INTRODUCTION
The intraoperative ultrasound (IOUS) was introduced in the 1960s and was initially used to evaluate choledocholithiasis. With advances in technology in the early 1980s, the development of IOUS accelerated[1,2]. In 1991, Reich et al[3] used laparoscopy to remove benign tumors on the liver surface and completed the world's first laparoscopic hepatectomy (LH). With advances in technology, laparoscopic or robotic hepatectomy is becoming increasingly important, as it combines precision with less surgical trauma.
However, given the loss of haptic feedback and the inability to observe internal conditions of the liver, LH involves a higher risk than open surgery, which limits its further development. Laparoscopic ultrasound (LUS) helps overcome the limitations of laparoscopic surgery and reduces the incidence of intraoperative hemorrhage and gas embolism, thus making laparoscopic liver resection safe and reliable. Compared with open liver resection, LH has a lower incidence of postoperative complications and shorter postoperative stay[4,5]. With the wide acceptance and use of laparoscopic liver surgery and increased use of robotic liver surgery, LUS plays an increasingly important role in laparoscopic liver surgery, particularly helping with exploring the liver parenchymal lesion, compensating for haptic feedback, improving surgical safety, reducing the positive rate of margin, improving the effect of radical cancer treatment, preventing extensive surgery, and reducing patient injury. Like in open surgery, IOUS was considered desirable for laparoscopic liver surgery as well[6].
Although LUS has numerous benefits, its clinical application still faces many challenges, such as significant technical difficulties, nonstandard operation, and limited popularity and adaptation. Currently, minimally invasive surgery is being increasingly favored by patients, leading to an increase in the proportion of laparoscopic surgeries[7], and with the continuous development of LUS and the increasing complexity of technology, it is imperative that liver surgeon develop their LUS skills. This review aims to introduce the role of LUS in LH and the new technology of LUS.
INSTRUMENTATION OF INTRAOPERATIVE LUS
Ultrasonic equipment has two components, a probe and a cable-connected scanner. There are two types of LUS probes, namely convex array probes and linear array probes. Linear array probes are suitable for intraoperative structure marking, whereas convex array probes have a larger scanning range that facilitates puncture procedure. Unlike transabdominal ultrasound, LUS involves directly placing the probe on the surface of the liver, which helps eliminate the artifacts caused by subcutaneous fat, ribs, and air from imaging. The commonly used ultrasonic probe is a high-frequency probe in the range 5-10 MHz with a maximum penetration depth of 13 cm[8]. Higher frequencies are associated with a higher image resolution and the ability to detect smaller lesions, albeit with reduced exploration depth. The scanner used during the operation should be easy to operate, portable, and provide high-quality images[9]. Ideally, the IOUS equipment should ensure the grayscale, pulse Doppler, and color Doppler capabilities, as well as the potential for contrast-enhanced (CE) ultrasound and elastography. In addition, these devices must be ergonomic, small, and easy to clean and sterilize regularly[10].
The direction of exploration using previously used laparoscopic probes has been limited by the fixed position of the laparoscopic trocar and the inability of laparoscopic probes to perform pitch swings in more than one direction. A few companies have now introduced LUS probes that can be rotated up, down, left, and right; in addition, they have puncture guide grooves, which considerably improve the convenience performing LUS and promote the development of LUS. To facilitate the observations of the surgeon in the operating room, the ultrasound display screen should be placed on the same side of the laparoscopic display screen, and if necessary, the brightness of the operating room should be reduced to visualize the abdominal cavity more clearly.
BASIC OPERATIONS OF INTRAOPERATIVE LUS
General selection of the position of trocar
The conventional abdominal trocar position of liver LUS includes the subxiphoid, lower costal margin of the left and right upper abdomen, intersection of the upper and lower horizontal lines of the umbilical plane, and the lateral margin of bilateral rectus abdominis[3]. The preferred channel can be chosen according to the hepatectomy location and operating conditions; generally, the subxiphoid trocar is chosen as it allows for comprehensive scanning of the whole liver. Occasionally, the transthoracic trocar is also a good choice, and it can be useful for LH of segments 7 and 8. A transthoracic trocar can be used not only for operation but also as an observation hole, offering a better field of vision and more operation space[11,12]. For the liver segment in the deep position, with the assistance of the traction of laparoscopic instruments, we can cut off the perihepatic ligament to free the liver and then extend into the bare liver area with the help of the flexible end of an ultrasonic probe.
Routine sequence of liver exploration
The probe is placed between the gallbladder and vena cava, and the middle hepatic vein is located to distinguish between the left and right liver. Then, the probe is moved upward and perpendicularly to the vena cava, which allows for the identification of the left, right, and middle hepatic veins. This was followed by scanning down along the middle hepatic vein to observe the possible fissure and short hepatic veins. According to the distribution of portal and hepatic veins, the eight liver segments (Couinaud segments) can be distinctly identified. When scanning the right half of the liver, the probe is placed on the diaphragmatic surface of the liver, followed by exploring from the middle hepatic vein to the right side, using the hepatic pedicle as the marker, and scanning S8, S5, S6, and S7 in that sequence. When scanning the left half of the liver, the probe is placed on the visceral surface of the liver, followed by exploring from the middle hepatic vein to the left side. Similarly, using the hepatic pedicle as the marker, S4, S2, and S3 were scanned in that sequence. During these explorations, it is important to be attentive and accurately locate space-occupying lesions like liver tumors and identify their attachment with peripheral blood vessels.
Identification and evaluation of important structures
The variation of important duct structures greatly affects the choice of anatomical hepatectomy. The invasion, obstruction, and space-occupying effect of liver lesions on important vessels can affect the resection margins, postoperative recurrence, and long-term survival[13]. Therefore, intraoperative reassessment of important hepatic duct structures is significant for accurate anatomical hepatectomy. The color Doppler probe can provide images of various ducts in the liver[14]. and change the two-dimensional laparoscopic visual field into three-dimensional visual field with depth information and stereoscopic vision.
The invasion of blood vessels malignant tumors leads to complete or incomplete occlusion of the lumen structure, and color Doppler images show interrupted or turbulent images. Lo Tesoriere et al[15] reported that for tumors involving the hepatocaval confluence, color Doppler intraoperative ultrasonography can evaluate congestion in the veno-occlusive parenchyma by assessing hepatic hemodynamics and provide real-time information of parenchyma-sparing surgery, thus improving the success rate of parenchyma-sparing surgery. In addition, when the anatomical position relationship between the cutting edge and vital duct structures cannot be clearly determined, LUS can assess the patency and continuity of important blood vessels and intrahepatic biliary tract structure. These are critical anatomical elements that surgeons must preserve to prevent complications. This can reduce the risk of tumor metastasis along the hepatic portal vein in the liver and play a key role in protecting the remaining liver parenchyma[16].
APPLICATION OF LUS IN LIVER TUMOR RESECTION
Evaluation of intrahepatic lesions and change of surgical strategy
LUS is now an integral part of LH, much like IOUS for open hepatectomy. Previous studies have shown that LUS for LH performs as well as IOUS for open hepatectomy in detecting new lesions and modifying surgical strategies[1,17].
LUS helps characterize lesions (solid or cystic and benign or malignant) before the operation. The echogenicity of hepatic tumors is different from that of normal liver parenchyma, which can be distinguished by LUS. Typically, LUS hypoechoic lesions are malignant (e.g., metastases of extraperitoneal origin or primary liver cancer), whereas hyperechoic lesions are benign (e.g., simple cysts like hemangiomas and anechoic lesions)[18]. Notably, LUS can also play a significant role in locating liver tumors as it can characterize small lesions because of its high spatial resolution; existing studies have shown that lesions smaller than 10 mm can be detected using high-resolution probes[19,20]. LUS can determine the structure and location of important vessels in the liver and effectively prevent their damage. Ferrero et al[21] showed that with the intraoperative use of LUS, the median intraoperative blood loss was approximately 100 mL, the intraoperative blood loss volume and transfusion rates were low, and there was no major bleeding (> 1 L). The use of LUS ensures accurate localization of tumor segments and identification of the relationship between the tumor and its adjacent vascular or biliary structures, excluding adjacent and adjunctive new lesions[22-24].
Currently, LUS is widely used in diagnosis and staging because it facilitates a pathologic diagnosis using biopsy or puncture under direct vision to provide a reliable basis for treatment and reduces the need for unnecessary exploratory laparotomy. According to existing literature, LUS contributes to better staging by detecting new hepatocellular carcinoma (HCC) lesions with an accuracy of 5%-27%[20,25-31]. In particular, for patients with colorectal cancer, routine intraoperative LUS for liver metastasis is of great importance because newly found lesions prompt the surgeon to avoid unnecessary exploratory laparotomy[23]. Despite preoperative planning, the final surgical strategy is based on intraoperative findings (i.e., the number, location, and anatomic relationship of lesions with vascular and biliary structures) and the presence of hepatic lymph node metastasis, which are important factors in evaluating resectability and planning surgical approaches. LUS provides surgeons with necessary information that can influence the operation. The use of LUS in laparoscopic liver surgery reportedly alters preoperative planning by 13%-27%[23,30-33]. Kose et al[20] showed that LUS detected 8.4% additional lesions (39/467) that were not found on preoperative magnetic resonance imaging (MRI) in 10% patients (14/147), and these additional findings changed the treatment plan of 8.8% patients (13/147). In addition, the more parenchyma-preserving surgical strategies are used, the more likely they are to be modified[1]. With advancements in imaging modalities, the rate of change in the surgical gradually decreased. However, the surgical strategy may still change due to the use of CE-IOUS, particularly in the operation of colorectal liver metastases (CRLM).
LH
The development of LH surgery has changed from the initial wedge-shaped local resection of benign liver tumors to the precise anatomic resection of any liver segment or combination with multiple liver segments for malignant liver tumors[34]. However, laparoscopic surgery has the disadvantage of not allowing for direct palpation of the liver; the use of LUS helps overcome this surgeon’s loss of tactile feedback during LH surgery and improves the safety of LH[1].
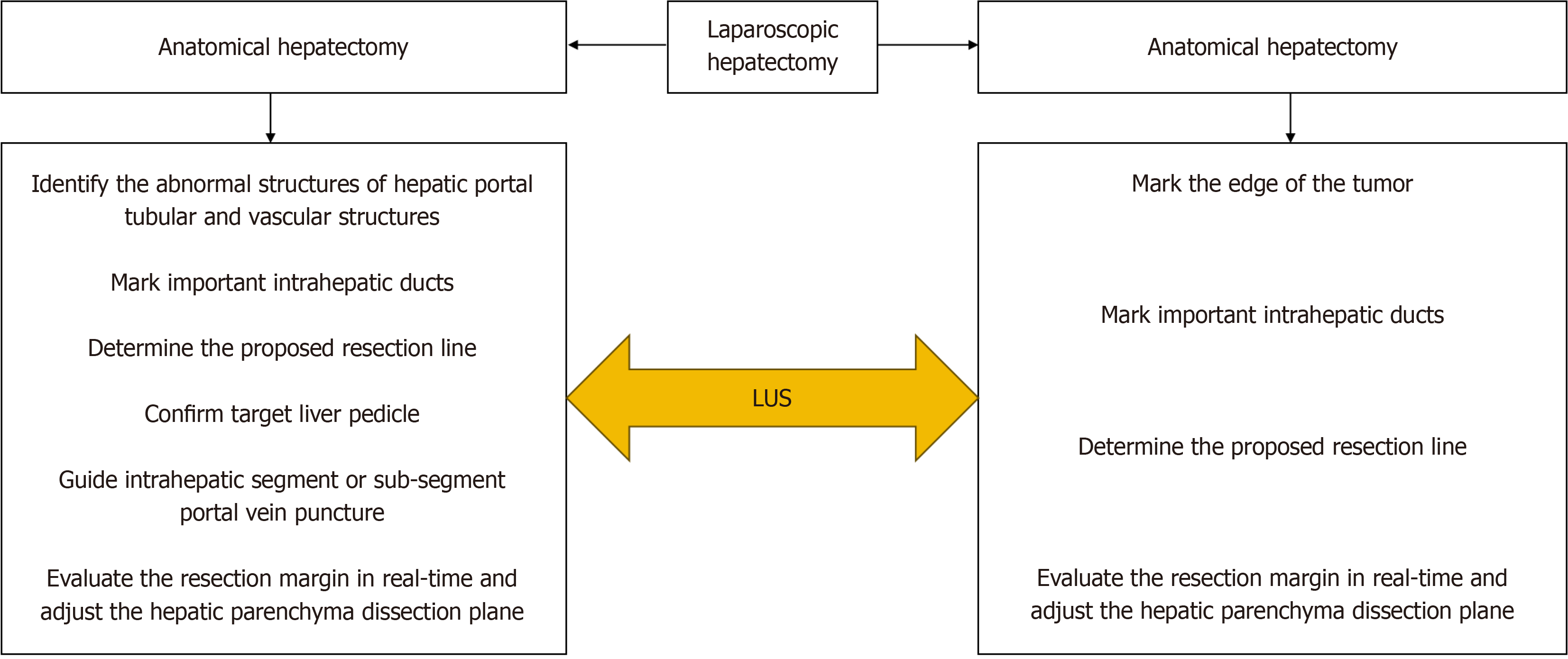
The basic intent underlying resection of malignant tumors is to ensure a sufficient tumor-free margin to avoid incomplete tumor resection and possible iatrogenic transmission. In addition, to increase the success rate of repeated hepatectomy and survival rate, healthy liver parenchyma should be preserved to the extent possible[35-38]. Therefore, it is particularly important to determine the surgical margin. The Southampton consensus[6]. recommended the use of IOUS in LH to accurately define resection margins and prevent injury to the major pedicles. There are usually two operation modes of liver resection that are described as follows (Figure 1).
Figure 1 The use of laparoscopic ultrasound in laparoscopic hepatectomy.
LUS: Laparoscopic ultrasound.
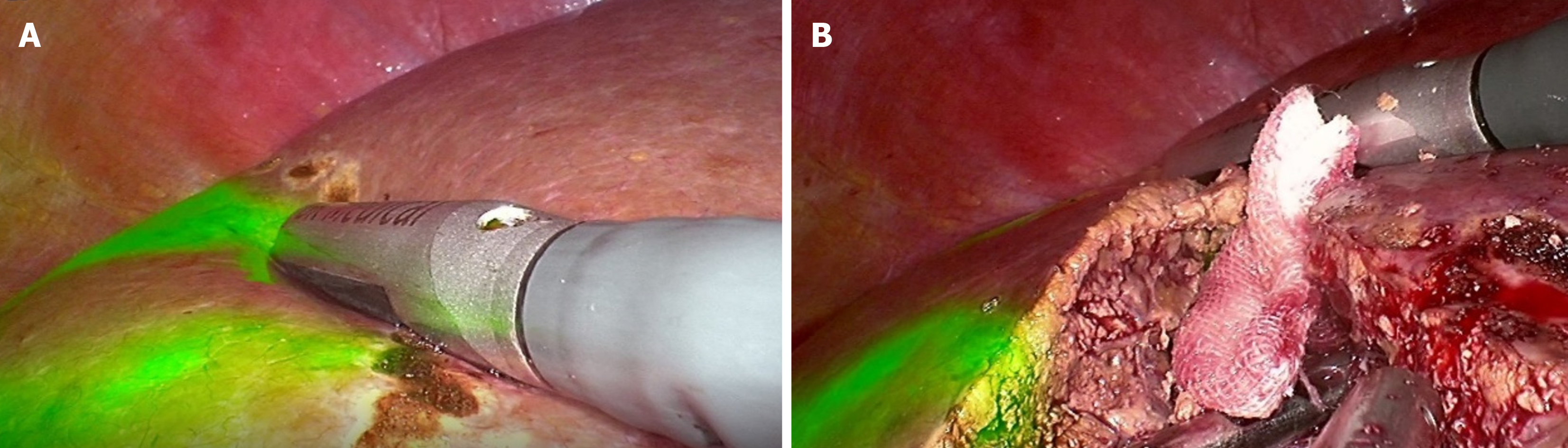
Laparoscopic non-anatomical hepatectomy: When performing the LUS, the LUS probe is moved to keep the linear array probe close to the liver surface and as perpendicular to the intrahepatic structure as possible. This is followed by identifying the tumor margins, adjacent important vessels, and vessels to be resected or retained and marking their projections on the liver surface using electrocautery (Figure 2A). According to the projection of the tumor margin and important duct structures, the proposed resection line is determined, and a 1 cm resection margin is pursued. Due to the adjustment of surgical position, rotation of the liver, and the fact that internal anatomical markers and their positional relationship are considerably different from preoperative images, the use of LUS can help evaluate the resection margin in real time and adjust the hepatic parenchyma dissection plane according to the evaluation results (Figure 2B). This helps ensure a negative resection margin and protect important duct structures from damage[21].
Figure 2 Intraoperative laparoscopic ultrasound images.
A: Laparoscopic ultrasound-guided delineation of the proposed resection margins in the liver; B: Adjust the liver transection plane using laparoscopic ultrasound under fluorescence laparoscopy.
Laparoscopic anatomical hepatectomy: Herein, color Doppler flow, which helps identify the hepatic portal tubular and vascular structures, is combined with the axial rotation of the LUS probe, which helps observe whether there are abnormal structures that affect anatomical hepatectomy. In hemihepatectomy, the middle hepatic vein needs to be marked on the liver surface along with the proposed resection line. According to the ischemia line and projection of the middle hepatic vein on the liver surface, we cut off the liver through the ischemia line; alternatively, the appropriate deviation from the ischemia line can be selected to ensure preservation of the middle hepatic vein. Laparoscopic anatomic segmental, subsegmental, or combined segmental resection requires precise preoperative planning combined with 3D reconstruction and relies on accurate real-time guidance of LUS during surgery. LUS should be used to confirm correctness before further blocking of or dye injection into the isolated liver pedicle. Laparoscopic anatomical hepatectomy also requires adjustment of the hepatic dissection plane through LUS to ensure preservation of the important hepatic vein while ensuring negative tumor margins. In addition, Lee et al[39] reported that during LLR, LUS probes can be used to compress hepatic vessels to block blood flow, thus revealing the boundaries of hepatic segments.
OTHER SURGICAL APPLICATIONS IN LIVER TUMOR
Laparoscopic liver tumor ablation
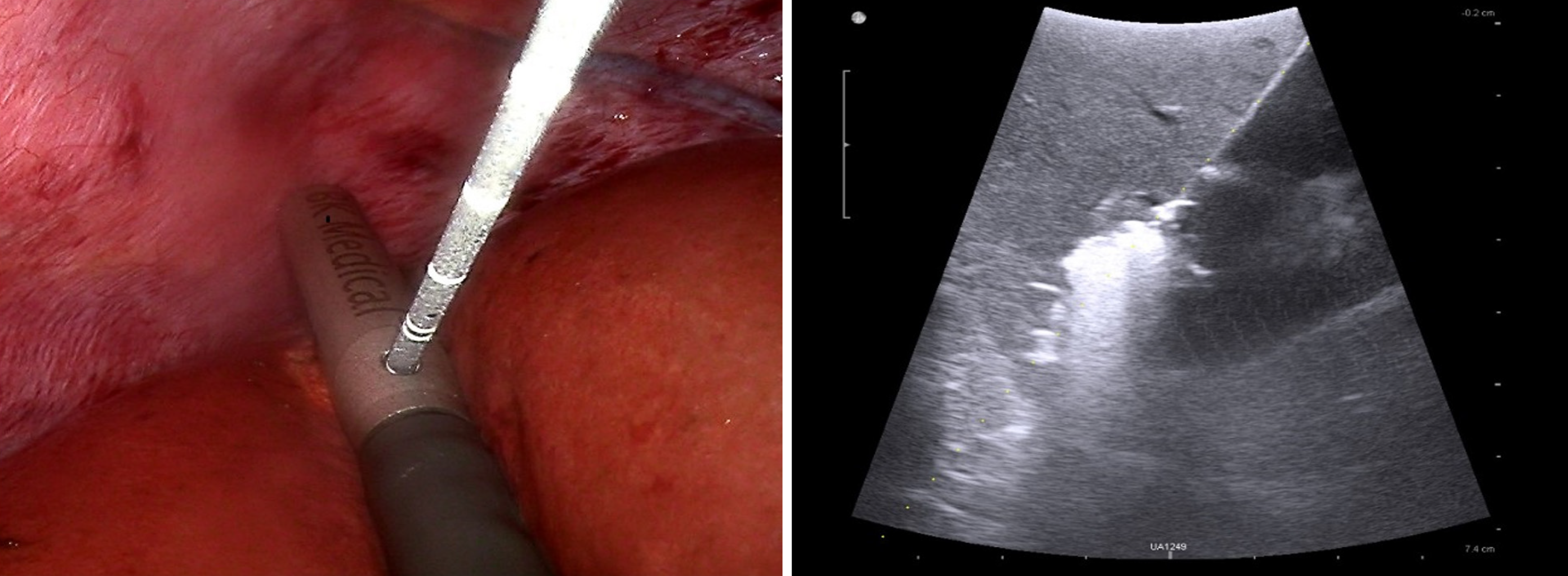
Ablation is a good option for patients with unresectable multifocal liver cancer, cirrhosis, reduced functional liver reserve, and unsuitable lesion location that is not eligible for operation[27,40-42]. Philips et al[43] reported that the survival results for one-stage hepatectomy and microwave ablation were similar to those for two-stage hepatectomy. Francone et al[35] performed LUS-guided liver resection with thermoablative precoagulation of the transection line in 20 cases and showed that this technique can achieve minimal blood loss during parenchymal transection and lower perioperative blood transfusions. This provides a non-quantifiable margin of oncological safety on the remaining liver. In addition, the loss of normal liver parenchyma is much lesser with ablation than with surgical resection[44]. The usual ablation approaches are open access, percutaneous, and laparoscopic, with percutaneous ablation being the most frequently used. However, as a necessary supplement to percutaneous ultrasound-guided liver tumor ablation, LUS-guided laparoscopic ablation has its unique advantages. Unlike percutaneous ablation, LUS-guided liver tumor ablation is visible during treatment, making its operation safer (Figure 3). The use of high-frequency ultrasound images can accurately monitor the needle trajectory in real time, guide needle placement to the tumor, and promptly manage potential complications during surgery. It also aids in protecting surrounding organs through the use of instruments. Particularly for tumors located in dangerous areas (e.g., protruding from the liver surface, top of the diaphragm, second hepatic hilum, caudate lobe of the liver, and near the gallbladder), LUS-guided laparoscopic ablation can provide relatively safe and effective treatment[27,42,45,46]. For ablation of perivascular tumors, LUS-guided ablation can well solve the “heat sink effect.” A retrospective study conducted by Kwak et al[47] showed that the 5-year cumulative local tumor progression rates of percutaneous radiofrequency ablation (PRFA) and laparoscopic radiofrequency ablation (LRFA) for tumors at the top of the diaphragm were significantly different (0.0% vs 23.4%, P = 0.015), and the 5-year overall survival rates were 71.6% and 95.7%, respectively. Eun et al[48] reported that LRFA provides superior survival outcomes to PRFA in HCC patients not suitable for surgery.
Figure 3 Laparoscopic ultrasound-guided microwave ablation imaging.
However, the operation of LUS-guided laparoscopic ablation is difficult. Due to the space-occupying effect of the trocar, ultrasonic probe and ablation needle outside the abdominal cavity[49], LUS-guided puncture needle insertion in the scanning plane of percutaneous ultrasound guidance, Therefore, the ultrasonic probe plane is angled to the needle entry plane. Consequently, it is necessary to slightly rotate the probe axially to adjust the intersection position of the two planes to better observe the needle path during the whole process and evaluate the depth and angle of the needle. Surgeons need to acquire a certain amount of experience before they can master LUS-guided laparoscopic ablation. A previous study showed that microwave ablation of liver tumors under LUS guidance can overcome the learning curve and achieve a low incomplete ablation rate after 93 liver tumor ablations[49].
Portal vein puncture under LUS
Segmental or subsegmental hepatectomy is a highly selective anatomical hepatectomy that is more difficult to perform. The basis of segment or subsegment delimitation is to obtain the corresponding segment or subsegment by blocking its blood flow or puncture staining[50,51]. For precisely injecting the dye to the hepatic segment for resection, LUS guidance is essential. In addition, it is necessary to use LUS to monitor the needle tip during the whole process to avoid other important structures and determine the relationship between the needle path and the target portal pedicle. To identify the anatomical relationship between the tumor and its surrounding structures before operation, 3D images of multidetector computed tomography are used for preoperative simulation to determine the anatomical relationship between the tumor and the portal vein branch[52-55].
In comparison with the negative staining technique, the positive staining technique of injecting indocyanine green into portal veins is more complex[53,56]. When performing laparoscopic puncture, both the ultrasound probe and puncture needle enter the abdominal cavity through same abdominal wall incision. This leads to the extrahepatic part of puncture needle being relatively fixed, making fine adjustments to reach the target site difficult. Therefore, it is more difficult than open surgery. Currently used puncture needles include a hard needle with a size of 18-22 G for entering the abdominal cavity from the abdominal wall and a soft biopsy needle from the puncture hole of the ultrasonic probe[52,53]. To puncture the portal branch accurately, surgeons need to be proficient in LUS. After extensive training and practice, surgeons should be able to significantly improve the success rate of portal vein branches with a puncture diameter of > 2 mm[51]. Due to the high technical requirements for the puncture of the intrahepatic portal vein under LUS, the existing LUS probe and puncture needle do not meet the requirements of surgeons. Therefore, positive staining of portal vein puncture will not be popular in a short time, and this special LUS probe and puncture needle need to be further developed in the near future.
ULTRASOUND COMBINED WITH NEW TECHNOLOGY
CE-IOUS
CE-IOUS is an advanced technology to improve the visualization of lesions using ultrasound contrast agents. One of the important functions of CE-IOUS is to depict the vascularization pattern of lesions. Benign pathologies and malignant lesions can be distinguished on the basis of vascularization patterns[57]. Particularly for HCC, its blood vessels are tortuous, and branches are excessive and short-circuited; this is significantly different from normal vessels and easy to be identified[58,59]. Sulfur hexafluoride microbubbles are the favored ultrasound contrast agents as they are smaller than erythrocytes and can reach vessels as narrow as 40 μm[60]. In addition, the contrast agent has no ionizing radiation and nephrotoxicity as it is not excreted by the kidneys, allowing for its use in patients with impaired renal function as well[61,62]. Following ultrasound contrast agent injection, a complete, segment-by-segment examination of the liver can be carried out to detect new lesions. The arterial, portal, and late venous phases can be recorded and analyzed. In the arterial phase, hepatocellular lesions take up contrast avidly, resulting in contrast enhancement relative to the normal liver parenchyma[63]. Conversely, CRLM do not enhance contrast and are hypoechoic compared to the normal hepatic parenchyma[64]. Previous studies have shown that even if preoperative imaging and IOUS are used to examine the liver, CE-IOUS can still find > 50% more lesions, thus potentially changing the operation strategy and leading to better therapeutic results[29,57].
CE-IOUS is helpful for immediate assessment of LRFA efficacy; a previous study showed that 50% of the ablated HCC nodules required a second ablation, and the use of CE-IOUS may achieve complete ablation by indicating residual neoplastic tissue in real time, thus reducing local recurrence[65]. Moreover, CE-IOUS should be conducted 20 min after completing LRFA as bubbles generated during RFA may affect the detection area. For CRLM resection, CE-IOUS should be considered for accurate detection of the presence and intrabiliary growth extent of CRLM and to enable accurate R0 resection[66]. A research showed that CE-IOUS performs better than computed tomography (CT), MRI, and IOUS on the tumor stage of CRLM by detecting 19% more lesions[67]. In comparison with other imaging technologies, CE-IOUS is relatively simple, inexpensive, and effective.
Real-time virtual sonography and augmented reality
Real-time virtual sonography (RVS) can realize accurate navigation of the surgical plane. Using RVS, LUS images and corresponding multiplanar reconstruction (MPR) images of preoperative CT and/or MRI can be displayed on a monitor synchronously. Moreover, 3D reconstruction images can be displayed on the same monitor[68]. By combining the 3D image with the RVS system, the actual resection plane can be easily compared with the virtual resection image provided by 3D simulation, and the relationship between the resection plane and main structures can be verified[69]. Previous studies have shown that the median time of spatial position registration was 3 min (1-12 minutes) on the basis of 26 patients who underwent liver resection with RVS navigation; with increasing number of cases, the required time is bound to decrease[68].
Presenting ultrasound images and laparoscopic videos to surgeons separately is often distracting and inefficient as the two imaging information types need to be integrated. Augmented reality (AR) laparoscopic systems improve efficiency and accuracy of the operation by integrating LUS images with laparoscopic videos in real time[70,71]. This collectively visualizes the critical internal structure and presents its 3D spatial relationship to the operator[8]. Moreover, as the application of artificial intelligence (AI) becomes increasingly mature and widespread, AI will enhance treatment precision and significantly improve surgical safety by learning from vast clinical data and integrating various imaging materials to assist surgeons in developing personalized treatment plans[72]. Liu et al[70] conducted pre-clinical experiments and showed that an AR navigation system (with electromagnetic tracking) improved the accuracy of needle positioning in LRFA by integrating LUS and ablation needle trajectory with a laparoscopic video in real time. This system also improved the efficiency and accuracy of the operation. However, more experiments are needed for further validation.
CONCLUSION
At present, LUS has many deficiencies in terms of technology and equipment. It requires advances in ultrasonic technology, as well as the development of improved operation methods and ultrasonic equipment, to allow surgeons to operate more conveniently and aid accurate intraoperative judgment. New technologies like CE-IOUS, multimodality image fusion, 3D visualization technology, virtual reality, and AR need further development to improve the safety of LUS-guided laparoscopic liver surgery. Studies have shown that surgeons need to work on 20-50 cases of LUS-assisted hepatectomy to achieve proficiency in LUS[23]. Surgeons still lack proficiency in operating LUS, and specialized technical training is essential for IOUS operators to enhance standardization in laparoscopic liver surgery. In conclusion, in the era of precision liver surgery, LUS is an important and indispensable device for hepatobiliary surgeons. With the assistance of LUS, the safety and efficacy of laparoscopic liver surgery can be improved; thus, LUS can be used throughout the entire surgical process. Surgeons should expand their knowledge on the basic operation of LUS while practicing the technique of laparoscopic liver surgery to ensure additional safety and efficacy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Committee Member of Chinese Society of Liver Cancer, Chinese Anti-Cancer Association, No. M160700544M.
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade A, Grade B
Creativity or Innovation: Grade A, Grade A
Scientific Significance: Grade A, Grade A
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Musa Y; Pannu MK S-Editor: Li L L-Editor: A P-Editor: Xu ZH