enhancement. AJR Am J Roentgenol. 2018;210:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Lenders JWM, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, Pacak K, Crona J, Zelinka T, Mannelli M, Deutschbein T, Timmers HJLM, Castinetti F, Dralle H, Widimský J, Gimenez-Roqueplo AP, Eisenhofer G. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38:1443-1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 292] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 61. | Gimenez-Roqueplo AP, Robledo M, Dahia PLM. Update on the genetics of paragangliomas. Endocr Relat Cancer. 2023;30:e220373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Fang F, Ding L, He Q, Liu M. Preoperative Management of Pheochromocytoma and Paraganglioma. Front Endocrinol (Lausanne). 2020;11:586795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Lima JV Júnior, Kater CE. The Pheochromocytoma/Paraganglioma syndrome: an overview on mechanisms, diagnosis and management. Int Braz J Urol. 2023;49:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 64. | Kim JH, Lee HC, Kim SJ, Lee KE, Jung KC. Characteristics of Intraoperative Hemodynamic Instability in Postoperatively Diagnosed Pheochromocytoma and Sympathetic Paraganglioma Patients. Front Endocrinol (Lausanne). 2022;13:816833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Aygun N, Uludag M. Pheochromocytoma and Paraganglioma: From Treatment to Follow-up. Sisli Etfal Hastan Tip Bul. 2020;54:391-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Nölting S, Bechmann N, Taieb D, Beuschlein F, Fassnacht M, Kroiss M, Eisenhofer G, Grossman A, Pacak K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr Rev. 2022;43:199-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/