Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.103340
Revised: March 6, 2025
Accepted: April 17, 2025
Published online: May 27, 2025
Processing time: 147 Days and 0.9 Hours
Gastric cancer predominantly affects the elderly, who face significant challenges due to high postoperative complications and stress. These challenges include co
To investigate the effects of ERAS-based continuity nursing care on postoperative satisfaction, inflammation, stress, and quality of life in elderly gastric cancer pa
A retrospective cohort analysis was conducted on 322 elderly patients who under
The ERAS-C group exhibited significantly lower postoperative interleukin-6 levels than the routine care group (12.97 ± 4.02 pg/mL vs 14.37 ± 3.86 pg/mL; P = 0.002). This finding indicates that the ERAS-C group experienced reduced inflammation. The ERAS-C group also had a higher cluster of differentiation (CD) 4:CD8 ratio than the routine care group (2.34 ± 0.35 vs 2.13 ± 0.61; P < 0.001), suggesting the former’s enhanced immune response. Postoperative stress markers, including norepinephrine, cortisol, and aldosterone, were significantly lower in the ERAS-C group than in the routine care group (P < 0.05 for all). Compared with the routine care group, the ERAS-C group showed increased nursing satisfaction scores (80.36 ± 7.24 vs 75.23 ± 7.03; P < 0.001) and improved quality of life indicators, such as reduced dysphagia and pain, (P < 0.05). The ERAS-C group also experienced fewer complications than the routine care group (5.42% vs 11.54%, P = 0.048).
Continuity nursing care within the ERAS framework significantly enhances postoperative outcomes for elderly gastric cancer patients by reducing inflammation, stress, and complications while improving satisfaction and qua
Core Tip: Enhanced recovery after surgery (ERAS) principles, when integrated with continuity nursing care, significantly improve postoperative outcomes in elderly gastric cancer patients. This study demonstrates that ERAS-based continuity nursing reduces inflammation and stress while enhancing immune response and patient satisfaction. Key findings include lower interleukin-6 levels, higher cluster of differentiation (CD) 4:CD8 ratios, reduced postoperative complications, and improved quality of life. These results highlight the potential of ERAS protocols to address the unique challenges faced by elderly patients undergoing gastric cancer resection, providing a robust foundation for future multi-center studies.
- Citation: Lu CP, Gao Y, Zhang ZH. Enhanced recovery after surgery continuity nursing in elderly gastric cancer patients. World J Gastrointest Surg 2025; 17(5): 103340
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/103340.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.103340
Gastric cancer remains a significant global health challenge, particularly among the elderly population, where the inci
In recent years, the enhanced recovery after surgery (ERAS) program has been heralded as a transformative approach to surgical care. Originating in colorectal surgery, the ERAS program is a multimodal, multidisciplinary approach aimed at reducing surgical stress and improving recovery times through optimized perioperative care. Its core principles include preoperative counseling and optimization, minimally invasive surgical techniques, and early postoperative mobilization and nutrition, all designed to harmonize the patients’ physiological state with the demands of surgery. Although the ERAS approach has been widely adopted across various surgical disciplines, its impact on the elderly undergoing gastric cancer surgery remains under investigated[4-6].
Continuity of care, an extension of ERAS, emphasizes sustained engagement between healthcare providers and patients. It carries out tailored interventions that span the preoperative, intraoperative, and postoperative phases. Such continuity is particularly crucial for elderly patients, who often possess unique physiological and psychological needs that can profoundly influence surgical outcomes. Nursing care, particularly within the ERAS framework, assumes an integral role in delivering this continuous support. Nurses serve as pivotal figures in perioperative management by providing not only direct medical care but also emotional support, education, and personalized guidance to encourage compliance with ERAS protocols[7-9].
Despite these promising insights, research exploring the intersection of continuity nursing care and the ERAS frame
This study is a retrospective cohort analysis involving 322 elderly patients who underwent gastric cancer resection at our institution between January 2020 and January 2022. The patients were divided into two groups: 156 patients who received routine care were placed in the routine group, whereas 166 patients who received continuity of care based on the ERAS principles were placed in the ERAS-control (ERAS-C) group. Data, including demographic information, general clinical details, postoperative complications, inflammation markers, stress responses, patient satisfaction, and quality of life, were collected from the medical records system for both groups.
This study was approved by the Ethics Committee of the Zhoukou First People’s Hospital. Informed consent was waived because the study utilized only de-identified patient data, which presented no risk or impact on patient care. This waiver adheres to the regulatory and ethical guidelines related to retrospective research as approved by our institution’s Institutional Review Board and Ethics Committee.
Inclusion criteria: (1) Patients with histologically confirmed gastric carcinoma, classified as resectable gastric cancer at clinical stages I–III based on the eighth edition of the tumor, node, metastasis (TNM) staging classification[12]; (2) Patients aged 60 years or older; and (3) Patients with comprehensive medical records.
Exclusion criteria: (1) Patients with a history of severe allergies or significant cardiovascular disease; (2) Patients with congenital immune dysfunction; (3) Patients undergoing multivisceral resection; and (4) Patients with psychiatric disor
In the routine group, patients received standard care, which involved the following steps. Before surgery, healthcare providers briefly introduced knowledge about the disease and the surgical procedure, informed patients about surgery-related precautions, and patiently alleviated any negative emotions. Postoperatively, they intensified monitoring of vital signs and recovery status, and rehabilitation activities based on recovery progress were coordinated. Before discharge, patients received reinforced guidance, including education about post discharge diet and exercise, and were followed up with weekly phone calls.
In the ERAS-C group, continuity of care based on the principles of ERAS was implemented through detailed methods discussed in the subsequent sections.
Multidisciplinary perioperative care under the ERAS framework: Healthcare professionals collectively managed patient admissions, gathered medical history, assessed the severity of the illness, and provided detailed information concerning gastric cancer resection.
On the day before surgery, anesthetists assessed the patient’s vital signs to determine the optimal anesthesia plan. They also educated the patient and their family on anesthesia-related knowledge and precautions.
Psychologists, along with medical staff, evaluated the patient’s psychological state, identified causes of negative emotions, and employed techniques such as distraction and relaxation exercises to adjust the psychological status.
Dieticians developed nutritional plans tailored to the patient’s condition and preferences. They emphasized high-quality proteins and vitamin-rich foods while ensuring sufficient daily nutritional intake. They strictly controlled salt and sodium intake to prevent diet-related blood pressure increases.
Postoperatively, rehabilitation therapists actively participated in rehabilitation treatment by creating tailored training regimens based on the patient’s condition and instructing them on training techniques.
Continuity of care: Establishing a health archive: Before discharge, nurses collected personal information, medical history, and treatment plans. These data were entered into electronic health records.
Before discharge, patients’ contact information and home addresses were double-checked. Then, the patients were encouraged to join a WeChat or QQ group. Healthcare providers regularly disseminated recovery knowledge about gastric cancer resection in these groups. Health education was provided to patients and their families to alleviate negative emotions, promote correct disease understanding, and increase recovery confidence. Personalized guidance on medica
Follow-up care: One week after discharge, patients received weekly phone follow-ups twice a week, along with monthly home visits to assess psychological status, medication adherence, diet, and recovery progress. Patients were advised on correcting any inappropriate behaviors, setting rehabilitation goals, and maintaining a recovery diary. They were also reminded to return for a first-month postoperative check-up.
Data on patients, encompassing demographic characteristics, pathological findings, surgical results, hospital courses, and postoperative outcomes, were systematically recorded in our electronic gastric cancer database. The demographic in
This study evaluated patient satisfaction one month post-surgery using the Chinese version of Ware’s patient satisfaction questionnaire-18 (PSQ-18). PSQ-18 consists of seven dimensions: Overall satisfaction, technical service quality, nurses’ interpersonal communication, nurse–patient communication, service cost-effectiveness, service timeliness, and service accessibility. Each dimension employs forward and reverse questioning techniques. Before statistical analysis, the res
One month post-surgery, fasting venous blood samples were collected from all patients in the morning for biochemical analysis.
Inflammatory markers: Serum interleukin-6 (IL-6) levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Ready-Set-Go; eBioscience, San Diego, CA, United States) by following the manufacturer’s instructions. Serum albumin was quantified via the bromocresol green method (AU-ALB, Beckman Coulter Inc., Brea, CA, United States). High-sensitivity C-reactive protein (CRP) levels were determined using the turbidimetric method (AU-CRP, Beckman Coulter Inc., Brea, CA, United States) in accordance with routine laboratory procedures.
Immune parameters: The immune profile was assessed by measuring the total number of leukocytes (μL), lymphocytes (μL), and lymphocyte subsets, including the percentage of natural killer (NK) cells and the cluster of differentiation (CD) 4:CD8 ratio. A minimum of 1 × 106 cells/mL was analyzed using a flow cytometer (FACScan; Becton Dickinson, Moun
Stress response: Postoperative levels of norepinephrine (NE), aldosterone (ALD), and cortisol (Cor) were quantified using ELISA. NE levels were measured with the NE ELISA kit (ABN-KA1877, Abnova, Taipei, Taiwan). ALD levels were assessed using the ALD ELISA kit (501090, Cayman, Ann Arbor, MI, United States), and serum Cor levels were determined with the Cor Parameter assay kit (KGE008B, Beckman Coulter Inc., Brea, CA, United States) according to the manufacturer’s protocols.
We utilized the validated version of the European Organization for Research and Treatment of Cancer 22-item Quality of Life Questionnaire specific to gastric cancer (EORTC-QLQ-STO22) to assess the changes in health-related quality of life (HRQOL) one month post-surgery[14]. The EORTC-QLQ-STO22 includes one functional item (body image), five symptom scales (dysphagia, eating restrictions, pain, reflux, and anxiety), and three individual symptom items (dry mouth, taste disturbance, and hair loss). Low scores indicate good HRQOL. The Cronbach’s alpha for this scale was 0.92, demonstrating high reliability[15].
Prior to data analysis, this study implemented a standardized data cleaning process to identify and rectify any inconsistencies, errors, or missing values. This process involved thoroughly examining the dataset, removing duplicate entries, correcting data entry errors, and addressing missing values. Missing data were imputed using the Multivariate Imputa
Missing data were kept within 5% to control potential selection bias, and sensitivity analyses were conducted. In these analyses, outcomes for cases lost to follow-up were calculated under worst-case and best-case scenarios. If the conclusions showed no significant differences, then the impact of the missing data on the conclusions was deemed minimal, thereby indicating the reliability of the findings. The final result output included the dataset with imputed missing values.
The sample size was calculated using G Power 3.1. Data analysis was conducted using Statistical Package for the Social Sciences (SPSS) version 29.0 (SPSS Inc., Chicago, IL, United States). Categorical data were represented as n (%). The χ2 test was applied to data with a sample size of ≥ 40 and a theoretical frequency (T) of ≥ 5, with the test statistic denoted by χ². When the sample size was ≥ 40 but the theoretical frequency was between 1 and < 5, an adjusted χ2 test was used with a correction formula. Fisher’s exact test was employed when the sample size was < 40 or the theoretical frequency (T) was < 1.
Continuous variables were first evaluated for normal distribution using the Shapiro-Wilk test. For normally distributed continuous data, values were expressed as mean ± SD. Nonnormally distributed data were assessed using the Wilcoxon rank-sum test and were presented as median (25% quantile, 75% quantile). A P value of < 0.05 was considered statistically significant. Correlation analyses were performed using Pearson correlation for continuous variables and Spearman correlation for categorical variables.
In the study comparing the impact of continuity nursing care based on ERAS principles on postoperative outcomes in elderly patients undergoing gastric cancer resection, 322 patients were divided into a routine care group (n = 156) and an ERAS-C group (n = 166) (Table 1). The analysis of demographic and baseline characteristics demonstrated no statistically significant differences between groups in terms of gender distribution [male: (routine) 68.59%, (ERAS-C) 65.66%; χ² = 0.312, P = 0.576], age [(routine) 70.84 ± 10.17 years, (ERAS-C) 69.66 ± 10.12 years; t = 1.043, P = 0.298], and BMI [(routine) 22.12 ± 3.40 kg/m², (ERAS-C) 22.51 ± 3.11 kg/m²; t = 1.070, P = 0.285]. Moreover, no significant variation was found in other factors, including the degree of education, smoking and drinking history, employment status, diabetes, hyperten
| Parameters | Routine group (n = 156) | ERAS-C group (n = 166) | t/χ² | P value |
| Gender (male/female) | 107 (68.59)/49 (31.41) | 109 (65.66)/57 (34.34) | 0.312 | 0.576 |
| Age (years) | 70.84 ± 10.17 | 69.66 ± 10.12 | 1.043 | 0.298 |
| BMI (kg/m²) | 22.12 ± 3.40 | 22.51 ± 3.11 | 1.070 | 0.285 |
| Degree of education | 0.874 | 0.928 | ||
| Junior high school and below | 68 (43.59) | 73 (43.98) | ||
| Technical secondary school | 36 (23.08) | 40 (24.10) | ||
| High school | 30 (19.23) | 26 (15.66) | ||
| Junior college | 13 (8.33) | 16 (9.64) | ||
| University and above | 9 (5.77) | 11 (6.63) | ||
| Smoking history | 59 (37.82) | 54 (32.53) | 0.988 | 0.320 |
| Drinking history | 71 (45.51) | 67 (40.36) | 0.871 | 0.351 |
| Employment | 113 (72.44) | 121 (72.89) | 0.008 | 0.927 |
| Diabetes | 52 (33.33) | 50 (30.12) | 0.384 | 0.536 |
| Hypertension | 111 (71.15) | 116 (69.88) | 0.063 | 0.802 |
| Residence area (town/rural) | 85 (54.49)/71 (45.51) | 93 (56.02)/73 (43.98) | 0.077 | 0.782 |
| Live alone | 22 (14.10) | 19 (11.45) | 0.511 | 0.475 |
| Tumor location | 5.538 | 0.136 | ||
| Upper | 32 (20.51) | 49 (29.52) | ||
| Middle | 73 (46.79) | 61 (36.75) | ||
| Lower | 40 (25.64) | 48 (28.92) | ||
| Remnant stomach | 11 (7.05) | 8 (4.82) | ||
| Clinical TNM stage | 0.363 | 0.834 | ||
| I | 11 (7.05) | 14 (8.43) | ||
| II | 105 (67.31) | 107 (64.46) | ||
| III | 40 (25.64) | 45 (27.11) |
Tumor histology, categorized by Lauren classification, showed similar distributions between the two groups [intestinal: (routine) 54.49%, (ERAS-C) 60.24%; diffuse: (routine) 14.10%, (ERAS-C) 19.28%; others: (routine) 31.41%, (ERAS-C) 20.48%; χ² = 5.474, P = 0.065] (Table 2). The type of gastrectomy performed did not significantly differ: Total gastrectomy was conducted in 26.28% and 27.71% of the routine group and the ERAS-C group, respectively, and distal gastrectomy was conducted in 54.49% and 47.59% of the routine group and the ERAS-C group, respectively (χ² = 5.729, P = 0.220). The proportion of patients undergoing an open surgical approach was comparable [(routine) 85.90%, (ERAS-C) 79.52%; χ² = 2.278, P = 0.131]. Operative time did not significantly differ between groups, with the routine group averaging 298.31 ± 40.94 minutes and the ERAS-C group averaging 300.14 ± 45.68 minutes (t = 0.377, P = 0.706). Similarly, the length of postoperative hospital stay was comparable, with the routine group averaging 13.85 ± 3.69 days and the ERAS-C group averaging 14.32 ± 3.11 days (t = 1.245, P = 0.214). Overall, these findings indicate homogeneity between the groups with respect to operative and pathological characteristics.
| Parameters | Routine group (n = 156) | ERAS-C group (n = 166) | t/χ² | P value |
| Tumor histology (Lauren) | 5.474 | 0.065 | ||
| Intestinal | 85 (54.49) | 100 (60.24) | ||
| Diffuse | 22 (14.10) | 32 (19.28) | ||
| Others | 49 (31.41) | 34 (20.48) | ||
| Type of gastrectomy | 5.729 | 0.220 | ||
| Total (including completion) | 41 (26.28) | 46 (27.71) | ||
| Distal | 85 (54.49) | 79 (47.59) | ||
| Proximal | 1 (0.64) | 5 (3.01) | ||
| Pylorus-preserving | 15 (9.62) | 25 (15.06) | ||
| Other | 14 (8.97) | 11 (6.63) | ||
| Open approach | 134 (85.90) | 132 (79.52) | 2.278 | 0.131 |
| Operative time (minutes) | 298.31 ± 40.94 | 300.14 ± 45.68 | 0.377 | 0.706 |
| Length of postoperative stay (days) | 13.85 ± 3.69 | 14.32 ± 3.11 | 1.245 | 0.214 |
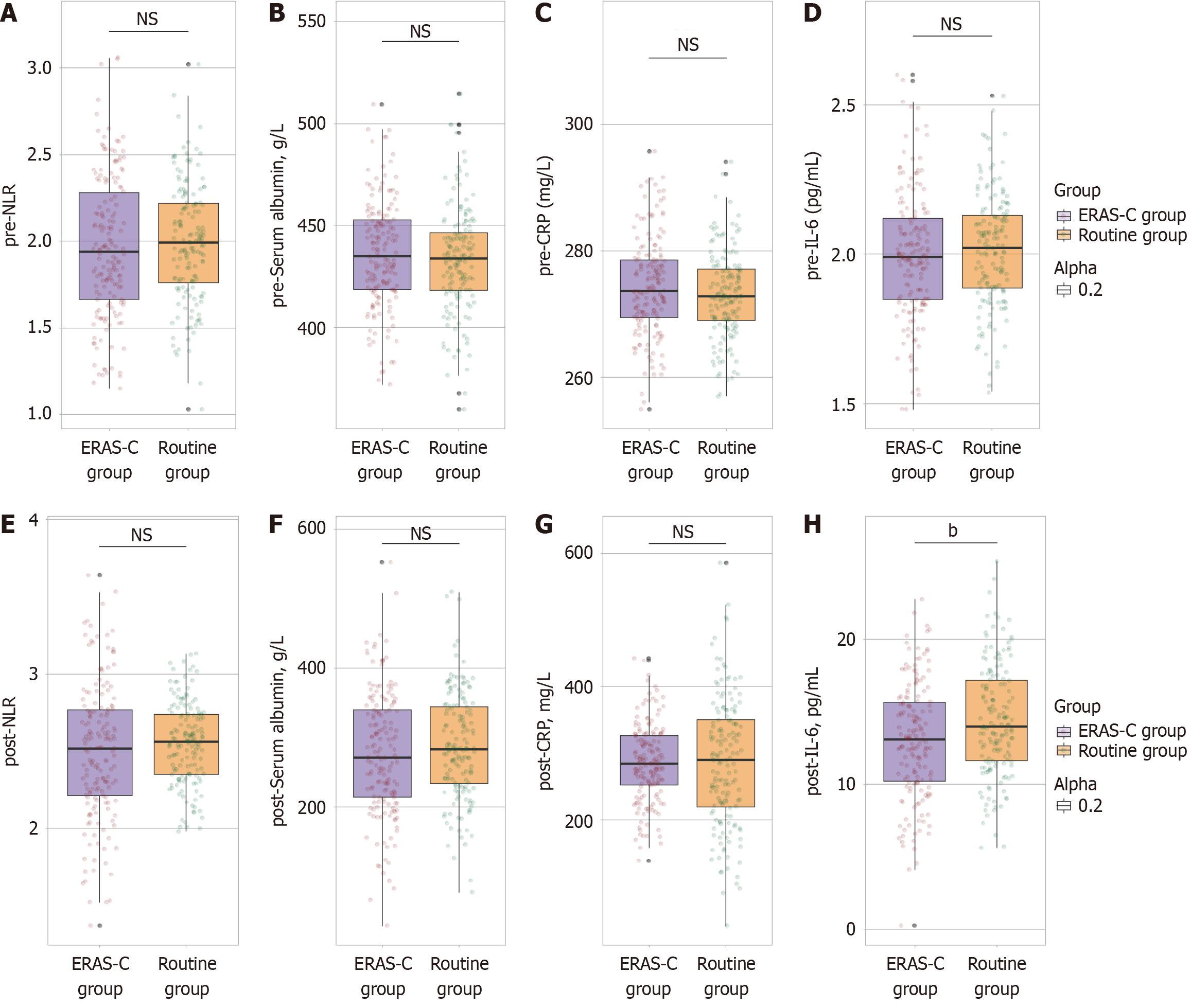
The neutrophil-to-lymphocyte ratio (NLR) was similar between the groups, with the routine group and the ERAS-C group showing an average of 1.99 ± 0.34 and 1.96 ± 0.41, respectively (t = 0.580, P = 0.562) (Figure 1A-D). Serum albumin levels were also comparable, with averages of 433.02 ± 25.86 and 435.74 ± 26.58 g/L for the routine group and the ERAS-C group, respectively (t = 0.930, P = 0.353). CRP levels were measured at 273.06 ± 6.87 and 274.38 ± 7.73 mg/L in the routine group and the ERAS-C group, respectively, with no significant difference observed (t = 1.622, P = 0.106). IL-6 levels were also not significantly different, with averages of 2.01 ± 0.19 and 2.00 ± 0.21 pg/mL in the routine group and the ERAS-C group, respectively (t = 0.700, P = 0.485). These results indicate that preoperative inflammatory status was equivalent between the two patient groups.
The IL-6 levels in the ERAS-C group (12.97 ± 4.02 pg/mL) were significantly lower than those in the routine group (14.37 ± 3.86 pg/mL). This finding indicates a significant reduction in postoperative inflammation with ERAS-C care (t = 3.182, P = 0.002) (Figure 1E-H). Other inflammatory markers did not exhibit statistically significant differences: The NLR in the ERAS-C group (2.49 ± 0.44) was marginally but not significantly lower than that in the routine group (2.55 ± 0.26) (t = 1.478, P = 0.141). Serum albumin levels, an indicator of nutritional and inflammatory status, were similar between the groups, with the routine group at 287.05 ± 76.88 g/L and the ERAS-C group at 276.14 ± 89.30 g/L (t = 1.171, P = 0.242). CRP levels remained consistent between the routine (285.12 ± 97.14 mg/L) and ERAS-C (284.54 ± 58.33 mg/L) groups (t = 0.065, P = 0.948), suggesting comparable systemic inflammatory response at this postoperative stage. These findings highlight the impact of ERAS-C on lowering IL-6 levels, potentially contributing to improved recovery.
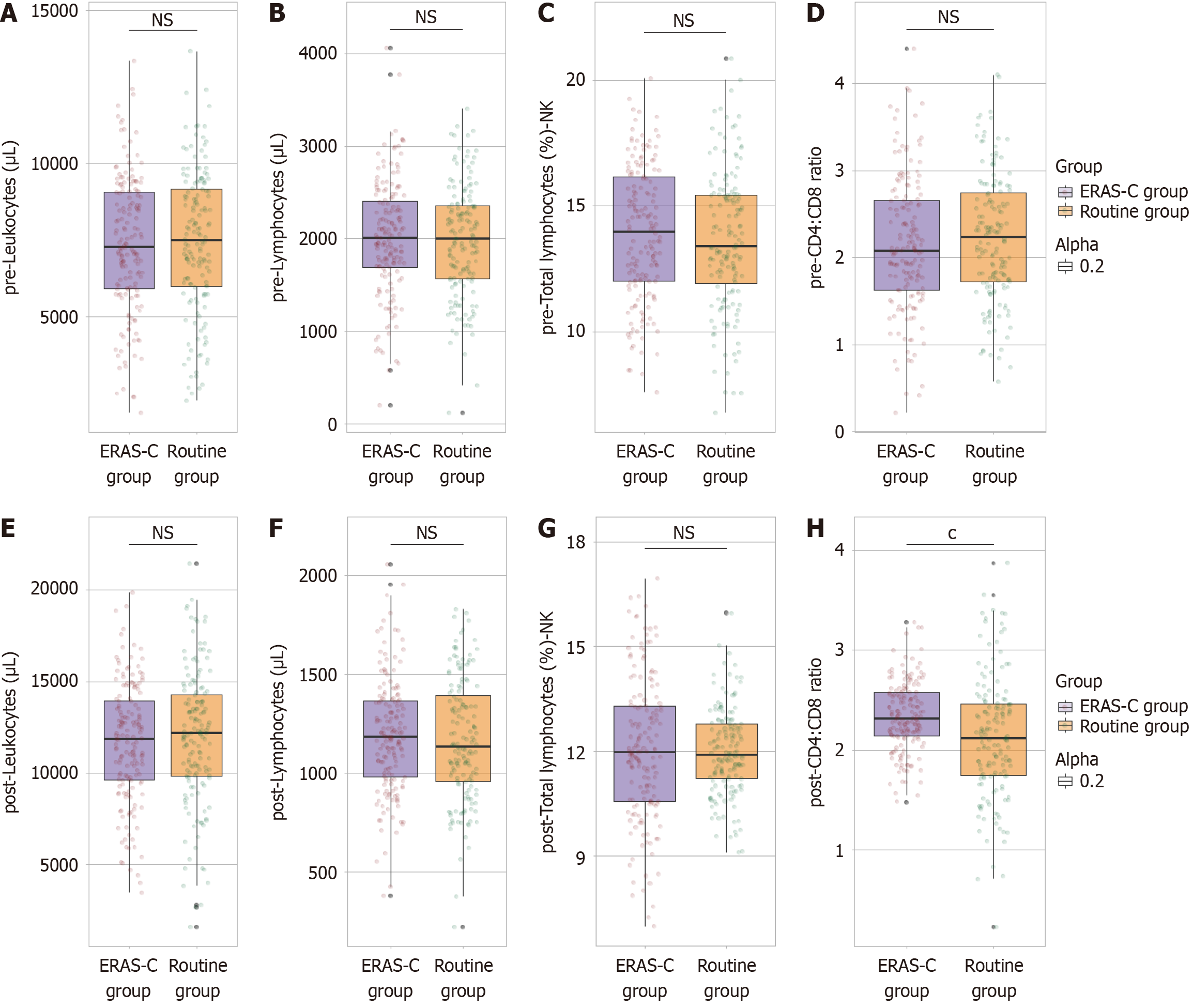
Leukocyte counts were similar between the groups, with the routine group averaging 7453.72 ± 2325.01 μL and the ERAS-C group averaging 7369.78 ± 2236.14 μL (t = 0.330, P = 0.741) (Figure 2A-D). Lymphocyte counts were also comparable, with the routine group at 1978.45 ± 602.17 μL and the ERAS-C group at 2004.36 ± 624.67 μL (t = 0.378, P = 0.705). The percentage of total lymphocytes classified as NK cells was consistent between groups, with the routine group at 13.65% ± 2.75% and the ERAS-C group at 13.89% ± 2.68% (t = 0.787, P = 0.432). Additionally, the CD4:CD8 ratio showed no significant difference, being 2.23 ± 0.73 and 2.14 ± 0.77 in the routine group and the ERAS-C group, respectively (t = 0.955, P = 0.340). Overall, these findings indicate a uniform preoperative immune status between the two patient groups.
One month postoperatively, the analysis of immune parameters between the routine care group and the ERAS-C group revealed a significant difference in the CD4:CD8 ratio, with the ERAS-C group exhibiting a higher ratio (2.34 ± 0.35) than the routine group (2.13 ± 0.61). This finding indicates a more favorable immune profile in the ERAS-C group than in the routine care (t = 3.748, P < 0.001) (Figure 2E-H). Other immune parameters showed no statistically significant differences. Leukocyte counts remained similar, with the routine group averaging 11983.64 ± 3645.18 μL and the ERAS-C group averaging 11647.15 ± 3254.97 μL (t = 0.875, P = 0.382). Lymphocyte counts were also comparable, with the routine group and the ERAS-C group at 1165.39 ± 307.26 and 1187.64 ± 289.17 μL, respectively (t = 0.669, P = 0.504). The percentage of total lymphocytes classified as NK cells was consistent between groups, with the routine group and the ERAS-C group at 11.98% ± 1.23% and 12.04% ± 2.06%, respectively (t = 0.356, P = 0.722). These results suggest that although most postope
Within the first postoperative month, complications were reported in 11.54% of the routine group and 5.42% of the ERAS-C group. This finding demonstrates a statistically significant decrease (χ² = 3.917, P = 0.048) (Table 3). Major complications in the ERAS-C group (1.20%) were significantly lower than those in the routine group (5.77%) (χ² = 5.078, P = 0.024). Specific major complications, such as pulmonary embolism, anastomosis leakage, intra-abdominal bleeding, and intra-abdominal abscess, showed numerically lower incidences in the ERAS-C group than in the routine care group, with no pulmonary embolisms or intra-abdominal bleedings reported. Minor complications, including wound infection, ileus, urinary tract infection, and atelectasis, were similar between the groups, with the routine group and the ERAS-C group experiencing 5.77% and 4.22%, respectively (χ² = 0.410, P = 0.522). These findings suggest the significant benefit of ERAS-based continuity nursing care in reducing postoperative complications, particularly major complications, in elderly pa
| Parameters | Routine group (n = 156) | ERAS-C group (n = 166) | χ² | P value |
| Overall complications | 18 (11.54) | 9 (5.42) | 3.917 | 0.048 |
| Major complications | 9 (5.77) | 2 (1.20) | 5.078 | 0.024 |
| Pulmonary embolism | 1 (0.64) | 0 (0.00) | ||
| Anastomosis leakage | 4 (2.56) | 1 (0.60) | ||
| Intra-abdominal bleeding | 2 (1.28) | 0 (0.00) | ||
| Intra-abdominal abscess | 2 (1.28) | 1 (0.60) | ||
| Minor complications | 9 (5.77) | 7 (4.22) | 0.410 | 0.522 |
| Wound infection | 2 (1.28) | 2 (1.20) | ||
| Ileus | 4 (2.56) | 3 (1.81) | ||
| Urinary tract infection | 2 (1.28) | 1 (0.60) | ||
| Atelectasis | 1 (0.64) | 1 (0.60) |
One month postoperatively, the total satisfaction score in the ERAS-C group (80.36 ± 7.24) was markedly higher than that in the routine group (75.23 ± 7.03). This finding reflects an overall enhanced nursing experience for patients under ERAS-C care (t = 6.450, P < 0.001) (Table 4). Specific aspects contributing to this increase included nurses’ interpersonal communication ability [which was rated at 9.24 ± 0.63 and 9.01 ± 0.67 in the ERAS-C group and the routine group, respectively (t = 3.301, P = 0.001)] and the nurse-patient communication level [with scores of 9.57 ± 0.74 vs 9.25 ± 0.58, respectively (t = 4.396, P < 0.001)]. Service timeliness in the ERAS-C group (9.36 ± 1.03) was also significantly better than that in the routine group (8.85 ± 1.20) (t = 4.130, P < 0.001). Furthermore, service accessibility in the ERAS-C group (17.35 ± 1.57) was rated higher than that in the routine group (16.98 ± 1.34) (t = 2.274, P = 0.024). Technical service quality and service economy showed no significant differences between groups, with comparable scores in technical service quality (t = 0.755, P = 0.451) and service economy (t = 0.738, P = 0.461). These data underline the advantages of continuity nursing care based on ERAS principles in enhancing patient satisfaction across multiple dimensions of nursing services.
| Parameters | Routine group (n = 156) | ERAS-C group (n = 166) | t value | P value |
| Technical service quality | 18.27 ± 1.44 | 18.16 ± 1.15 | 0.755 | 0.451 |
| Nurses’ interpersonal communication ability | 9.01 ± 0.67 | 9.24 ± 0.63 | 3.301 | 0.001 |
| Nurse-patient communication level | 9.25 ± 0.58 | 9.57 ± 0.74 | 4.396 | < 0.001 |
| Service economy | 8.33 ± 1.56 | 8.45 ± 1.34 | 0.738 | 0.461 |
| Service timeliness | 8.85 ± 1.20 | 9.36 ± 1.03 | 4.130 | < 0.001 |
| Service accessibility | 16.98 ± 1.34 | 17.35 ± 1.57 | 2.274 | 0.024 |
| Total | 75.23 ± 7.03 | 80.36 ± 7.24 | 6.450 | < 0.001 |
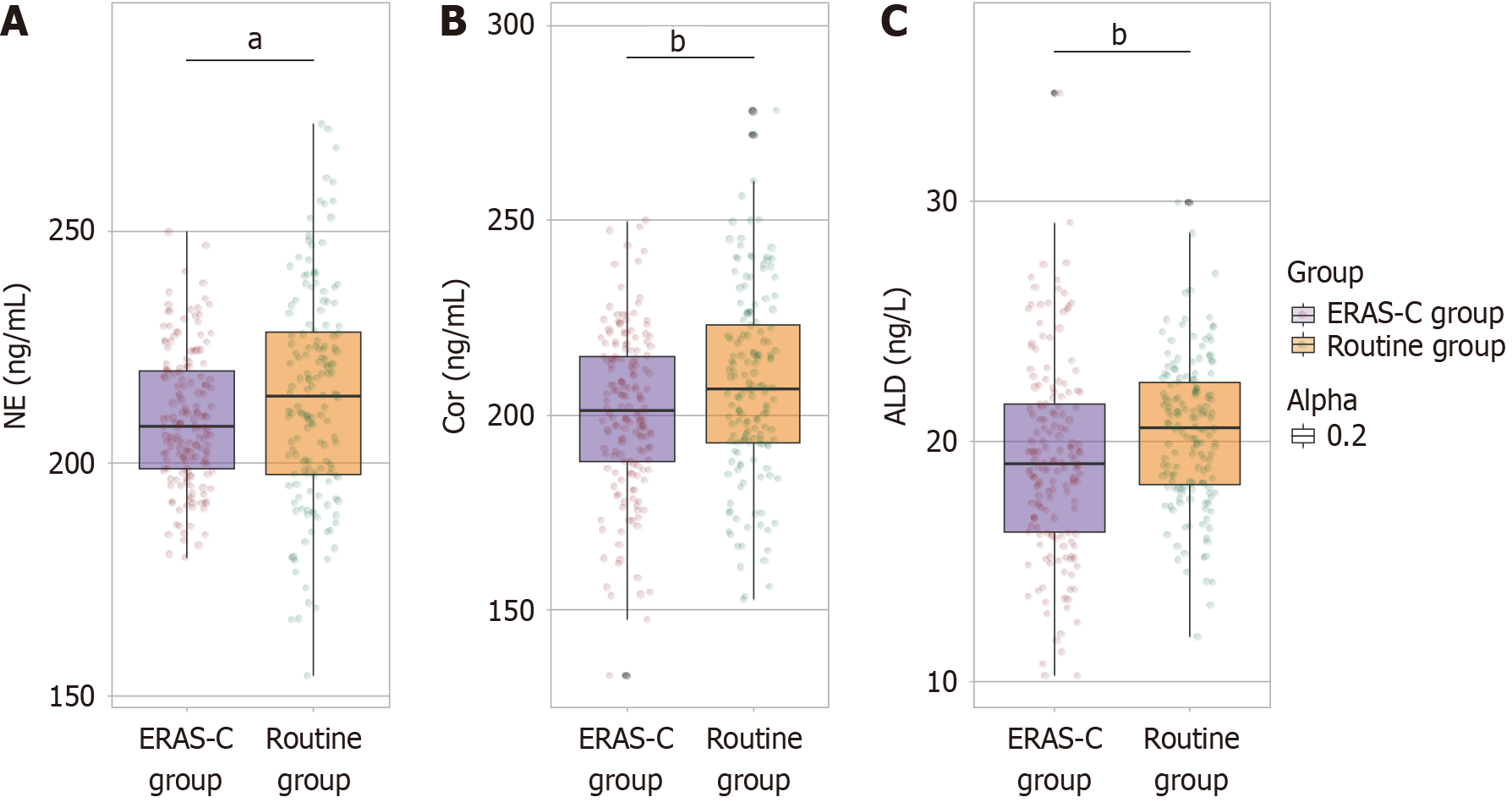
One month postoperatively, the level of NE in the ERAS-C group (209.48 ± 14.48 ng/mL) was significantly reduced than that in the routine group (214.15 ± 23.08 ng/mL), indicating a decreased stress response (t = 2.159, P = 0.032) (Figure 3). Similarly, Cor levels in the ERAS-C group (200.65 ± 20.97 ng/mL) were lower than those in the routine group (207.54 ± 24.19 ng/mL). This finding further suggested reduced stress (t = 2.734, P = 0.007). Additionally, the ALD levels in the ERAS-C group (19.22 ± 4.05 ng/L) were significantly lower than those in the routine group (20.37 ± 3.04 ng/L) (t = 2.891, P = 0.004). These findings highlight the effectiveness of continuity nursing care based on ERAS principles in mitigating postoperative stress responses in elderly patients following gastric cancer resection.
One month postoperatively, dysphagia scores in the ERAS-C group (19.18 ± 2.54) were significantly lower than those in the routine group (20.26 ± 3.77). This finding indicates that the former experienced less difficulty in swallowing than the latter (t = 2.992, P = 0.003) (Table 5). Pain levels in the ERAS-C group (24.74 ± 3.68) were also reduced relative to those in the routine group (25.77 ± 3.49) (t = 2.585, P = 0.010). Participants in the ERAS-C group reported fewer eating restrictions (19.96 ± 1.77) than those in the routine group (22.18 ± 2.85), demonstrating a substantial improvement (t = 8.335, P < 0.001). Anxiety scores, reflecting emotional well-being, in the ERAS-C group (38.67 ± 3.46) were lower than those in the routine group (40.52 ± 6.98) (t = 2.977, P = 0.003). Additionally, dry mouth severity in the ERAS-C group (28.72 ± 2.15) was reduced compared with that in the routine group (30.66 ± 5.43) (t = 4.166, P < 0.001). Body image perceptions also improved, with lower concerns reported in the ERAS-C group (38.96 ± 2.54) than those reported in the routine group (40.03 ± 4.15) (t = 2.760, P = 0.006). These findings underscore the enhanced quality of life experienced by patients receiving ERAS-based continuity nursing care postoperatively.
| Parameters | Routine group (n = 156) | ERAS-C group (n = 166) | t value | P value |
| Dysphagia | 20.26 ± 3.77 | 19.18 ± 2.54 | 2.992 | 0.003 |
| Pain | 25.77 ± 3.49 | 24.74 ± 3.68 | 2.585 | 0.010 |
| Eating restrictions | 22.18 ± 2.85 | 19.96 ± 1.77 | 8.335 | < 0.001 |
| Anxiety | 40.52 ± 6.98 | 38.67 ± 3.46 | 2.977 | 0.003 |
| Dry mouth | 30.66 ± 5.43 | 28.72 ± 2.15 | 4.166 | < 0.001 |
| Body image | 40.03 ± 4.15 | 38.96 ± 2.54 | 2.760 | 0.006 |
The study explores the impact of continuity nursing care rooted in ERAS principles on postoperative outcomes, particularly satisfaction, inflammation, immune response, stress, and quality of life in elderly gastric cancer patients.
The superior outcomes in the ERAS-C group were due to the core principles of ERAS. These principles focused on multidisciplinary and perioperative care. This comprehensive approach optimizes physiological responses to surgery, reduces stress, and curtails inflammatory cascades, thereby resulting in decreased postoperative complications, improved satisfaction, and enhanced quality of life[9,11,16].
Patient satisfaction, markedly higher in the ERAS-C group than in the routine care group, reflects enhanced communi
Postoperative inflammation was intrinsically linked to surgical stress and immune function. In the ERAS-C group, IL-6 levels were much lower, indicating less inflammation. This reduction may be due to better preoperative preparation of patients’ health and mental state. Synchronization of nutritional support, anesthetic care, and early mobilization further attenuates the inflammatory response, as evidenced by controlled CRP levels and stable serum albumin. Nutrition, a pivotal element of ERAS, improves healing capacity and immune function, thereby diminishing inflammatory markers and aiding in a smooth recovery[19,20].
The ERAS protocols’ influence on stress was underscored by the reduced NE, Cor, and ALD levels observed in the ERAS-C group. The ERAS model minimizes the hypothalamic–pituitary–adrenal axis activation, which was responsible for the physiological stress response, by alleviating anxiety through educational initiatives and psychological support. The low stress marker levels signify reduced physiological stress and correlate with improved pain management and decreased pain perception, contributing to improved psychological well-being and patient satisfaction[21-23].
Immune function, vital for postoperative recovery, also appears to be effectively preserved under the ERAS frame
The reduction in postoperative complications, particularly major ones, highlights the practical benefits of ERAS prin
The improvement in quality of life parameters, such as reduced dysphagia, pain, and anxiety, along with improved results in eating restrictions and body image, conveys the broad impact of ERAS-based care. The ERAS protocols foster a holistic healing process by collectively addressing the physical, psychological, and social dimensions of recovery. Emo
Mechanistically, ERAS protocols seem to attenuate the typical stress and inflammatory pathways activated by surgery. The strategic combination of preoperative optimization, including nutrition and mental health preparations, coupled with postoperative care such as pain management, early mobilization, and continuous education, disrupts the traditional trajectory of surgical recovery. This comprehensive care model expedites physiological recovery and mentally equips patients to engage positively with their health outcomes[34,35].
Although this study demonstrates the benefits of continuity nursing care rooted in ERAS principles, several limitations must be acknowledged. First, the study’s sample size was relatively small and drawn from a single institution. Thus, the generalizability of our findings may be limited to broad populations. To address this limitation, future research could adopt a multi-center collaborative approach, pooling data from multiple institutions to enhance the feasibility and prospective validity of the study. Such an approach would allow for a larger and more diverse patient cohort, thereby increasing the robustness of the findings. Additionally, the assessment of patient satisfaction and quality of life relied on self-reported measures, which can be subject to bias. Future studies should consider incorporating objective measures, such as clinical assessments and biomarkers, to complement self-reported data. This dual approach would provide a more comprehensive understanding of patient outcomes. The study also did not account for potential confounding factors, such as comorbidities and varying degrees of surgical complexity, which may influence postoperative outcomes. Future research should include detailed stratification of patients based on these factors to control for potential biases and ensure more accurate comparisons. Additionally, employing statistical techniques such as propensity score matching or multivariate regression analysis could help mitigate the impact of these confounders. The observational design precludes any causal inferences, thereby underscoring the need for future randomized controlled trials (RCTs) to effectively establish the efficacy of ERAS-based continuity nursing care. RCTs would enable researchers to draw stronger conclu
Moreover, the integration of ERAS principles into clinical practice faces unique challenges depending on the local context. For instance, resource constraints, varying levels of staff training, and differences in patient demographics can influence the implementation and effectiveness of ERAS protocols. By analyzing additional clinical practice cases, we can gain deeper insights into how these factors impact the outcomes of ERAS-based care. This approach not only enhances the depth and breadth of our discussion but also provides practical guidance for adapting ERAS principles to meet the specific needs of different institutions and patient groups.
The implementation of continuity nursing care based on ERAS principles in elderly gastric cancer patients undergoing surgery has demonstrated significant improvements in various postoperative measures. The ERAS protocol stands out as a superior alternative to conventional care approaches by optimizing inflammation, stress, immune response, and overall patient satisfaction and recovery. Future research should continue exploring specific components of ERAS that yield the greatest benefits to refine and enhance these protocols further. Ultimately, the integration of ERAS-based strategies holds the potential to reform surgical care paradigms, thereby promoting healthy and rapid recoveries and improved long-term outcomes for patients.
| 1. | Röcken C. Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol. 2023;149:467-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 116] [Reference Citation Analysis (5)] |
| 2. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 235] [Reference Citation Analysis (3)] |
| 3. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 594] [Reference Citation Analysis (5)] |
| 4. | Tazreean R, Nelson G, Twomey R. Early mobilization in enhanced recovery after surgery pathways: current evidence and recent advancements. J Comp Eff Res. 2022;11:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 5. | Sorabella LL, Bauchat JR. Enhanced Recovery after Surgery: Cesarean Delivery. Anesthesiol Clin. 2021;39:743-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Nelson G, Fotopoulou C, Taylor J, Glaser G, Bakkum-Gamez J, Meyer LA, Stone R, Mena G, Elias KM, Altman AD, Bisch SP, Ramirez PT, Dowdy SC. Enhanced recovery after surgery (ERAS®) society guidelines for gynecologic oncology: Addressing implementation challenges - 2023 update. Gynecol Oncol. 2023;173:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
| 7. | Stenberg E, Dos Reis Falcão LF, O'Kane M, Liem R, Pournaras DJ, Salminen P, Urman RD, Wadhwa A, Gustafsson UO, Thorell A. Correction to: Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J Surg. 2022;46:752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Joliat GR, Kobayashi K, Hasegawa K, Thomson JE, Padbury R, Scott M, Brustia R, Scatton O, Tran Cao HS, Vauthey JN, Dincler S, Clavien PA, Wigmore SJ, Demartines N, Melloul E. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations 2022. World J Surg. 2023;47:11-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 9. | Debono B, Wainwright TW, Wang MY, Sigmundsson FG, Yang MMH, Smid-Nanninga H, Bonnal A, Le Huec JC, Fawcett WJ, Ljungqvist O, Lonjon G, de Boer HD. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Spine J. 2021;21:729-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 10. | Scott MJ, Aggarwal G, Aitken RJ, Anderson ID, Balfour A, Foss NB, Cooper Z, Dhesi JK, French WB, Grant MC, Hammarqvist F, Hare SP, Havens JM, Holena DN, Hübner M, Johnston C, Kim JS, Lees NP, Ljungqvist O, Lobo DN, Mohseni S, Ordoñez CA, Quiney N, Sharoky C, Urman RD, Wick E, Wu CL, Young-Fadok T, Peden CJ. Consensus Guidelines for Perioperative Care for Emergency Laparotomy Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations Part 2-Emergency Laparotomy: Intra- and Postoperative Care. World J Surg. 2023;47:1850-1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 11. | Naftalovich R, Singal A, Iskander AJ. Enhanced Recovery After Surgery (ERAS) protocols for spine surgery - review of literature. Anaesthesiol Intensive Ther. 2022;54:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol. 2017;24:3683-3691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 13. | Wang J, Hu H, Sun J, Zhang Q, Chen Z, Wang Q, Zhu M, Yao J, Yuan H, Zhang X. The effectiveness of health education based on the 5Ts for teach-back on oral nutritional supplements compliance of post-discharge patients after surgery for gastric cancer: a randomized controlled trial. Support Care Cancer. 2023;31:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 14. | Blazeby JM, Currie E, Zee BC, Chie WC, Poon RT, Garden OJ; EORTC Quality of Life Group. Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer. 2004;40:2439-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S, Kvikstad A. The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer. 1995;31A:2260-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 303] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Bogani G, Sarpietro G, Ferrandina G, Gallotta V, DI Donato V, Ditto A, Pinelli C, Casarin J, Ghezzi F, Scambia G, Raspagliesi F. Enhanced recovery after surgery (ERAS) in gynecology oncology. Eur J Surg Oncol. 2021;47:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, Wellge EB, Kunzler F, Besselink MG, Asbun H, Scott MJ, Dejong CHC, Vrochides D, Aloia T, Izbicki JR, Demartines N. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) Recommendations 2019. World J Surg. 2020;44:2056-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 341] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 18. | Kitchin S, Raman VT, Javens T, Jatana KR. Enhanced Recovery After Surgery: A Quality Improvement Approach. Otolaryngol Clin North Am. 2022;55:1271-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Peng J, Dong R, Jiao J, Liu M, Zhang X, Bu H, Dong P, Zhao S, Xing N, Feng S, Yang X, Kong B. Enhanced Recovery After Surgery Impact on the Systemic Inflammatory Response of Patients Following Gynecological Oncology Surgery: A Prospective Randomized Study. Cancer Manag Res. 2021;13:4383-4392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 20. | Gumusoglu AY, Kabuli HA, Cikot M, Isıksacan N, Kasapoglu P, Cayırcı EC, Binboga S, Karabulut M, Alıs H. The importance of inflammatory markers in detection of complications in patients with gastric cancer undergoing the Enhanced Recovery After Surgery (ERAS) protocol: a prospective cohort study. Wideochir Inne Tech Maloinwazyjne. 2022;17:688-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Zhao H, Sun W. Effect of Enhanced Recovery after Surgery with Integrated Traditional Chinese and Western Medicine on Postoperative Stress Response of Patients with Gastrointestinal Tumors. Comput Math Methods Med. 2022;2022:3663246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Cheng W, Jiang ZW. [Digitalization of perioperative traumatic stress in enhanced recovery after surgery program: current application and future prospect]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Chen JX, Lin R, Fan X, Zong MH, Feng L, Wang Y. [Effect of enhanced recovery after surgery on surgical stress response in patients with gastric cancer complicated with type 2 diabetes mellitus]. Zhonghua Yi Xue Za Zhi. 2022;102:847-852. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Zhang W, Cong X, Zhang L, Sun M, Li B, Geng H, Gu J, Zhang J. Effects of thoracic nerve block on perioperative lung injury, immune function, and recovery after thoracic surgery. Clin Transl Med. 2020;10:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Ding H, Xu J, You J, Qin H, Ma H. Effects of enteral nutrition support combined with enhanced recovery after surgery on the nutritional status, immune function, and prognosis of patients with esophageal cancer after Ivor-Lewis operation. J Thorac Dis. 2020;12:7337-7345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Albers KI, Polat F, Helder L, Panhuizen IF, Snoeck MMJ, Polle SBW, de Vries H, Dias EM, Slooter GD, de Boer HD, Diaz-Cambronero O, Mazzinari G, Scheffer GJ, Keijzer C, Warlé MC; RECOVER Study Collaborators. Quality of Recovery and Innate Immune Homeostasis in Patients Undergoing Low-pressure Versus Standard-pressure Pneumoperitoneum During Laparoscopic Colorectal Surgery (RECOVER): A Randomized Controlled Trial. Ann Surg. 2022;276:e664-e673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 27. | Wang YL, Zhang FB, Zheng LE, Yang WW, Ke LL. Enhanced recovery after surgery care to reduce surgical site wound infection and postoperative complications for patients undergoing liver surgery. Int Wound J. 2023;20:3540-3549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | M Íximo MA, Santos D, F Lix-Oliveira A, Pereira M, Carmona C. Association between enhanced recovery after surgery protocol compliance and clinical complications: a cohort study. Braz J Anesthesiol. 2023;73:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 29. | Lannes F, Walz J, Maubon T, Rybikowski S, Fakhfakh S, Picini M, Tourret M, Brun C, Gravis G, Pignot G. Enhanced Recovery after Surgery for Radical Cystectomy Decreases Postoperative Complications at Different Times. Urol Int. 2022;106:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Somoza-Fernández B, Ribed-Sánchez A, Martín-Lozano S, de Vega-San Vicente FM, Menéndez-Tarín R, Giménez-Manzorro Á, Sanz-Ruiz P, Garutti-Martínez I, Herranz-Alonso A, Vaquero-Martín J, Sanjurjo-Sáez M. Implementation of an enhanced recovery after surgery program including a patient school: Impact on quality of life results. Injury. 2022;53:3987-3992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Molenaar CJ, van Rooijen SJ, Fokkenrood HJ, Roumen RM, Janssen L, Slooter GD. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database Syst Rev. 2022;5:CD013259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 32. | Miao C, Yu A, Yuan H, Gu M, Wang Z. Effect of Enhanced Recovery After Surgery on Postoperative Recovery and Quality of Life in Patients Undergoing Laparoscopic Partial Nephrectomy. Front Oncol. 2020;10:513874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Hao J. Enhanced rehabilitation intervention improves postoperative recovery and quality of life of patients after heart valve replacement surgery. Am J Transl Res. 2022;14:5132-5138. [PubMed] |
| 34. | Peden CJ, Aggarwal G, Aitken RJ, Anderson ID, Bang Foss N, Cooper Z, Dhesi JK, French WB, Grant MC, Hammarqvist F, Hare SP, Havens JM, Holena DN, Hübner M, Kim JS, Lees NP, Ljungqvist O, Lobo DN, Mohseni S, Ordoñez CA, Quiney N, Urman RD, Wick E, Wu CL, Young-Fadok T, Scott M. Guidelines for Perioperative Care for Emergency Laparotomy Enhanced Recovery After Surgery (ERAS) Society Recommendations: Part 1-Preoperative: Diagnosis, Rapid Assessment and Optimization. World J Surg. 2021;45:1272-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 35. | Jiao S, Feng Z, Huang J, Dai T, Liu R, Meng Q. Enhanced recovery after surgery combined with quantitative rehabilitation training in early rehabilitation after total knee replacement: a randomized controlled trial. Eur J Phys Rehabil Med. 2024;60:74-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/