Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.105136
Revised: March 16, 2025
Accepted: May 19, 2025
Published online: July 27, 2025
Processing time: 189 Days and 21.9 Hours
The diagnosis of gastric inflammatory fibroid polyps (IFPs) mainly depends on pathological confirmation after endoscopic or surgical treatment. Gastric IFP have typical manifestations under endoscopic ultrasonography (EUS), but atypical EUS features have also been reported. Previous studies have found that atypical features of gastric IFPs observed under EUS have corresponding histological manifestations. At present, there is no study elaborating the EUS manifestations of gastric IFPs at different pathological stages. We hypothesize that gastric IFPs at different pathological stages may have different EUS features.
To describe EUS features of gastric IFPs and compare with their pathological characteristics.
Clinical data of 53 inpatients with pathologically diagnosed gastric IFPs after endoscopic treatment were collected. All patients underwent preoperative EUS. We analyzed the EUS characteristics of the lesions and compared with the pathological characteristics and staging of the resected specimens.
Most gastric IFPs showed medium-low echo (67.9%), homogeneous echo (90.6%), and unclear boundaries (83%), and involved the second and third layers of the gastric wall (69.8%) under EUS. The echogenicity level and echo homogeneity were significantly correlated with the pathological stage of gastric IFP. Gastric IFPs in the nodular stage presented hypoechoic and homogeneous echo. Gastric IFPs in the fibrovascular stage mostly showed medium-low echo and homogeneous echo. Gastric IFPs in the sclerotic stage showed different echogenicity levels and echo homogeneity. The accuracy of EUS in diagnosing gastric IFPs was 66.0% (35/53), and the accuracy in determining the origin layer of gastric IFPs was 73.4% (39/53).
Gastric IFPs at different pathological stages have different EUS features. In order to improve the diagnostic rate, it is necessary to combine EUS with EUS-guided fine-needle aspiration or artificial intelligence.
Core Tip: This is believed to be the first study analyzing the endoscopic ultrasonographic (EUS) manifestations of gastric inflammatory fibroid polyps (IFPs) at different pathological stages. It was found that echogenicity level and echo homogeneity were significantly correlated with the pathological stage of gastric IFPs. Nodular stage gastric IFPs presented hypoechoic and homogeneous echo. Fibrovascular stage gastric IFPs mostly showed medium-low echo and homogeneous echo. Sclerotic stage showed different echogenicity levels and echo homogeneity. The accuracy of EUS in diagnosing gastric IFPs was 66.0%, and the accuracy in determining the origin layer of gastric IFPs was 73.4%.
- Citation: Zhang FM, Ning LG, Wang XX, Du HJ, Zhu HT, Chen HT. Comparison of endoscopic ultrasonography features and pathological staging of gastric inflammatory fibroid polyps. World J Gastrointest Surg 2025; 17(7): 105136
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/105136.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.105136
Inflammatory fibroid polyp (IFP) is a benign tumor of mesenchymal origin and its pathogenesis remains unclear. Pathologically, it is characterized by the specific arrangement of fibrous tissue and vascular infiltration, as well as eosinophil infiltration[1,2]. The diagnosis of gastric IFP is mainly based on surgical or endoscopic specimens combined with pathological examination[3-6]. Histologically, gastric IFP is divided into three stages: nodular, fibroangiogenic and sclerotic[1]. The nodular stage is mainly composed of immature fibroblastic-like spindle cells, with a small number of inflammatory cells such as eosinophils and thin-walled vascular proliferation. In the fibrovascular stage, mature fibroblastic-like spindle cells proliferate, but collagenization is poor, thin- and thick-walled vessels are visible, and there is a significant increase in eosinophil proliferation around the vessels, arranged in concentric circles. The sclerotic stage is characterized by collagen fiber proliferation, which may be accompanied by hyaline degeneration, a significant increase in thick-walled blood vessels, and a large number of eosinophil infiltrations.
Gastric IFP usually presents as polypoid elevation or submucosal elevation under endoscopy. However, gastric IFP by routine mucosal biopsy under endoscopy is prone to misdiagnosis as hyperplastic polyps, and the definitive diagnosis mainly depends on pathological examination after excision. Endoscopic ultrasonography (EUS) can be used to evaluate the focal location, tumor size, layer origin, edge condition, and relationship with surrounding organs. It is a valuable tool in establishing diagnosis and determining treatment plan[7-10]. Gastric IFPs have typical manifestations under EUS which are characterized by an indistinct margin, a hypoechoic homogeneous lesion, and location within the second and/or third echolayer, without involvement of the fourth echolayer[2]. However, there are also reports on the atypical EUS features of gastric IFP. Aydin et al[11] reported a gastric IFP which showed a hypoechoic homogenous lesion within the second and third echolayers under EUS, and a distinct hyperechoic area within the lesion. Matsushita et al[2] analyzed the EUS features of 10 cases of pathologically diagnosed gastric IFPs and they found that the internal echo was hyperechoic or heterogeneous in two cases, which corresponded to numerous small blood vessels in the specimen. At present, there is no study elaborating the EUS manifestations of gastric IFP at different pathological stages. We speculate that gastric IFPs at different pathological stages may have different EUS features.
This study retrospectively analyzed 53 patients with pathologically diagnosed gastric IFP who were treated in the Department of Gastroenterology of the First Affiliated Hospital of Zhejiang University School of Medicine over the past 10 years. This is the study with the largest sample size so far in comparing the pathological stages of gastric IFPs with the features of EUS. By analyzing the EUS features of pathologically diagnosed gastric IFPs, this study aims to evaluate the accuracy of EUS in diagnosing gastric IFP, and explore the different EUS manifestations of gastric IFPs at different pathological stages.
Fifty-three patients with gastric IFPs who were treated in the Department of Gastroenterology of the First Affiliated Hospital of Zhejiang University School of Medicine from January 1, 2013 to May 1, 2023 were selected as the study subjects. Examinations and treatments were provided with informed consent from the patients. This study was approved by the Ethics Committee of our hospital. Inclusion criteria were: Patients with gastric polyps/submucosal tumors found by endoscopy, further examined by EUS, and pathologically diagnosed with gastric IFPs after endoscopic treatment. Exclusion criteria were: Patients with incomplete or missing medical records on examination, diagnosis, and treatment.
All patients underwent gastroscopy and EUS before surgery. The instrument used for gastroscopy was the GIF-H-290 gastroscope (Olympus). The instruments used for EUS were: EU-ME2, GF-UCT260, the ultrasonic probe driver MAJ-1720, and the small ultrasonic probe UM-DP12-25R. The frequency of the conventional EUS ranged from 5 to 12 MHz. When using the miniature ultrasonic probe, we used frequencies 20 MHz and 12 MHz. The lower the frequency, the deeper it can penetrate the tissues, allowing us to observe the structures and lesions outside the digestive tract wall. The higher the frequency, the relatively higher the resolution it can provide, enabling a clearer display of the structures of each layer of the digestive tract wall and smaller lesions.
In conventional EUS, we often adopted the water balloon method or the water injection method. When performing examinations with a miniature ultrasonic probe, we used the water injection method. For some parts of the stomach such as the gastric fundus and gastric angle, where water is not easy to fill or the observation angle is not ideal due to the effect of gravity during the water injection, the water balloon method can better conform to the gastric wall of these parts by adjusting the position and shape of the water balloon, so as to obtain clearer images and help detect and observe the lesions in these parts. For lesions in other parts, we adopted the water injection method as it can evenly fill the gastric cavity with water, better eliminate the interference of gas, and provide a broader and continuous field of view. The water injection method enables the ultrasonic probe to fully contact the gastric wall, reduces the interface reflection, and makes the image clearer. In addition, the water injection method only requires injecting an appropriate amount of water through the endoscopic biopsy channel, and the operation is simple and quick. Moreover, since it creates an examination environment by naturally filling the gastric cavity, it exerts less pressure on the gastric wall, and patients feel less discomfort during the examination.
EUS showed that the five-layer structure of the normal gastric wall was closely related to the histological layer. The first layer was the mucosal interface and the superficial mucosa, which showed high echo; the second layer was the deep mucosa, which showed low echo; the third layer was the submucosa and the interface between the submucosa and the proper muscularis, which showed high echo; the fourth layer was the proper muscularis, which showed low echo; and the fifth layer was the serosa and the subserosal fat, which showed high echo. The size of the lesion was expressed as the maximum diameter measured by EUS, rounded to the nearest millimeter (mm). The echo of the lesion was divided into five grades: Level 0 corresponds to the anechoic pattern, level 1 corresponds to the fourth layer of the gastric wall, presenting as hypoechoic, level 2 corresponds to the echo of the splenic parenchyma, showing medium-low echo, the echo of level 3 is between level 2 and level 4, presenting as medium-high echo, level 4 corresponds to the third layer of the gastric wall, showing high echo. The EUS findings of the lesion were recorded: Layer of origin, size, echogenicity and homogeneity, margin condition, and the EUS diagnosis.
Criteria for EUS diagnosis of IFPs: Most of the lesions present as spherical, hemispherical or broad-based polypoid protrusions, involving the second and third layers of the gastric wall, with internal low echo, homogeneous echo and indistinct margins.
Criteria for EUS diagnosis of ectopic pancreas in the stomach: It is mostly located in the gastric antrum, and there is a typical umbilical-like depression or opening at the center of the lesion. Under EUS, the lesion often originates from the submucosa, involves the muscularis propria and mucosa, with indistinct margins, mainly showing medium-low echo, heterogeneous internal echo, and scattered high-intensity echo. In some cases, duct-like echo structures can be seen within the lesion, with clear margins, but no definite capsule.
Criteria for EUS diagnosis of gastric gastrointestinal stromal tumors: It mostly occurs in the gastric fundus in a round or round-like shape. Most of the tumors originate from the muscularis propria, mostly showing uniform low echo. In larger tumors, anechoic areas or hyperechoic areas may appear due to bleeding, necrosis, etc., with clear margins and a complete capsule.
Criteria for EUS diagnosis of gastric neuroendocrine tumors: It presents as single or multiple polypoid protrusions, mostly seen in the gastric body and gastric fundus. Under EUS, the tumor mostly originates from the submucosa, and can also be located in the mucosa or muscular layer, with low echo and homogeneous echo. When the lesion is large, it may show heterogeneous echo, generally with clear margins. Some tumors may invade the surrounding tissues, and the margins become blurred.
Criteria for EUS diagnosis of gastric adenomas: It is usually round or oval, and can also be flat or broad-based polypoid. It mostly originates from the mucosa, with homogeneous internal echo, generally medium echo or slightly hyperechoic echo, similar to the echo of the surrounding normal gastric mucosa, with clear margins.
Criteria for EUS diagnosis of gastric polyps: The polyps have diverse shapes, including spherical, hemispherical, pedunculated or broad-based types, etc. Most of them originate from the mucosa. Hyperplastic polyps mostly show uniform medium echo, and inflammatory polyps may present slightly lower echo, with clear margins.
Criteria for EUS diagnosis of inflammatory protrusions in the stomach: It has an irregular shape, which can be patchy or nodular, mainly located in the mucosa, and can involve the submucosa in severe cases. The internal echo is heterogeneous, mostly low echo, and sometimes strong echo spots can be seen, with blurred margins.
Statistical analysis was performed using SPSS 26.0 statistical software. When comparing the lesion sizes at different pathological stages, interquartile range was used for representation, and Kruskal-Wallis test was used for comparison between groups. Categorical variables were expressed as the number of cases and percentage. When comparing the EUS manifestations (origin layer, echo intensity, whether the echo was uniform, and whether the boundary was clear) of gastric IFPs at different pathological stages, Fisher’s exact test was used. A P value less than 0.05 was considered statistically significant.
All 53 patients with gastric IFPs underwent preoperative EUS. EUS showed that seven cases originated from the second layer of the gastric wall (13.2%); nine originated from the third layer of the gastric wall (17.0%); and 37 involved both the second and third layers simultaneously (69.8%). Ten cases (18.9%) showed low echo; 36 (67.9%) showed medium-low echo; four (7.5%) showed medium-high echo; and three (5.7%) showed low echo lesions with high echo areas. Forty-eight cases (90.6%) had homogeneous echo, and five (9.4%) had heterogeneous echo. Forty-four cases (83%) had indistinct margins, and nine (17%) had distinct margins. The lesion size ranged from 5 mm to 40 mm, with a mean size of 14.3 mm ± 8.2 mm. Most IFPs under EUS showed medium-low echo (67.9%), homogeneous echo (90.6%), and indistinct margin (83%), and involved both the second and third layers of the gastric wall (69.8%). The EUS characteristics of gastric IFP are shown in Table 1 and Figure 1.
Pathological results showed that one case of gastric IFP involved only the mucosal layer; one involved the submucosal layer; and 51 involved both the mucosal and submucosal layers. There were four cases of nodular stage gastric IFP; 43 of fibrovascular stage gastric IFPs; and six of sclerosing stage gastric IFPs.
There were 35 cases of gastric IFPs diagnosed by EUS, with an accuracy rate of 66.0% (35/53). EUS misdiagnosed 18 cases of gastric IFPs as other diseases, including nine cases diagnosed as gastric ectopic pancreas, two diagnosed as gastric stromal tumor, two diagnosed as gastric neuroendocrine tumor; two diagnosed as gastric adenoma, two diagnosed as gastric polyp and one diagnosed as inflammatory protrusion. After comparing with pathological results, five cases of sclerotic stage gastric IFPs were misdiagnosed, with a misdiagnosis rate of 83.3% (5/6); 12 cases of fibrovascular stage gastric IFPs were misdiagnosed, with a misdiagnosis rate of 27.9% (12/43); and one case of nodular stage gastric IFP was misdiagnosed, with a misdiagnosis rate of 25% (1/4). EUS revealed that seven cases originated from the second layer of the gastric wall, postoperative pathology found that one case originated from the second layer of the gastric wall and the remaining six cases involved both the mucosal layer and the submucosa layer. EUS revealed that nine cases originated from the third layer of the gastric wall, postoperative pathology found that one case originated from the third layer of the gastric wall and the remaining eight cases involved both the mucosal layer and the submucosa layer. EUS and postoperative pathology both revealed that 37 cases involved the second and third layers. The accuracy of preoperative EUS examination in determining the layer of origin of the lesion was 73.4% (39/53).
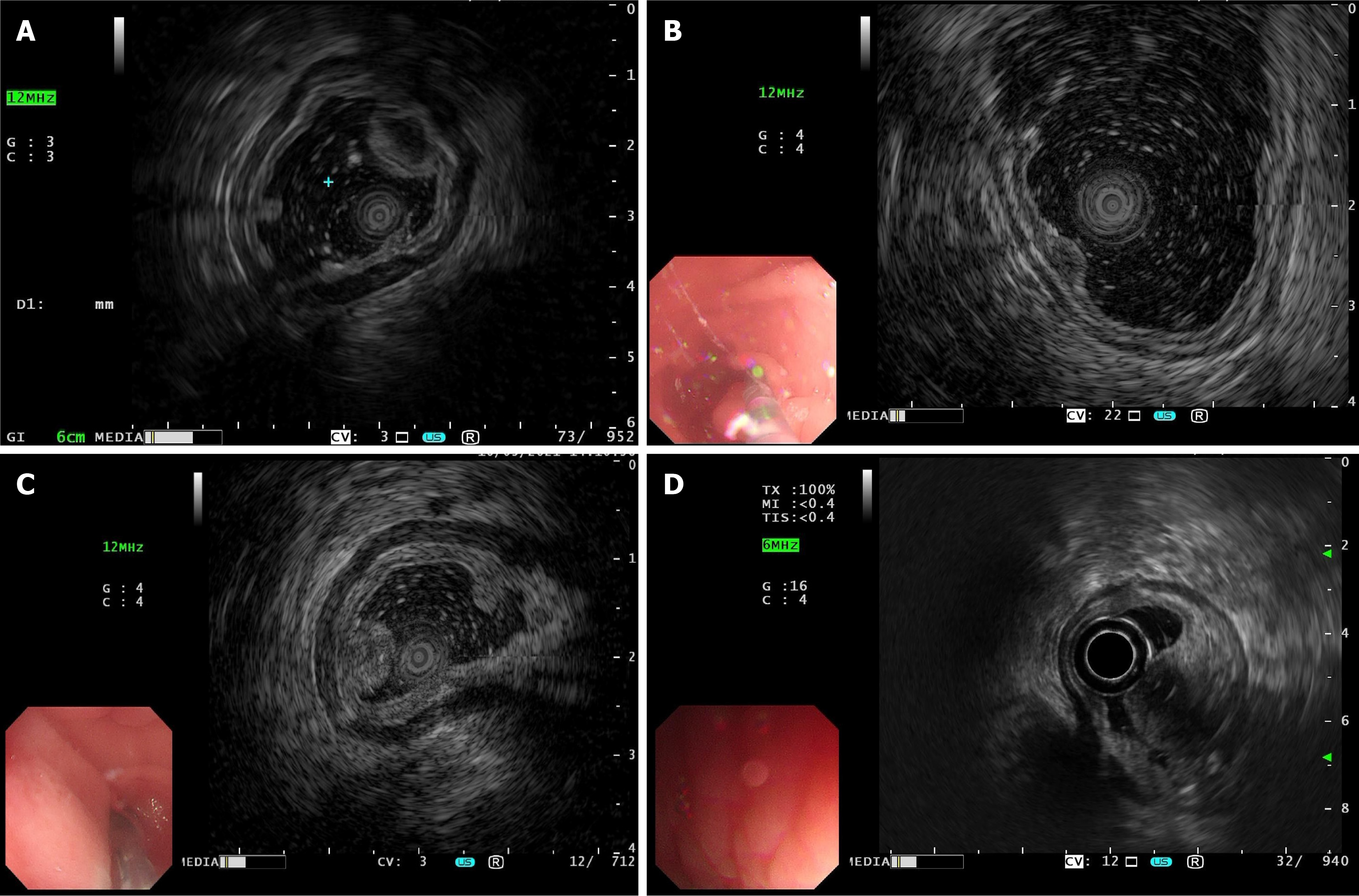
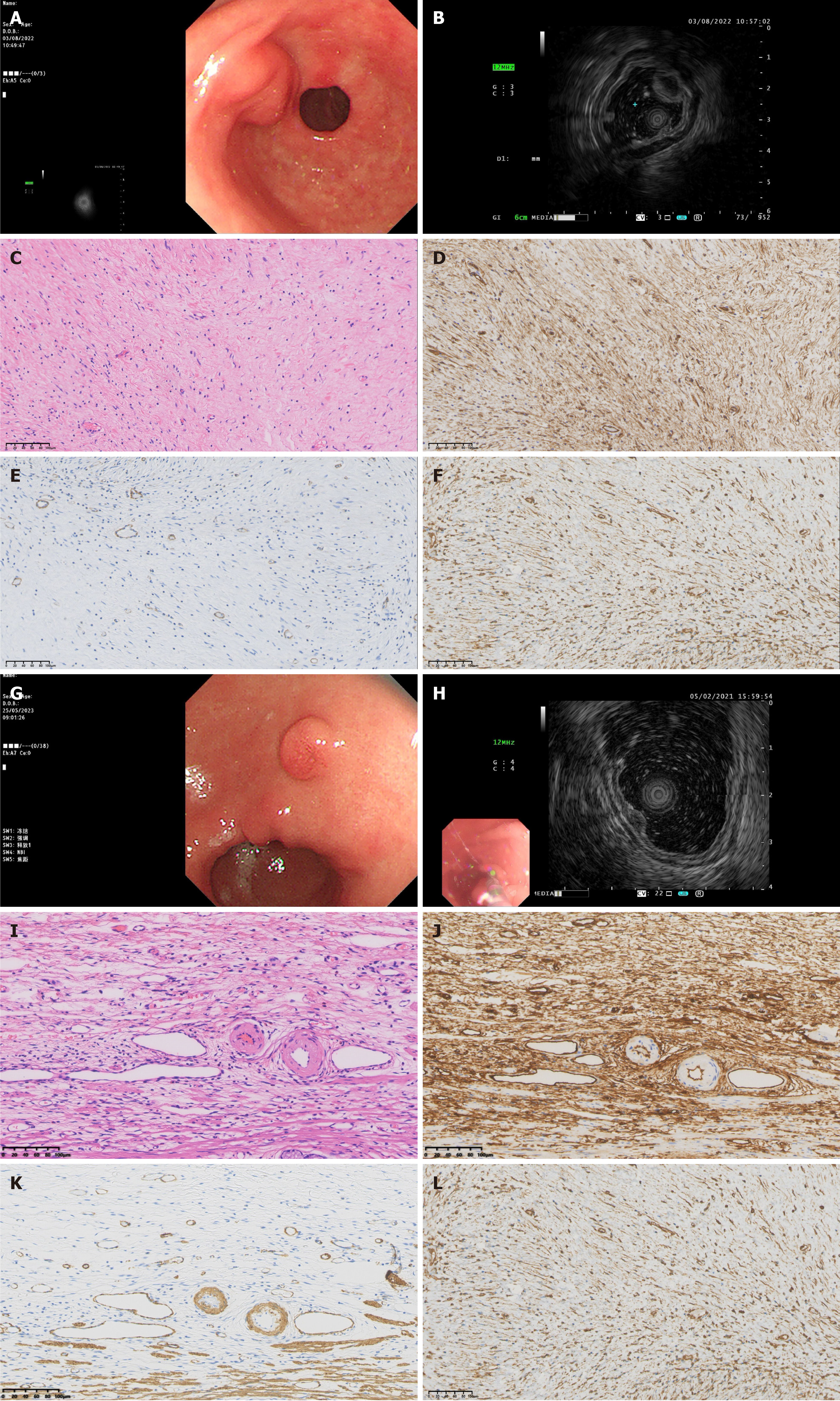
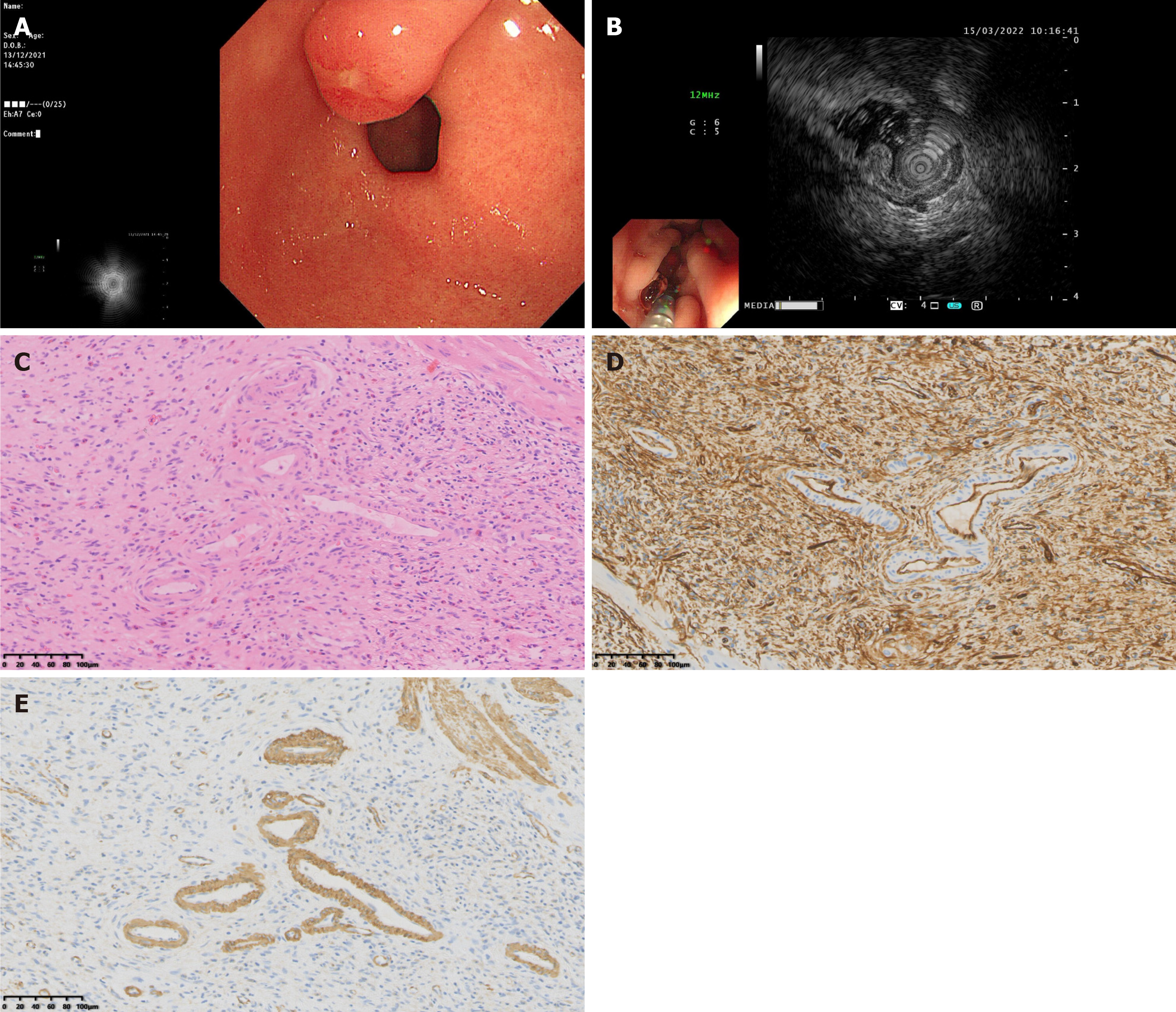
The EUS findings of gastric IFPs were closely related to the pathological nature and staging of the lesions. Pathological results showed that there were four cases of nodular stage gastric IFPs, they were low echo, homogeneous echo, indistinct margin, and involved the second and third layers on EUS (Figure 2A-F and Table 2). There were 43 cases of fibrovascular stage gastric IFPs, most of them showed medium-low echo (35 cases), homogeneous echo (42 cases), indistinct margin (34 cases), and involved both the second and third layers (29 cases). A few cases showed low echo (5 cases) or medium-high echo (2 cases) or low echo lesions with high echo areas (1 case), 9 cases with distinct margins; 7 cases involved the second layer and 7 cases involved the third layer (Figure 2G-L and Table 2). There were 6 cases of sclerotic stage gastric IFP, 2 cases showed medium-high echo, 1 case showed medium-low echo, 1 case showed low echo, 2 cases showed low echo lesions with high echo areas; 4 cases had heterogeneous echo and 2 cases had homogeneous echo; all 6 cases had indistinct margins; 4 cases involved both the second and third layers and 2 cases involved the third layer (Figure 3 and Table 2). The results of statistical analysis showed that the echogenicity level (P < 0.001) and echo homogeneity (P < 0.001) of the lesions under EUS were significantly correlated with the pathological stages of gastric IFPs (Table 2). Gastric IFPs in the nodular stage all originated from the second and third layers, and showed low echo and homogeneous echo. Gastric IFPs in the fibrovascular stage mostly originated from the second and third layers, with medium-low echo and homogeneous echo. Gastric IFPs in the sclerotic stage originated from the third layer, or from both the second and third layers, and the echo intensity and homogeneity varied (Tables 1 and 2). However, there is no obvious correlation between the size and margin of the lesion and the pathological stage.
| Pathological stage | |||||
| EUS features | Nodular stage | Fibrovascular stage | Sclerotic stage | χ2/H value | P value |
| Origin, layer1 | 2.504 | 0.667 | |||
| 2nd | 0 | 7 | 0 | ||
| 3rd | 0 | 7 | 2 | ||
| 2nd + 3rd | 4 | 29 | 4 | ||
| Echogenicity, level2 | |||||
| 1 | 4 | 5 | 1 | 25.942 | < 0.001 |
| 2 | 0 | 35 | 1 | ||
| 3 | 0 | 2 | 2 | ||
| 1 + 43 | 0 | 1 | 2 | ||
| Homogeneticy | |||||
| Homogeneous | 4 | 42 | 2 | 15.076 | < 0.001 |
| Heterogeneous | 0 | 1 | 4 | ||
| Margin | |||||
| Distinct | 0 | 9 | 0 | 1.397 | 0.500 |
| Indistinct | 4 | 34 | 6 | ||
| Size (mm), interquartile range | 9.35 (6.45, 10.98) | 12.00 (8.00, 18.00) | 12.80 (10.45, 21.65) | 3.059 | 0.217 |
IFP was first reported by Vanek[12] in 1949 and was once known as “gastrointestinal submucosal granuloma with eosinophilic infiltration”. In 1953, Helwig and Ranier[13] first used the name IFP and described its histological features in detail. The cause of IFP remains unknown. Previously, IFP was thought to be a non-neoplastic lesion caused by infection, trauma, and inflammatory repair[14,15]. In 2008, Schildhaus et al[16] found frequent activating mutations in exons 12 and 18 of the PDGFRA gene in IFP, providing evidence for the tumorous nature of IFP[16]. Subsequent studies have also reported similar findings[17-20]. Recently, Huss et al[21] discovered a site-specific mutation pattern, indicating that exon 12 mutations dominate in small intestinal IFPs, while exon 18 mutations frequently are common in gastric IFPs[21]. In addition, there are some reports of recurrent and familial IFP in the literature supporting the genetic background[22,23]. Currently, it is widely accepted that IFP is a benign mesenchymal tumor of the digestive tract with PDGFRA gene mutations.
Current literature reports and clinical experience suggest that the diagnosis of gastric IFP is mainly based on surgical resection or endoscopic treatment specimens combined with pathological diagnosis[3-6]. Microscopically, the lesions of IFP are located in the mucosa and submucosa; mainly composed of short spindle cells arranged in interwoven bundles and sheets. The background shows many blood vessels and various forms of inflammatory cells, usually mainly eosinophil infiltration. Spindle cells often form a whirlpool-like arrangement around blood vessels, known as the characteristic “onion skin” change[24,25]. Immunohistochemical staining is helpful in the pathological and differential diagnosis of this disease. Almost all cases express vimentin, 82%-100% of cases express cluster of differentiation (CD) 34, and smooth muscle actin is expressed with varying degrees of focal expression. CD117, CD10, CD23, CD35, CD99, bcl-2, DOG-1, desmin, S-100, creatine kinase, neuron-specific enolase, factor VIII, and anaplastic lymphoma kinase are all negative[25,26]. Development of IFP lesions in the stomach mainly goes through three stages: Nodular, fibrovascular and sclerotic. Kim and Kim[1] reported that the pathological stage of IFP may represent evolutionary changes with increasing size. They analyzed 15 cases of digestive tract IFP (9 of gastric IFP and 6 of small intestinal IFP) and found that the average size in the nodular stage was 0.4 cm (all gastric IFP), and the average size of digestive tract IFP in the fibrovascular stage was 1.5 cm (all gastric IFP). The average size of IFP in the digestive tract during the sclerotic stage was 4.8 cm (all IFP were located in the small intestine). Our pathological study showed that there were 4 cases of nodular stage gastric IFP, 43 cases of fibrovascular stage gastric IFP, and 6 cases of sclerotic stage gastric IFP. The average size of IFPs was 9.35 mm in the nodular stage, 12.00 mm in the fibrous nodular stage, and 12.80 mm in the sclerotic stage. There was no significant statistical difference in the size of gastric IFP in each stage. The possible reason is that our study objects are all gastric IFPs, not involving other parts of the digestive tract, and the overall size is not large. However, the IFPs of the small intestine is often larger, which can also easily lead to symptoms such as intestinal obstruction and bleeding.
EUS can detect most gastric IFP and provide more evaluation information on the location, size, origin layer, margin status of the lesions and their relationship with surrounding organs, which has good reference and guiding significance for clinically determining a scientific and reasonable treatment plan[2,9-11]. A previous study[2] summarized the EUS features of 10 cases of gastric IFPs and found that the most common EUS features of the lesions were indistinct margins, low and homogeneous echo, and being located in the second and/or third ultrasonic layers, without involving the fourth ultrasonic layer. The authors indicated that that the low and homogeneous echo of gastric IFP under EUS was related to the presence of proliferative fibrous tissue, and the indistinct margins were related to the presence of fibrous tissue histologically at the edge of the lesion without a capsule. However, there are also reports on the atypical EUS features of gastric IFPs. Aydin et al[11] reported a case of gastric IFP, where a low and homogeneous echo lesion was found located within the second and third ultrasonic layers under EUS, and there was also a distinct hyperechoic area within the lesion. Among the 10 cases of pathologically diagnosed gastric IFPs reported by Matsushita et al[2], the internal echo was hyperechoic or heterogeneous in two cases under EUS. In contrast with the pathological tissues, these heterogeneous echoes or hyperechoes corresponded to a large numbers of small blood vessels in the specimens. Recently, Harima et al[26] also reported a case of IFP with gastric outlet obstruction. After surgical resection, the specimen showed that the lesion originated from the submucosa, invaded the muscularis propria, and extended to the subserosa, suggesting that gastric IFPs may have potential invasiveness. The above research results indicate that the manifestations of gastric IFPs under EUS may be diverse. We found that most gastric IFPs under EUS showed medium-low echo, homogeneous echo, indistinct margins, and involved the second and third layers of the gastric wall, which is consistent with the previous research results[27]. In addition, we found that the echogenicity level and echo homogeneity of the lesions were significantly correlated with the pathological stages of gastric IFPs. However, there is no obvious correlation between the size and margin of the lesion and the pathological stage. This provides us with the following clinical implications: When performing EUS examinations, endoscopists can accurately diagnose gastric IFPs, determine their pathological stages, and formulate subsequent treatment strategies. Although the margin characteristics do not show a significant correlation, they still play a role in evaluating the nature and extent of the lesions. Moreover, since there is no significant difference between the tumor size and the pathological stage, we cannot simply judge the tumor status and determine the treatment strategy based on the size alone, so as to avoid excessive or inappropriate treatment.
Pathologically, the nodular stage is mainly composed of immature fibroblast-like spindle cells, accompanied by a small amount of inflammatory cells such as eosinophils and the proliferation of thin-walled blood vessels. In the fibrovascular stage, mature fibroblast-like spindle cells proliferate, but the degree of collagenization is poor, and thin-walled and thick-walled blood vessels can be seen. Eosinophils around the blood vessels proliferate significantly and are arranged concentrically. The sclerotic stage is characterized by the proliferation of collagen fibers, which may be accompanied by hyaline degeneration, a significant increase in thick-walled blood vessels, and a large numbers of eosinophil infiltrations. Our present study shows that the EUS features are related to the specific manifestations of different pathological stages. Understanding the characteristics of different pathological stages combined with the results of EUS examinations is helpful in accurately determining the stage of the disease. For example, when the EUS reveals specific echo and vascular characteristics, clinicians can infer whether the gastric IFP is in the nodular stage, the fibrovascular stage, or the sclerotic stage, thus providing a basis for further diagnosis. If the EUS examination suggests the possibility of a specific pathological stage, further examinations such as tissue biopsy should be performed to confirm an accurate pathological diagnosis and avoid missed or misdiagnoses.
The accuracy of EUS in determining the origin layer of the lesions was 73.4%, and the sensitivity of EUS in diagnosing IFPs was 66.0%. Among them, 18 cases were misdiagnosed as other diseases by EUS [9 cases were misdiagnosed as ectopic pancreas in the stomach, 2 cases were misdiagnosed as gastric gastrointestinal stromal tumors (GISTs), 2 cases were misdiagnosed as gastric neuroendocrine tumors, 2 cases were misdiagnosed as gastric adenomas, 2 cases were misdiagnosed as gastric polyps, and 1 case was misdiagnosed as inflammation]. Our study found that EUS misdiagnosed 5 cases of gastric IFPs in the sclerotic stage, with a misdiagnosis rate of 83.3% (5/6), misdiagnosed 12 cases of gastric IFPs in the fibrovascular stage, with a misdiagnosis rate of 27.9% (12/43), and misdiagnosed 1 case of gastric IFP in the nodular stage, with a misdiagnosis rate of 25% (1/4). The factors contributing to misdiagnosis may be as follows: Firstly, under EUS, gastric IFPs share similarities with other gastric diseases, such as ectopic pancreas, GISTs, gastric neuroendocrine tumors, gastric adenomas, gastric polyps, and gastric inflammation. The echo characteristics and origin layers of these diseases may be similar, making it difficult to accurately distinguish gastric IFP from other diseases based solely on the results of EUS examination. Secondly, gastric IFP has different EUS features at different pathological stages, which increases the difficulty of accurate diagnosis. Gastric IFP in the sclerotic stage is more likely to be misdiagnosed due to the diverse echo intensity and echo homogeneity under EUS. In addition, this is a retrospective study, gastric IFP was examined by different endoscopists before pathological confirmation. The accuracy of EUS diagnosis is also related to the experience and technical proficiency of the operators. Inexperienced operators may have difficulty accurately identifying the features of gastric IFP and distinguishing it from other diseases, thus leading to misdiagnosis. Finally, although EUS is an effective tool for diagnosing gastric diseases, it has certain limitations. It may not be able to clearly display the fine structure of the lesions, to accurately determine the depth and extent of the lesions, and perform real-time pathological diagnosis, and is affected by gas and fluid in the stomach.
In view of the fact, the gastric IFPs have different pathological stages, and gastric IFPs at different pathological stages have different manifestations on EUS, it is difficult to accurately diagnose gastric IFP merely by EUS, and the misdiagnosis rate remains high. This is also true for other gastric submucosal tumors. Many studies have found that EUS combined with EUS-guided fine needle aspiration (EUS-FNA) has important value for the diagnosis of gastric submucosal protrusions[28,29]. In recent years, artificial intelligence (AI) diagnostic systems based on deep learning have achieved groundbreaking developments in the medical field, especially in the aspects of radiological imaging, pathology, and endoscopy, and have yielded abundant achievements. A meta-analysis has found that AI-assisted EUS has a high accuracy in the automatic diagnosis of GISTs under endoscopy, and can serve as a valuable supplementary method for differentiating GISTs in the future. A study has developed a system named multimodal, multipath AI system (MMP-AI), which can be used not only for the recognition of white-light endoscope images but also for the analysis of EUS images. They found that the areas under the curve of MMP-AI for classifying gastric GISTs, gastric leiomyomas, and gastric ectopic pancreas are 0.896, 0.890, and 0.999 respectively. Compared with endoscopists, MMP-AI has a higher recognition accuracy for submucosal lesions[30]. In order to further improve the diagnostic rate of gastric IFP and other gastric submucosal protrusions, the implementation of EUS-FNA and AI-assisted EUS is of great significance in future clinical practice.
Our study has several limitations. Firstly, the sample size of gastric IFPs at each pathological stage is small. Specifically, there were only four cases in the nodular stage and six cases in the sclerotic stage. Such a small sample size may limit the generalizability of our research findings regarding the relationship between EUS features and pathological stages. With a larger sample size, more precise associations or additional characteristics could be identified. Secondly, our cohort was composed solely of gastric IFP cases, lacking IFP cases from other parts of the digestive tract. This may lead to a one-sided understanding of the disease, as IFPs in different parts of the digestive tract may have distinct pathological and EUS characteristics. Endoscopists made diagnoses based on the previously reported EUS features of IFP, and were varied in experience, which may impact the results. Additionally, we did not use the combine techniques such as EUS elastography, contrast-enhanced EUS, color Doppler imaging and FNA, all of which could have provided more comprehensive information for accurate diagnoses. Our research scope is also limited. We mainly focused on the correlations among the EUS features, pathological stages, and pathological characteristics of gastric IFPs, and did not explore the long-term clinical outcomes of patients with gastric IFPs at different pathological stages, such as the recurrence rate, survival rate, and the impact of different treatment methods on prognosis.
Gastric IFPs at different pathological stages have different EUS features. Most gastric IFPs show medium-low echo, homogeneous echo, unclear boundaries, and involve the second and third layers of the gastric wall under EUS. The echogenicity level and echo homogeneity are significantly correlated with the pathological stage of gastric IFP. Gastric IFPs in the nodular stage present hypoechoic and homogeneous echo. Gastric IFPs in the fibrovascular stage mostly show medium-low echo and homogeneous echo. The echogenicity levels and echo homogeneity of gastric IFPs in the sclerotic stage vary. If endoscopic operators can understand the EUS manifestations corresponding to different pathological stages of gastric IFPs, the preoperative diagnosis rate of gastric IFP can be improved. In order to further improve the diagnostic rate of gastric IFP, the implementation of EUS-FNA and AI-assisted EUS is of great significance in future clinical practice.
| 1. | Kim YI, Kim WH. Inflammatory fibroid polyps of gastrointestinal tract. Evolution of histologic patterns. Am J Clin Pathol. 1988;89:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric inflammatory fibroid polyps: endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc. 1997;46:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Inayat F, Ur Rahman A, Wahab A, Riaz A, Zahid E, Bejarano P, Pimentel R. Gastric Inflammatory Fibroid Polyp: A Rare Cause of Occult Upper Gastrointestinal Bleeding. J Investig Med High Impact Case Rep. 2020;8:2324709620936840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Nagao S, Tsuji Y, Sakaguchi Y, Ushiku T, Koike K. Inflammatory fibroid polyp mimicking an early gastric cancer. Gastrointest Endosc. 2020;92:217-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Garmpis N, Damaskos C, Garmpi A, Georgakopoulou VE, Sakellariou S, Liakea A, Schizas D, Diamantis E, Farmaki P, Voutyritsa E, Syllaios A, Patsouras A, Sypsa G, Agorogianni A, Stelianidi A, Antoniou EA, Kontzoglou K, Trakas N, Dimitroulis D. Inflammatory Fibroid Polyp of the Gastrointestinal Tract: A Systematic Review for a Benign Tumor. In Vivo. 2021;35:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Kawai A, Matsumoto H, Haruma K, Kanzaki T, Sugawara Y, Akiyama T, Hirai T. Rare case of gastric inflammatory fibroid polyp located at the fornix of the stomach and mimicking gastric cancer: a case report. Surg Case Rep. 2020;6:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Pinto-Pais T, Fernandes S, Proença L, Fernandes C, Ribeiro I, Sanches A, Carvalho J, Fraga J. A Large Gastric Inflammatory Fibroid Polyp. GE Port J Gastroenterol. 2015;22:61-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Matsushita M, Okazaki K. Atypical EUS features of gastric inflammatory fibroid polyps. Gastrointest Endosc. 2005;61:637-8; author reply 638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Matsushita M, Uchida K, Nishio A, Okazaki K. Endoscopic and EUS features of gastric inflammatory fibroid polyps. Gastrointest Endosc. 2009;69:188; author reply 188-188; author reply 189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Enestvedt BK, Chandrasekhara V, Ginsberg GG. Endoscopic ultrasonographic assessment of gastric polyps and endoscopic mucosal resection. Curr Gastroenterol Rep. 2012;14:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Aydin A, Tekin F, Gunsar F, Tuncyurek M. Gastric inflammatory fibroid polyp. Gastrointest Endosc. 2004;60:802-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Vanek J. Gastric submucosal granuloma with eosinophilic infiltration. Am J Pathol. 1949;25:397-411. [PubMed] |
| 13. | Helwig EB, Ranier A. Inflammatory fibroid polyps of the stomach. Surg Gynecol Obstet. 1953;96:335-367. [PubMed] |
| 14. | Bullock WK, Moran ET. Inflammatory fibroid polyps of the stomach. Cancer. 1953;6:488-493. [PubMed] [DOI] [Full Text] |
| 15. | Shimer GR, Helwig EB. Inflammatory fibroid polyps of the intestine. Am J Clin Pathol. 1984;81:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 60] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Schildhaus HU, Cavlar T, Binot E, Büttner R, Wardelmann E, Merkelbach-Bruse S. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J Pathol. 2008;216:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Schildhaus HU, Büttner R, Binot E, Merkelbach-Bruse S, Wardelmann E. [Inflammatory fibroid polyps are true neoplasms with PDGFRA mutations]. Pathologe. 2009;30 Suppl 2:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Lasota J, Wang ZF, Sobin LH, Miettinen M. Gain-of-function PDGFRA mutations, earlier reported in gastrointestinal stromal tumors, are common in small intestinal inflammatory fibroid polyps. A study of 60 cases. Mod Pathol. 2009;22:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Yamashita K, Arimura Y, Tanuma T, Endo T, Hasegawa T, Shinomura Y. Pattern of growth of a gastric inflammatory fibroid polyp with PDGFRA overexpression. Endoscopy. 2011;43 Suppl 2 UCTN:E171-E172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Daum O, Hatlova J, Mandys V, Grossmann P, Mukensnabl P, Benes Z, Michal M. Comparison of morphological, immunohistochemical, and molecular genetic features of inflammatory fibroid polyps (Vanek's tumors). Virchows Arch. 2010;456:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Huss S, Wardelmann E, Goltz D, Binot E, Hartmann W, Merkelbach-Bruse S, Büttner R, Schildhaus HU. Activating PDGFRA mutations in inflammatory fibroid polyps occur in exons 12, 14 and 18 and are associated with tumour localization. Histopathology. 2012;61:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Anthony PP, Morris DS, Vowles KD. Multiple and recurrent inflammatory fibroid polyps in three generations of a Devon family: a new syndrome. Gut. 1984;25:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Allibone RO, Nanson JK, Anthony PP. Multiple and recurrent inflammatory fibroid polyps in a Devon family ('Devon polyposis syndrome'): an update. Gut. 1992;33:1004-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Liu TC, Lin MT, Montgomery EA, Singhi AD. Inflammatory fibroid polyps of the gastrointestinal tract: spectrum of clinical, morphologic, and immunohistochemistry features. Am J Surg Pathol. 2013;37:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Pantanowitz L, Antonioli DA, Pinkus GS, Shahsafaei A, Odze RD. Inflammatory fibroid polyps of the gastrointestinal tract: evidence for a dendritic cell origin. Am J Surg Pathol. 2004;28:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Harima H, Kimura T, Hamabe K, Hisano F, Matsuzaki Y, Sanuki K, Itoh T, Tada K, Sakaida I. Invasive inflammatory fibroid polyp of the stomach: a case report and literature review. BMC Gastroenterol. 2018;18:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Unal Kocabey D, Cakir E, Dirilenoglu F, Bolat Kucukzeybek B, Ekinci N, Akder Sari A. Analysis of clinical and pathological findings in inflammatory fibroid polyps of the gastrointestinal system: A series of 69 cases. Ann Diagn Pathol. 2018;37:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Okasha HH, Naguib M, El Nady M, Ezzat R, Al-Gemeie E, Al-Nabawy W, Aref W, Abdel-Moaty A, Essam K, Hamdy A. Role of endoscopic ultrasound and endoscopic-ultrasound-guided fine-needle aspiration in endoscopic biopsy negative gastrointestinal lesions. Endosc Ultrasound. 2017;6:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Kamata K, Kurita A, Yasukawa S, Chiba Y, Nebiki H, Asada M, Yasuda H, Shiomi H, Ogura T, Takaoka M, Hoki N, Ashida R, Shigekawa M, Yanagisawa A, Kudo M, Kitano M. Utility of a 20G needle with a core trap in EUS-guided fine-needle biopsy for gastric submucosal tumors: A multicentric prospective trial. Endosc Ultrasound. 2021;10:134-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Zhu C, Hua Y, Zhang M, Wang Y, Li W, Ding Y, She Q, Zhang W, Si X, Kong Z, Liu B, Chen W, Wu J, Dang Y, Zhang G. A Multimodal Multipath Artificial Intelligence System for Diagnosing Gastric Protruded Lesions on Endoscopy and Endoscopic Ultrasonography Images. Clin Transl Gastroenterol. 2023;14:e00551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/