Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.105023
Revised: February 25, 2025
Accepted: March 14, 2025
Published online: May 27, 2025
Processing time: 134 Days and 17 Hours
Veno-venous extracorporeal membrane oxygenation (V-V ECMO) is a lifesaving intervention for severe respiratory failure; however, its effectiveness depends on accurate cannulation-patients with anatomical variations present with significant challenges during the procedure.
We describe the case of a 56-year-old woman with severe pulmonary infection and acute respiratory failure managed with V-V ECMO. During the initial cannulation, a 23Fr venous drainage cannula was inadvertently inserted into the middle hepatic vein (HV) instead of the inferior vena cava (IVC) owing to the enlargement of the HV (1.02 cm diameter) and its acute angle (77.78°) relative to the IVC. This misplacement led to extracorporeal membrane oxygenation (ECMO) flow issues which were resolved after repositioning the cannula under real-time ultrasonographic and fluoroscopic guidance. This correction stabilized the patient’s condition and restored effective ECMO function, preventing severe complications such as liver injury and liver failure.
In clinical practice, real-time ultrasonography and fluoroscopy are critical in preventing cannulation errors in patients with anatomical variations. Vigilant imaging and precise techniques are essential for optimizing ECMO management and effectively addressing complications.
Core Tip: Veno-venous extracorporeal membrane oxygenation (ECMO) is a vital intervention for severe respiratory failure, but anatomical variations can complicate the procedure. Anatomical variations of the middle hepatic vein, for example, may hinder cannula placement, leading to insufficient flow and severe complications such as liver injury or failure. Real-time ultrasonography and fluoroscopy are essential for detecting and correcting cannula misplacement, ensuring optimal ECMO function, and preventing serious complications. Careful imaging and precise technique are crucial, particularly in patients with anatomical anomalies.
- Citation: Li K, Pan XJ, Liu TT, Guo HY, Fang XL. Rare complication of extracorporeal membrane oxygenation cannula misplacement into the hepatic vein: A case report. World J Gastrointest Surg 2025; 17(5): 105023
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/105023.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.105023
Extracorporeal membrane oxygenation (ECMO) is a crucial intervention in patients with severe respiratory or cardiac failure[1]. Veno-venous ECMO (V-V ECMO) is recommended for patients with severe, acute, and reversible respiratory failure [partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) < 80 mmHg] who do not respond to optimal medical management[2]. Recent randomized trials advocate for the timely initiation of V-V ECMO in cases of severe respiratory failure that do not respond to conventional treatments[2,3].
With the survival rate approaching 60% for adult patients undergoing elective or emergency V-V-ECMO, it is essential to thoroughly understand the management, cannulation techniques, and potential complications associated with this increasingly utilized respiratory support modality[2]. However, the success of ECMO depends heavily on the precise cannulation of correct vascular structures. Typically, accurate cannulation is achieved under fluoroscopic and/or echocardiographic guidance using the percutaneous Seldinger technique[4]. Cannulation errors, particularly in patients with atypical anatomical features, can result in severe complications and undermine the effectiveness of therapy.
Complications associated with ECMO include hemorrhage and embolism, whereas those related to cannulation may involve perforation, arterial dissection, or cannula malposition[5-7]. Venous catheters used for extracorporeal circulation are typically inserted through the femoral vein (FV) during ECMO. However, there have been reports of ECMO cannulas inadvertently entering the right hepatic vein (HV)[8] or accessory HV[7] after insertion via the FV; in these cases, catheter misplacement was not detected until one week later. Although venous catheters occasionally migrate into the HV following surgical insertion from the right atrium (RA), this misplacement is rare, given its anatomical structure and blood flow dynamics[9,10]. However, incorrect positioning of the venous cannulas in the HV can lead to serious complications, including inadequate venous drainage and potential vascular injury[9-11].
Here, we describe the case of a critically ill patient with severe pulmonary infection and respiratory failure who experienced complications during V-V ECMO cannulation. The catheter tip was inadvertently advanced into the middle HV during the procedure instead of the inferior vena cava (IVC), owing to an anatomical anomaly in the middle HV. This error was promptly identified within a few hours of ECMO initiation using bedside chest radiography and corrected under ultrasonographic guidance. Importantly, the patient did not experience liver rupture, liver dysfunction, or intra-abdominal hemorrhage because of this misplacement.
A 56-year-old woman (height, 160 cm; weight, 49 kg) presented with recurrent fever and dry cough for more than one month, progressive dyspnea for two weeks, and worsening of symptoms over the past four days.
The patient had developed a recurrent fever one month previously, although her temperature was not recorded. The fever temporarily subsided after antipyretic therapy. She also experienced dry cough without significant sputum production. Over the past two weeks, progressive dyspnea developed and gradually worsened. Four days before admission, she experienced marked shortness of breath, even at rest. She sought medical attention and was admitted to the respiratory department.
The patient had a 10-year history of hypertension that was not regularly monitored or controlled. The patient had no history of diabetes, cardiovascular disease, kidney disease, pulmonary tuberculosis, viral hepatitis, or other infectious diseases. She denied food or drug allergies and had no history of trauma or abdominal surgery.
The patient was born in this province and lived here most of her life. She worked as a general laborer and neither consumed alcohol nor smoked. Additionally, she had no history of residing in endemic areas or exposure to toxic substances and denied any family history of specific genetic diseases.
Upon admission, the patient’s vital signs included a temperature of 38 °C, pulse rate of 133 beats per minute, respiratory rate of 33 breaths per minute, and blood pressure of 136/104 mmHg. The oxygen saturation was maintained at 89% with an oxygen mask. Physical examination revealed coarse breathing sounds bilaterally but no significant rales.
Laboratory investigations showed a white blood cell count of 7.92 × 109/L, with 81.9% neutrophils, red blood cell count of 2.52 × 10¹²/L, hemoglobin level of 75 g/L, hematocrit of 22.6%, and platelet count of 86 × 109/L. Alanine aminotransferase (ALT) activity was 61 U/L (reference range: 7-40 U/L), aspartate aminotransferase (AST) was 28 U/L (reference range: 13-35 U/L), and total bilirubin was 13.3 μmol/L (reference range: 0.0-21.0 μmol/L). Arterial blood gas analysis revealed hypoxemia and respiratory acidosis.
Chest radiography and computed tomography (CT) scans revealed diffuse bilateral pulmonary infiltrates and pleural effusion.
Based on the patient’s history, laboratory examination results upon admission, and imaging findings, she was diagnosed with severe pulmonary infection and respiratory failure.
Initial management included administering meropenem (1.0 g every 8 hours) for antimicrobial therapy, methylprednisolone for anti-inflammatory treatment, and high-flow nasal cannula oxygen therapy at 60% oxygen. Despite these interventions, her respiratory distress continued to worsen, leading to her transfer to the intensive care unit (ICU) on day 11.
In the ICU, the patient was placed on pressure control mode mechanical ventilation with settings of 50% FiO2, 15 cm H2O driving pressure, and 5 cm H2O positive end-expiratory pressure. Additionally, deep sedation and analgesia (Richmond Agitation Sedation Scale score of –5), neuromuscular blockade, and prone position ventilation (17-20 hours daily) were implemented. Bronchoscopy revealed white fungal-like material in the airways. Bronchoalveolar lavage fluid was sent for next-generation sequencing, identifying infections with Mucor, Aspergillus fumigatus, and Pneumocystis jirovecii. Consequently, the treatment regimen was adjusted to include isavuconazole and trimethoprim-sulfamethoxazole with nebulized amphotericin B for localized antifungal therapy.
As respiratory failure worsened, with the oxygenation index (PaO2/FiO2) declining to 59 mmHg, and at the insistence of the patient’s family, V-V ECMO was initiated on hospital day 19 to provide advanced respiratory support.
During ECMO initiation, an Edwards 23Fr venous drainage cannula (Edwards Lifesciences, United States) was inserted into the right FV to a depth of 43 cm. A Medos 18Fr arterial infusion cannula (Beijing Medos Medical Technology Co., Ltd., China) was placed into the right internal jugular vein to a depth of 13 cm under ultrasonographic guidance. Notably, during insertion of the venous drainage cannula via the right FV, transthoracic echocardiography (TTE) confirmed that the guidewire was positioned in the IVC and not in the RA. The initial ECMO settings included a rotational speed of 2590 rpm, flow rate of 3.39 L/min, oxygen concentration of 100%, and gas flow rate of 4 L/min.
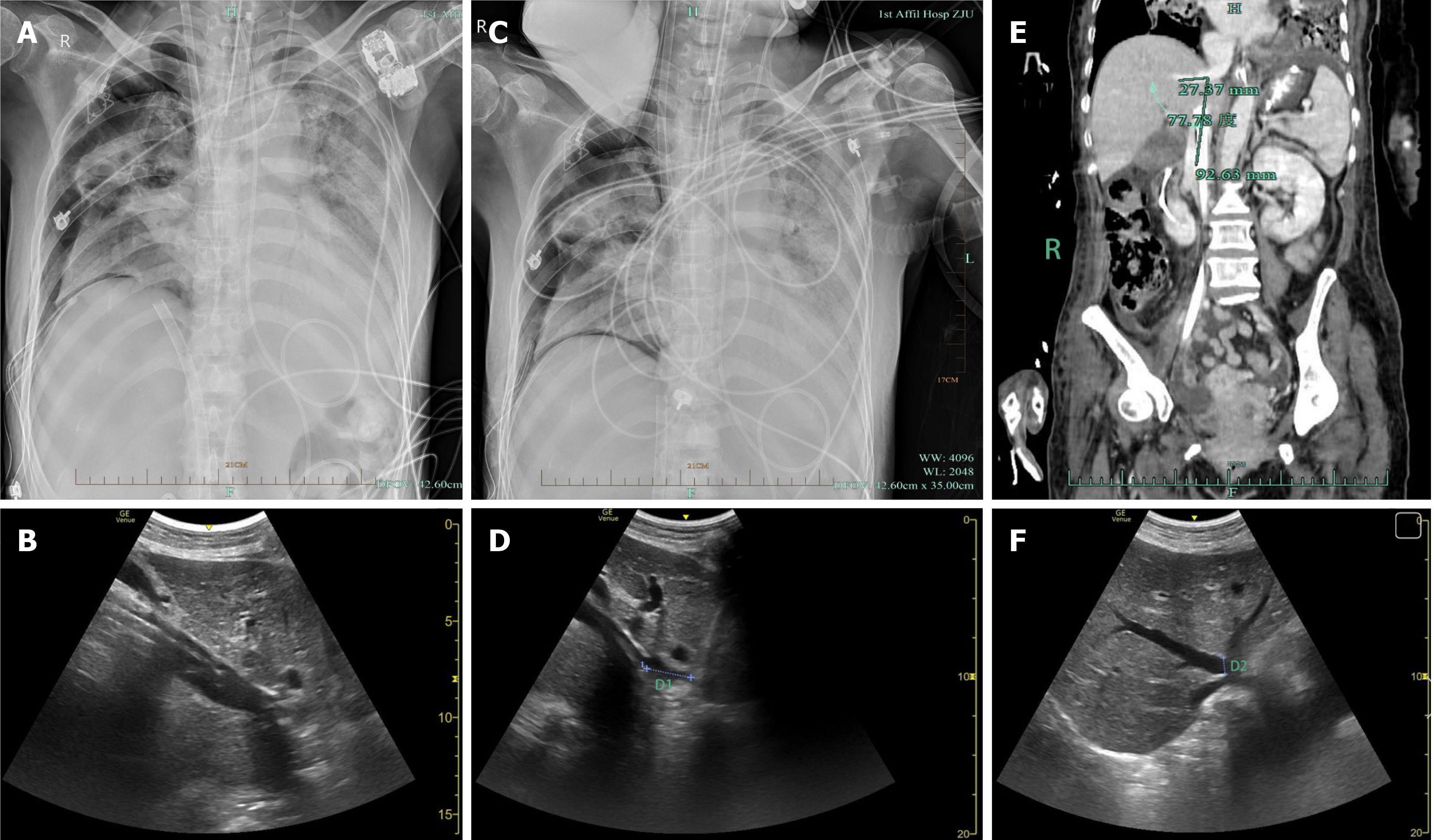
Several hours later, ECMO flow became unstable. Bedside radiography and ultrasonography revealed that the tip of the ECMO venous drainage cannula was misplaced in the middle HV instead of the IVC (Figure 1A and B). The ECMO team immediately initiated corrective action. After ultrasonography confirmed that there was no hepatic rupture, HV injury, or significant hemorrhage, the cannula was carefully withdrawn from the IVC under ultrasonographic guidance. To ensure optimal venous drainage and ECMO preloading, the cannula tip was advanced to the intended position at the junction of the IVC and RA. However, the cannula entered the middle HV again, forcing us to abandon further attempts. Finally, the cannula was placed at a depth of 36 cm, securing its position within the IVC, approximately 2.71 cm from the RA (Figure 1C and D).
After this adjustment, the ECMO parameters were recalibrated to a rotational speed of 3070 rpm, flow rate of 4.16 L/min, gas flow rate of 3 L/min, and oxygen concentration of 90% to stabilize the ECMO flow. Subsequent contrast-enhanced abdominal CT angiography and ultrasonography were performed to further assess the patient’s vascular anatomy. Additional imaging revealed a 77.78° angle between the middle HV and the IVC (Figure 1E) and an unusually wide opening of the middle HV, approximately 1.02 cm in diameter (Figure 1F). Fortunately, multiple subsequent evaluations did not reveal hepatic rupture, HV injury, or intra-abdominal hemorrhage (Figure 1E and F), and liver function was not significantly affected, with ALT at 11 U/L, AST at 21 U/L, total bilirubin at 21.5 μmol/L, and direct bilirubin at 10.1 μmol/L.
Despite intensive treatment, the condition of the patient continued to worsen. Follow-up chest CT showed progressive pulmonary lesions, whereas cranial CT revealed multiple hyperdense lesions, confirming intracranial hemorrhage. After a thorough discussion with the family, a decision was made to withdraw ECMO support. ECMO was discontinued on day 24, and the patient’s condition rapidly declined, leading to her death shortly thereafter.
V-V ECMO serves as a lifesaving rescue therapy for patients experiencing severe respiratory failure[12,13]. Optimal ECMO performance critically depends on the accurate positioning of both the arterial and venous cannulas. Previous reports have documented various venous cannula malpositions[8,14], including placement in the coronary sinus[15] and in transseptal or trans-atrial positions[16]. Additionally, obstruction of the HV by the outflow of an ECMO double-lumen cannula has been noted[17].
In this case report, we describe a patient in whom both a guidewire and venous cannula were inadvertently inserted into the middle HV during cannulation via the FV. This complication was likely influenced by the enlarged middle HV of the patient and the acute angle (77.78°) between the middle HV and the IVC. Under typical anatomical conditions, catheter misplacement is less likely when advancing from the FV to the RA, because of the generally obtuse angle between the IVC and HV. However, anatomical variations, such as those observed in this patient, can significantly increase the risk of such errors.
Previous reports have noted that guidewires inserted via the FV can occasionally deviate into the ascending lumbar vein owing to the acute angle between the ascending lumbar and iliac veins[18]. Similarly, structures within the RA, such as the Eustachian valve, can obstruct the passage of a guidewire or venous cannula inserted from the FV, potentially diverting these devices into the HV. The Eustachian valve, a membranous remnant of embryonic development located at the junction of the IVC and the RA, typically partially covers the IVC orifice[19]. However, in some cases, a prominent valve may cause obstruction[19]. The Eustachian valve has been implicated in obstructing venous cannula insertion during ECMO, leading to complications such as suck-down events[20].
In this case, no Eustachian valve was identified, and the angle between the middle HV and the IVC was 77.78°, which was acute. We observed only a mild widening of the middle HV. These findings suggest that even a mildly widened HV combined with an acute angle relative to the IVC can pose anatomical challenges leading to accidental cannulation of the HV. Although the exact angle at which the guidewire or cannula deviated into the HV could not be determined, these anatomical factors complicated the ECMO cannulation in this patient. This underscores the importance of ultrasonographic guidance in ensuring accurate guidewire placement and cannula placement at the junction of the IVC and RA.
For optimal venous drainage and ECMO preloading, the venous cannula should ideally be positioned at the junction of the IVC and RA or slightly towards the RA. Placement of a venous cannula in the IVC may result in inadequate venous return to the ECMO pump, particularly in cases of hypovolemia. Additionally, misplacement within the IVC can cause collapse owing to excessive negative pressure, which can reduce venous return and compromise ECMO blood flow.
We suspected cannula malposition upon observing inadequate ECMO pump flow in our patient. Bedside radiography and ultrasonography confirmed that the venous cannula inadvertently entered the middle HV. Repositioning the cannula in such cases increases the risk of infection at the femoral insertion site and secondary bloodstream infections. It poses a high risk of hepatic laceration and subsequent hemoperitoneum[7]. In severe cases, liver laceration with potentially fatal hemoperitoneum may necessitate emergency surgical intervention[21,22].
Although the ECMO pump flow stabilized following fluid resuscitation, additional precautions were taken to prevent fluid overload, which could worsen respiratory failure but prevent liver injury. A thorough bedside ultrasonographic assessment confirmed the absence of hepatic rupture (with the hepatic capsule remaining intact), HV rupture, or perihepatic or intra-abdominal bleeding. The venous cannula was then carefully withdrawn from the HV and placed into the IVC as soon as possible. Under ultrasonographic guidance, we attempted to position the cannula tip at the opening of the RA, but the cannula tip inadvertently entered the middle HV. Consequently, we decided to abandon further attempts and position the cannula tip within the IVC. Fortunately, the ECMO pump flow remained stable, and the patient’s oxygenation and circulation were maintained. The patient’s liver function remained unaffected throughout the hospital stay.
Echocardiography is an essential tool for accurately positioning venous cannulas, and healthcare professionals, including perfusionists and intensivists in the ICU, must remain vigilant to avoid potential pitfalls[23-25]. It is crucial to perform all necessary checks to ensure smooth cannula insertion and ECMO initiation. During V-V ECMO implantation, real-time echocardiographic monitoring plays a key role in guiding proper placement of the venous cannula in the RA. Merely visualizing the guidewire within the IVC without confirming its position in the RA is inadequate to ensure correct cannula placement. As demonstrated in this case, accidental catheterization of the middle HV can occur, particularly when TTE provides limited visualization of the IVC.
Therefore, combining fluoroscopy with ultrasonographic guidance to confirm the proper positioning of the cannula for ECMO, followed by continuous monitoring in the ICU, is increasingly recommended[26]. Martin-Villen et al[27] highlighted that thoracic ultrasonography, when combined with echocardiography, provides comprehensive monitoring of patients undergoing V-V ECMO. The absence of an experienced echocardiography operator in the ECMO team may have increased the likelihood of cannulation errors. Thus, the ECMO team must include a skilled echocardiography operator who can offer real-time guidance to ensure accurate cannula placement.
In conclusion, as ECMO has become increasingly prevalent in ICUs, a multimodal and multidisciplinary approach is essential for managing patients with complex conditions. Precision and ultrasonographic guidance in ECMO cannulation are critical, particularly for patients with atypical vascular anatomy, such as those with abnormal hepatic venous structures. At the same time, proper training and vigilant monitoring are imperative to prevent major complications and ensure adherence to best practices in ECMO management.
The authors would like to thank their colleagues from the Department of Critical Care Medicine, First Affiliated Hospital of Zhejiang University School of Medicine for their kind support of this project.
| 1. | Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, Pesenti A, Gori A. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo-Sidron JA, Usman A, Fan E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021;67:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 409] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 3. | Li X, Hu M, Zheng R, Wang Y, Kang H, Jiang L, Zhong M, Sang L, Zheng X, Pan C, Zhang W, Qiu H, Du B, Tong Z. Delayed Initiation of ECMO Is Associated With Poor Outcomes in Patients With Severe COVID-19: A Multicenter Retrospective Cohort Study. Front Med (Lausanne). 2021;8:716086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Bazan VM, Taylor EM, Gunn TM, Zwischenberger JB. Overview of the bicaval dual lumen cannula. Indian J Thorac Cardiovasc Surg. 2021;37:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013;59:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 474] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 6. | Bisdas T, Beutel G, Warnecke G, Hoeper MM, Kuehn C, Haverich A, Teebken OE. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. 2011;92:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Winiszewski H, Perrotti A, Chocron S, Capellier G, Piton G. Malposition of the Extracorporeal Membrane Oxygenation Venous Cannula in an Accessory Hepatic Vein. J Extra Corpor Technol. 2018;50:167-169. [PubMed] |
| 8. | Serck N, Lheureux O, Creteur J, Taccone FS. Hepatic vein cannulation during veno-arterial extracorporeal membrane oxygenation. Intensive Care Med. 2018;44:1571-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Raut MS, Maheshwari A, Dubey S, Shivnani G, Joshi S, Verma A, Das S. Inadequate venous drainage-transesophageal echocardiography as rescue. Ann Card Anaesth. 2017;20:333-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kirkeby-Garstad I, Tromsdal A, Sellevold OFM, Bjørngaard M, Bjella LK, Berg EM, Karevold A, Haaverstad R, Wahba A, Tjomsland O, Astudillo R, Krogstad A, Stenseth R. Guiding surgical cannulation of the inferior vena cava with transesophageal echocardiography. Anesth Analg. 2003;96:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Tempe DK, Kiro KL, Satyarthy S, Virmani S, Kumar P, Betigiri VM, Minhas HS. Evaluation of different types of inferior vena cava cannulae placement by transesophageal echocardiography and its impact on hepatic dysfunction. Perfusion. 2016;31:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, MacLaren G, Brodie D, Shekar K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8:518-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 13. | Cho HJ, Heinsar S, Jeong IS, Shekar K, Li Bassi G, Jung JS, Suen JY, Fraser JF. ECMO use in COVID-19: lessons from past respiratory virus outbreaks-a narrative review. Crit Care. 2020;24:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Lee S, Chaturvedi A. Imaging adults on extracorporeal membrane oxygenation (ECMO). Insights Imaging. 2014;5:731-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Thomas TH, Price R, Ramaciotti C, Thompson M, Megison S, Lemler MS. Echocardiography, not chest radiography, for evaluation of cannula placement during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2009;10:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Reichelt A, Pichlmaier M, Hagl C, Khaladj N. Malposition of a venous extracorporal life support cannula in a patent foramen ovale. Eur J Cardiothorac Surg. 2013;43:441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Yastrebov K, Kapalli T. Malposition of Double Lumen Bicaval Venovenous Extracorporeal Membrane Oxygenation (VV ECMO) cannula resulting in hepatic venous congestion. Australas J Ultrasound Med. 2013;16:193-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Göcze I, Müller-Wille R, Stroszczynski C, Schlitt HJ, Bein T. Accidental cannulation of the left ascending lumbar vein through femoral access-still often unrecognized. ASAIO J. 2012;58:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Klimek-Piotrowska W, Hołda MK, Koziej M, Strona M. Anatomical barriers in the right atrium to the coronary sinus cannulation. PeerJ. 2015;3:e1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ram D, Gillham M, Sibal AK. Prominent eustachian valve: An uncommon cause of a common problem during extracorporeal membrane oxygenation support. J Card Surg. 2021;36:374-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Thomas MN, Whaba R, Datta RR, Bunck AC, Stippel DL, Bruns CJ. [Management and treatment of liver injuries after blunt abdominal trauma]. Chirurgie (Heidelb). 2023;94:669-674. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Hetherington A, Cardoso FS, Lester ELW, Karvellas CJ. Liver trauma in the intensive care unit. Curr Opin Crit Care. 2022;28:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Victor K, Barrett NA, Gillon S, Gowland A, Meadows CI, Ioannou N. CRITICAL CARE ECHO ROUNDS: Extracorporeal membrane oxygenation. Echo Res Pract. 2015;2:D1-D11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Hussey PT, von Mering G, Nanda NC, Ahmed MI, Addis DR. Echocardiography for extracorporeal membrane oxygenation. Echocardiography. 2022;39:339-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Douflé G, Roscoe A, Billia F, Fan E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care. 2015;19:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Tignanelli CJ, Weinberg A, Napolitano LM. Optimal Methods to Secure Extracorporeal Membrane Oxygenation Bicaval Dual-Lumen Cannulae: What Works? ASAIO J. 2019;65:628-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Martin-Villen L, Martin-Bermudez R, Perez-Chomon H, Fuset Cabanes MP. Role of ultrasound in the critical ill patient with ECMO. Med Intensiva (Engl Ed). 2024;48:46-55. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |