Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.103653
Revised: March 1, 2025
Accepted: April 2, 2025
Published online: May 27, 2025
Processing time: 176 Days and 8.8 Hours
For locally advanced gallbladder cancer, previous clinical studies have demon
To investigate the survival benefit of chemotherapy in patients with stage II gallbladder cancer.
We performed a retrospective multivariable analysis of the National Cancer Database from 2010 to 2017 to evaluate the effect that chemotherapy has on the survival of patients with stage II gallbladder cancer. Our objective was to de
Of the 899 patients with stage II gallbladder cancer, 328 patients had undergone chemotherapy and surgery. The average overall survival for those who had surgery and chemotherapy vs only surgery was 52.6 months and 51.1 months, respectively. This difference was not statistically significant (P = 0.2). In the secondary analysis, the surgical group who had a liver resection had better overall survival (P < 0.0001).
Practitioners should carefully consider chemotherapy for early-stage gallbladder cancer, as risks may outweigh survival benefits, and surgeons should also consider liver resections as part of their surgical management.
Core Tip: In this retrospective study, we utilized the National Cancer Database to evaluate the effect that chemotherapy has on survival of patients with stage II gallbladder cancer. While the role of chemotherapy is being investigated for stage II gallbladder cancer, surgeons should exercise caution when recommending chemotherapy to patients with early-stage gallbladder cancer as the risks may outweigh the survival benefits. Our study showed that there was no statistically significant difference in survival between the those who underwent surgery and chemotherapy compared to those who only had surgery for stage II gallbladder cancer.
- Citation: Kim M, Vengatesan K, Aploks K, Thompson K, Dong X, Seshadri R. National Cancer Database analysis of gallbladder cancer: Evaluating survival benefit of chemotherapy in early-stage gallbladder cancer. World J Gastrointest Surg 2025; 17(5): 103653
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/103653.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.103653
Gallbladder cancer is the sixth most common gastrointestinal malignancy in the United States, effecting 1.2/100000 people per year[1-4]. Like other gastrointestinal malignancies, therapy revolves around complete surgical resection for localized disease. However, given that most patients have advanced disease at the time of diagnosis, systemic therapies like chemotherapy have become an increasingly popular modality of treatment[5]. Based on the results of the ABC-02 trial, the combination of gemcitabine and cisplatin is now considered to be a viable first-line therapy for unresectable gallbladder cancer[6]. More recently, focus has shifted towards investigating the potential benefit that comes with combining surgery with chemotherapy in patients with resectable disease. Hakeem et al[7] reviewed the available literature and determined there is insufficient data to support the routine use of neoadjuvant chemotherapy and chemoradiotherapy in the treatment of advanced gallbladder cancer due to patients eventually receiving a R0 resection. With regard to adjuvant therapy, studies based out of Japan and a meta-analysis have demonstrated survival benefit in adjuvant therapy in the treatment of gallbladder cancer[8-10]. One recent study, using a large national database, identified that surgery with adjuvant chemotherapy was associated with increased survival among individuals with stage II and stage III gallbladder cancer[11].
At this time, little research has published done to evaluate the role chemotherapy plays in the treatment of early-stage gallbladder cancer. A retrospective study using data from the National Cancer Database (NCDB) identified that adjuvant chemotherapy had no effect on survival among patients with T1-T3, N1-N2 gallbladder cancer when compared with surgery alone[12]. Conversely, a retrospective study from the Mayo Clinic found that the use of adjuvant chemotherapy for T2 disease actually increased overall survival (OS)[13].
The objective of this study was to evaluate whether chemotherapy and surgery impacted survival in patients with early-stage gallbladder cancer compared to surgery alone using the NCDB.
This is a retrospective cohort study using the NCDB from 2010 to 2017. The NCDB is a clinical oncology database sourced from hospital registry data collected in more than 1500 Commission on Cancer accredited facilities across the country[14].
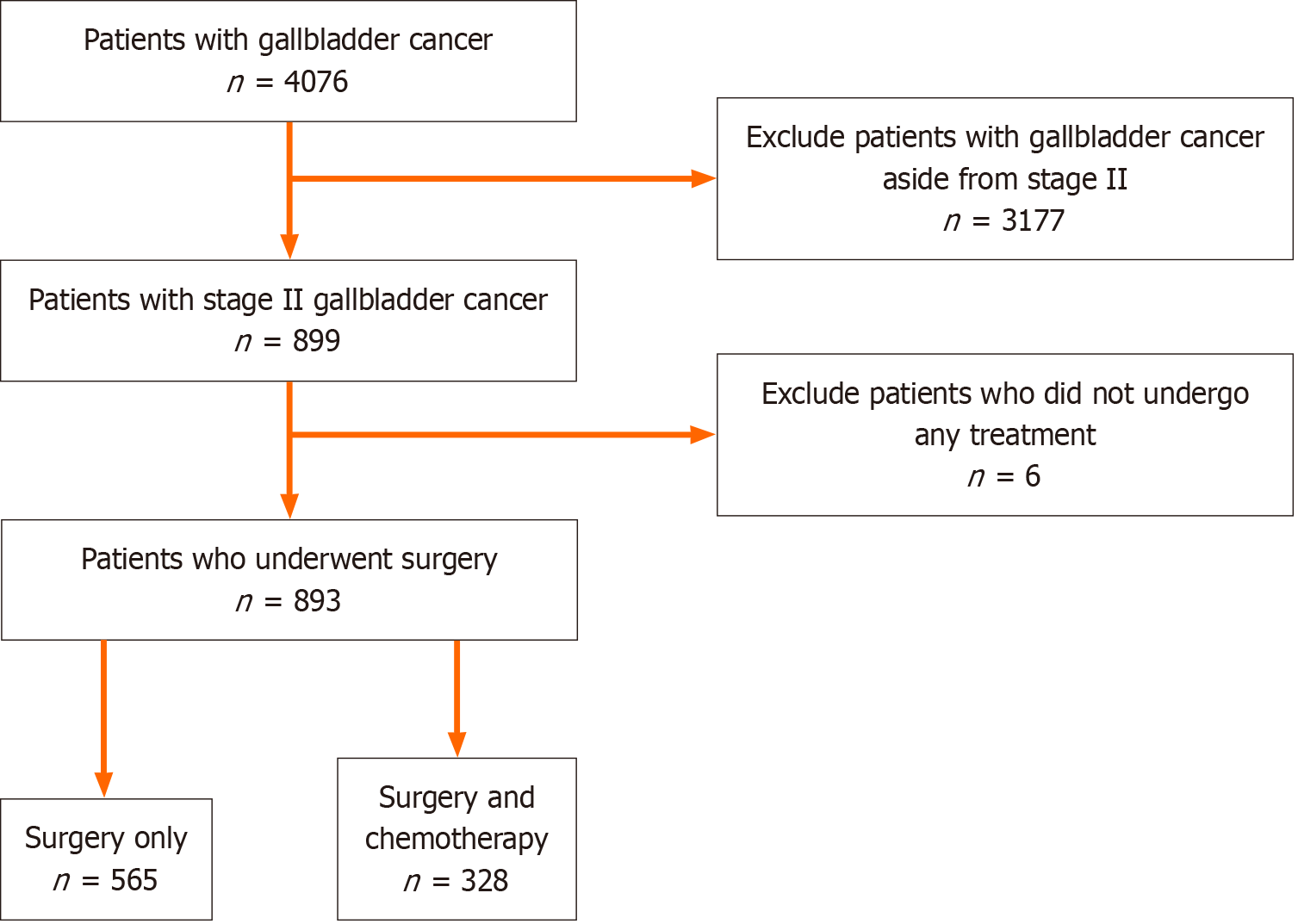
We included patients who were 18 years of age and older and were diagnosed with cancer using the International Classification of Diseases for Oncology 3rd edition. The study included only those with stage II gallbladder cancer since the effects of chemotherapy in stage II gallbladder cancer are limited compared to stage III and stage IV. Stage I was excluded from the study since surgical management is usually curative. We excluded those who did not receive any treatment with stage II gallbladder cancer. We applied the American Joint Committee on Cancer (AJCC) edition 7 to define stage II gallbladder cancer. The study selection criteria are outlined in Figure 1.
The NCDB Participant User File dictionary was used to define variables for the study[15]. Patient demographic data included age, gender, race (white, black, Hispanic, or other/unknown), geographic regions (rural, urban, or metro
| Characteristic | Surgery only (n = 565) | Surgery and chemotherapy (n = 328) | P value |
| Age (years) | < 0.0001 | ||
| 75-90 | 267 (47.2) | 59 (18.0) | |
| 65-74 | 153 (27.1) | 112 (34.1) | |
| 55-64 | 95 (16.8) | 90 (27.4) | |
| < 55 | 50 (8.8) | 67 (20.4) | |
| Sex | 0.09 | ||
| Male | 164 (29.0) | 98 (29.6) | |
| Female | 401 (71.0) | 231 (70.4) | |
| Race | 0.46 | ||
| Black | 82 (15.6) | 57 (17.4) | |
| White | 442 (78.2) | 245 (74.7) | |
| Other/unknown | 41 (0.1) | 26 (7.9) | |
| Hispanic ethnicity | 0.69 | ||
| Non-Hispanic | 476 (84.4) | 269 (85.1) | |
| Hispanic | 71 (12.6) | 47 (14.9) | |
| Geography | 0.80 | ||
| Urban/rural | 65 (11.9) | 40 (12.5) | |
| Metro | 480 (88.1) | 280 (87.5) | |
| Facility type | 0.12 | ||
| Community cancer | 52 (9.3) | 22 (6.8) | |
| Comprehensive community | 214 (38.3) | 110 (34.2) | |
| Integrated network | 104 (18.6) | 79 (24.5) | |
| Academic/research | 189 (33.8) | 111 (34.5) | |
| Insurance | < 0.0001 | ||
| Medicaid/Medicare/government | 406 (71.9) | 194 (59.1) | |
| Private | 128 (22.7) | 121 (36.9) | |
| None/unknown | 31 (5.5) | 13 (4.0) | |
| Charlson-Deyo score | 0.07 | ||
| 0 | 351 (62.1) | 230 (70.1) | |
| 1 | 150 (26.5) | 75 (22.9) | |
| 2 | 42 (7.4) | 16 (4.9) | |
| 3 + | 22 (3.9) | 7 (2.1) | |
| Surgical management1 | < 0.0001 | ||
| Partial hepatectomy | 113 (20.0) | 124 (37.8) | |
| Wedge resection | 56 (9.9) | 49 (14.9) | |
| Lobectomy | 6 (1.1) | 2 (0.6) | |
| Non liver resection | (69.0) | 153 (46.6) |
We conducted a descriptive analysis of the demographic characteristics of the patients with stage II gallbladder cancer and used χ2 test to compare the characteristics of patients by subgroups. The primary outcome was analyzing the OS between those who had surgery and those who had surgery and chemotherapy. OS was defined as the number of months between the patient’s date of diagnosis to their date of death or when they were lost to follow up. OS was estimated by using the Kaplan-Meier analysis. Multivariable logistic regression analysis was performed to analyze specific factors (i.e., age, sex, race, comorbidity, treatment facility type) that affected survival.
Of the 4076 patients with gallbladder cancer in the NCDB, 899 patients were diagnosed with stage II gallbladder cancer from 2010 to 2017. Of the 899 patients, 565 (63.27%) had surgery (cholecystectomy with or without hepatic resection) and 328 (36.73%) had surgery and chemotherapy as shown in Figure 1. Table 1 shows the baseline characteristics of the two cohorts. In both cohorts, the majority of the patients were non-Hispanic (84.4% surgery only vs 85.1% surgery and chemotherapy, P = 0.69), white (78.2% surgery only vs 74.7% surgery and chemotherapy, P = 0.46) females (71.0% surgery only vs 70.4% surgery and chemotherapy, P = 0.09) who lived in metropolitan areas (88.1% surgery only vs 87.5% surgery and chemotherapy, P = 0.80). Patients over the age of 65 were significantly more likely to receive surgery alone (74.3%) vs surgery and chemotherapy (52.1%) (P < 0.0001). Patients with Medicare/Medicaid/Government insurance were significantly more likely to undergo surgery only (71.9%) vs surgery and chemotherapy (59.1%), which contrasted with private insurance patients who more likely to receive surgery and chemotherapy (36.9%) vs surgery alone (22.7%) (P < 0.0001). There were no significant differences between cohorts when comparing facility type or extent of comorbidities (Charlson-Deyo score). Table 1 also presents the surgical intervention each cohort had undergone. In the surgery only group, significantly more patients had a cholecystectomy without a liver resection (69.0%), compared to the surgery and chemotherapy group, wherein the majority of patients had a cholecystectomy with liver resection (53.4%) (P < 0.0001).
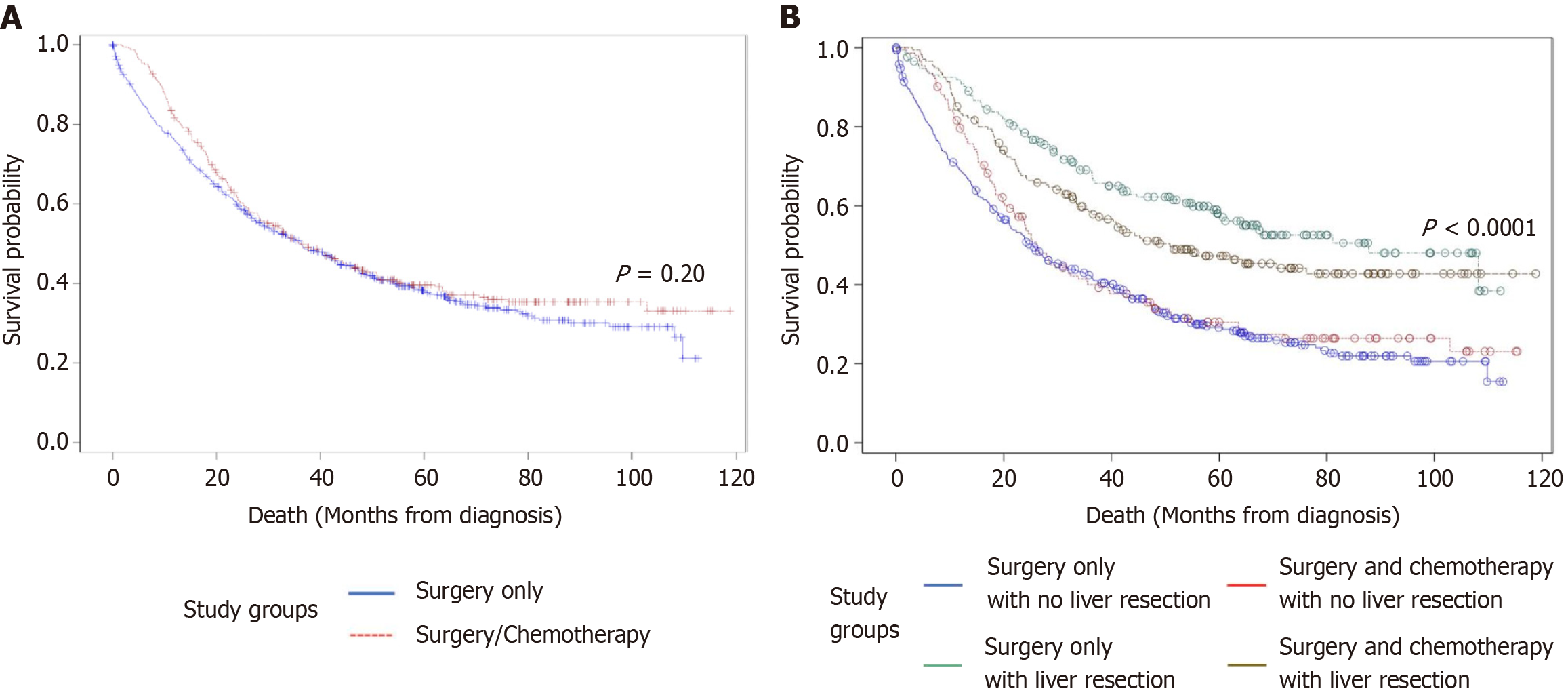
In the unadjusted Kaplan-Meier curve, the mean OS for the surgery only cohort was 51.06 months and the mean OS for the surgery and chemotherapy cohort was 52.58 months. This difference was not significant (P = 0.20) (Figure 2A).
The survival outcomes between the surgical groups were analyzed with the Kaplan-Meier analysis. The groups were surgery without a liver resection (cholecystectomy), surgery including liver resection, surgery without a liver resection and chemotherapy (cholecystectomy and chemotherapy), and surgery including liver resection and chemotherapy. The median survival of the groups were 42.34 months, 69.76 months, 44.47 months, and 48.01 months, respectively. The group with the best overall median survival was that of surgery with a liver resection (Figure 2B).
The management of gallbladder cancer remains controversial in regard to use of chemotherapy and the choice of chemotherapy regimen. Neoadjuvant chemotherapy is mostly considered for locoregionally advanced disease. There is no standard regimen for neoadjuvant chemotherapy due to limited availability of clinical data[16]. However, gemcitabine and cisplatin are commonly used for neoadjuvant chemotherapy based on the results from the ABC-02 trial, which demonstrated significant survival benefit in advanced biliary cancers[6].
Adjuvant chemotherapy is being considered for gallbladder cancer stage II and higher. The goal for adjuvant therapy is to reduce the recurrence rate in those with residual or node positive disease[14]. The survival benefit for adjuvant therapy have been evaluated by mostly retrospective studies and have shown varying results. Shroff et al[17] performed a systematic review and meta-analysis to determine the impact adjuvant therapy had on survival for biliary tract cancers. Their analysis demonstrated those who received adjuvant chemotherapy showed no significant improvement in survival. Ma et al[10] performed a meta-analysis on adjuvant therapy in gallbladder cancer and showed survival benefits of adjuvant chemotherapy with those with R1 disease, lymph node positive disease, and stage II or higher.
Prospective randomized control studies have also provided additional support for adjuvant chemotherapy. Takada et al[8] performed a phase III prospective randomized control study and demonstrated the 5-year OS and 5-year disease free survival were higher in the adjuvant therapy group compared to the surgery only group. The BILCAP phase III randomized control study conducted in the United Kingdom demonstrated improved median OS and recurrence free survival in patients treated with adjuvant capecitabine compared to those who were observed[18].
Patients enrolled in the BILCAP study had gallbladder cancer involving at least the muscularis and underwent radical surgical approach, which may have involved liver resection, pancreatic resection, or both[19]. Although current guidelines recommend radical cholecystectomy for T1b and higher tumors, our data from the NCDB showed that out of 893 patients who underwent surgical resection, 543 patients had a cholecystectomy without further resection. The benefit of adjuvant chemotherapy for those who only underwent cholecystectomy is unclear since these patients were not included in the BILCAP study. Therefore, the goal of this study was to evaluate whether chemotherapy and surgery impacted survival in patients with T2 gallbladder cancer compared to surgery alone.
T2 gallbladder cancer involves the perimuscular connective tissue. In the eighth edition of the AJCC staging classification for gallbladder cancer, T2 was categorized as cancer involving the perimuscular connective tissue on the peritoneal side without involvement of the visceral peritoneum (T2a) and hepatic side without invading the liver (T2b). The classification is based on studies that reported T2b gallbladder cancer has worse prognosis than T2a due to increase likelihood of recurrence in the liver parenchyma[20]. The survival difference between T2a and T2b is being debated. For the purposes of this study, the seventh edition AJCC staging classification, which did not categorize T2 into T2a and T2b, was used.
In our study, we included patients who had received chemotherapy (neoadjuvant and/or adjuvant chemotherapy). The majority of patients who received chemotherapy had only adjuvant chemotherapy. Patients older than 65 more often underwent surgery only and had no co-morbidities, whereas younger patients tended to undergo chemotherapy in addition to surgery. This may be because younger patients are able to tolerate the side effects associated with chemotherapy better than older patients. Despite the younger patients being treated with chemotherapy, the OS between the two groups was not statistically significant. As expected, older patients, especially older than 75 years of age, and those with higher Charlson-Deyo score had decreased OS.
Liver resection was also a significant factor in survival. In the secondary analysis, we found that those who underwent liver resection and did not receive chemotherapy had the best survival. Compared to the cohort that underwent liver resection and chemotherapy, the cohort that underwent a liver resection only lived approximately 22 months longer. Survival in the cohort that underwent liver resection and chemotherapy group may have been impacted by the side effects of the chemotherapy or the extent of their disease. The cohort with the lowest median survival was the cohort who only had cholecystectomy. Comparing the two cohorts that had surgery (with or without liver resection) and received chemotherapy, our data show that there was no significant survival benefit. Those who underwent a liver resection and chemotherapy had an additional 4-month survival compared to the cohort who underwent cholecystectomy and chemotherapy. The data from our secondary analysis support the current practice that stage II gallbladder cancers should undergo a radical cholecystectomy and not all patients with stage II disease should undergo chemotherapy.
One of the limitations of this study is the pre-defined variables in the NCDB. NCDB does not have a variable for disease-free survival, so it was not possible to assess for recurrence. Also, the type of chemotherapy is not defined so patients were treated with different chemotherapy regimens. Despite these limitations, our study provides additional insight into the role of adjuvant chemotherapy in early-stage gallbladder cancer. Based on our results, chemotherapy does not provide additional survival benefit for those with stage II gallbladder cancer.
Chemotherapy in early-stage gallbladder cancer does not provide additional survival benefit as it has shown in clinical trials for advanced gallbladder cancer. Therefore, physicians should carefully consider the use of chemotherapy in patients with early-stage gallbladder cancer. As clinical studies are being conducted to show the effect of adjuvant chemotherapy in gallbladder cancer, physicians will need to continue to use their clinical judgement and individualize their care for patients.
| 1. | Liebe R, Milkiewicz P, Krawczyk M, Bonfrate L, Portincasa P, Krawczyk M. Modifiable Factors and Genetic Predisposition Associated with Gallbladder Cancer. A Concise Review. J Gastrointestin Liver Dis. 2015;24:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014;94:343-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 3. | Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp Hepatol. 2019;5:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (1)] |
| 4. | Schmidt MA, Marcano-Bonilla L, Roberts LR. Gallbladder cancer: epidemiology and genetic risk associations. Chin Clin Oncol. 2019;8:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Søreide K, Harrison EM, Wigmore SJ. Research gaps and unanswered questions in gallbladder cancer. HPB (Oxford). 2018;20:685-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3332] [Article Influence: 208.3] [Reference Citation Analysis (15)] |
| 7. | Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - A systematic review. Eur J Surg Oncol. 2019;45:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 8. | Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T; Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 447] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Hanazaki K. Systemic chemotherapy following non-curative resection may increase survival for people with gall bladder carcinoma, but not carcinomas of the pancreas, bile duct or ampulla of Vater. Cancer Treat Rev. 2003;29:135-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Ma N, Cheng H, Qin B, Zhong R, Wang B. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. 2015;15:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Kemp Bohan PM, Kirby DT, Chick RC, Bader JO, Clifton GT, Vreeland TJ, Nelson DW. Adjuvant Chemotherapy in Resectable Gallbladder Cancer is Underutilized Despite Benefits in Node-Positive Patients. Ann Surg Oncol. 2021;28:1466-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Mantripragada KC, Hamid F, Shafqat H, Olszewski AJ. Adjuvant Therapy for Resected Gallbladder Cancer: Analysis of the National Cancer Data Base. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Gold DG, Miller RC, Haddock MG, Gunderson LL, Quevedo F, Donohue JH, Bhatia S, Nagorney DM. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys. 2009;75:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 14. | American College of Surgeons. National Cancer Database. [cited March 19, 2025]. Available from: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/. |
| 15. | American College of Surgeons. National Cancer Database PUF Dictionary. [cited March 19, 2025]. Available from: https://www.facs.org/media/ujjlhni4/puf-2021-data-dictionary.pdf. |
| 16. | National Comprehensive Cancer Network. Hepatobiliary Cancer (Version 5.2022). [cited March 19, 2025]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. |
| 17. | Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, El-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37:1015-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 18. | Bridgewater J, Fletcher P, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans TR, Stocken D, Stubbs C, Praseedom R, Ma YT, Davidson B, Neoptolemos J, Iveson T, Cunningham D, Garden OJ, Valle JW, Primrose J; BILCAP study group. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J Clin Oncol. 2022;40:2048-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 19. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 903] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 20. | Sung MK, Lee W, Lee JH, Song KB, Kim SC, Kwak BJ, Hwang DW. Comparing survival rate and appropriate surgery methods according to tumor location in T2 gallbladder cancer. Surg Oncol. 2022;40:101693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/