Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.102335
Revised: January 31, 2025
Accepted: March 13, 2025
Published online: May 27, 2025
Processing time: 220 Days and 4.3 Hours
Post-hepatectomy liver failure (PHLF), represents a serious complication after liver resection, significantly impacting the long-term outcomes for patients who undergo such surgeries. There exists a strong correlation between intraoperative hemorrhage and transfusion requirements with the development of PHLF. Presently, a combination of hepatic portal occlusion techniques alongside con
To develop and validate a nomogram that predicts the risk factors associated with the development of PHLF patients undergoing liver resection with CLCVP.
We conducted a retrospective analysis of 285 patients who underwent hepatectomy for the first time and had no history of prior non-index abdominal surgeries, with hepatic inflow occlusion combined with CLCVP from January to December 2019 in Hunan Provincial People’s Hospital. Univariate and multivariate regression analyses were used to identify preoperative and intraoperative risk factors for PHLF. Eligible patients were randomly divided into training and validation groups in a 7:3 ratio, and a nomogram prediction model was constructed.
The incidence of PHLF in these patients was 22.46%. Multiple logistic analysis showed that preoperative serum albumin level, causes of liver resection (cancer or others), and cirrhosis were independent preoperative risk factors for PHLF (P < 0.05) and that only post-blocking blood potassium concentration was an independent intraoperative risk factor for PHLF (P < 0.05). Least absolute shrinkage and selection operator regression analysis revealed that preoperative serum albumin level, direct bilirubin level (DBIL), platelet count, causes of liver resection (cancer or others), and cirrhosis were significant predictors of PHLF. The nomogram risk prediction model based on preoperative serum albumin level, DBIL, platelet count, causes of liver resection (cancer or others), cirrhosis and post-blocking blood potassium concentration can better predict the occurrence of PHLF.
For patients undergoing liver resection with CLCVP, serum albumin level, DBIL, platelet count, causes of liver resection (cancer or others), and cirrhosis are independent preoperative risk factors for PHLF.
Core Tip: This is the first study to establish and validate a model for predicting the risk of post-hepatectomy liver failure (PHLF) in patients undergoing hepatectomy with controlled low central venous pressure. In this work, we determined that serum albumin level, direct bilirubin level, platelet count, causes of liver resection (cancer or others), and cirrhosis were the main preoperative risk factors associated with PHLF in patients undergoing hepatectomy with controlled low central venous pressure. There is no evidence showing that intraoperative variables other than post-blocking blood potassium concentration may affect PHLF in these patients. The predictive model established in this study holds significant potential for enhancing the identification and risk stratification of patients, thereby aiding in postoperative management and improving clinical outcomes.
- Citation: Tang L, Chen LX, Luo CC, Zhao Y. Predicting risk of post-hepatectomy liver failure in patients undergoing liver resection with controlled low central venous pressure. World J Gastrointest Surg 2025; 17(5): 102335
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/102335.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.102335
Partial hepatectomy is the main treatment for a variety of liver and biliary diseases, but it also causes many complications, such as infection, biliary leakage, and gastrointestinal bleeding. The most important complication is post-hepatectomy liver failure (PHLF). It is a serious postoperative complication and is considered to be a marker of poor liver regeneration[1]. It is also one of the main causes of death after partial hepatectomy[2]. The incidence of PHLF ranges from 1.2% to 32%, and the mortality rate is approximately 1.6% to 2.8%[3-6].
Due to the liver’s rich blood supply, complex vascular distribution, numerous sinusoids, and high tissue fragility, it could become very difficult to control intraoperative bleeding, once the damage to hepatic vessels happens, especially the injuries of the hepatic and inferior vena cava. PHLF directly affecting patient outcomes, is related to both the amount of intraoperative bleeding and blood transfusions[7,8]. To reduce intraoperative bleeding, techniques such as hepatic portal occlusion combined with controlled low central venous pressure (CLCVP) technique were increasingly used in hepatectomy[8-10]. However, their effect on the incidence of PHLF is still controversial. Some studies point out that CLCVP can effectively reduce the amount of blood loss and blood transfusion during liver resection, and shorten the operation time[10,11]. There are many studies focused on the risk factors for PHLF, but fewer studies on the risk factors for PHLF under CLCVP. Therefore, based on the application of CLCVP in hepatectomy patients, we explored the preoperative and intraoperative risk factors for PHLF, and developed a nomogram in order to predict the incidence of PHLF. This model is good to figure out the possible effect of CLCVP on PHLF.
A total of 1000 patients underwent liver resection for the first time from January to December 2019 in Hunan Provincial People’s Hospital. All patients underwent partial hepatectomy alone with intraoperative CLCVP [measured central venous pressure (CVP) ≤ 5 cm H2O].
The inclusion criteria were: (1) Patients undergoing their first laparoscopic or open liver resection; (2) Age between 18 and 65 years; and (3) Preoperative assessment indicating suitability for surgery. The exclusion criteria included: (1) Emergency surgery; (2) Preoperative presence of significant organ dysfunction such as heart, kidney, or liver failure (Child-Pugh > C), untreated obstructive jaundice, or liver function not meeting surgical standards by the time of surgery; (3) History of major surgery or cancer; (4) Previous liver ablation or embolization; (5) History of radiotherapy or chemotherapy; (6) Combined non-liver surgery; and (7) Incomplete clinical data. According to the diagnostic criteria proposed by the International Liver Surgery Study Group in 2011, PHLF was defined as an increase in the international normalized ratio (INR) accompanied by hyperbilirubinemia on or after the fifth postoperative day[3].
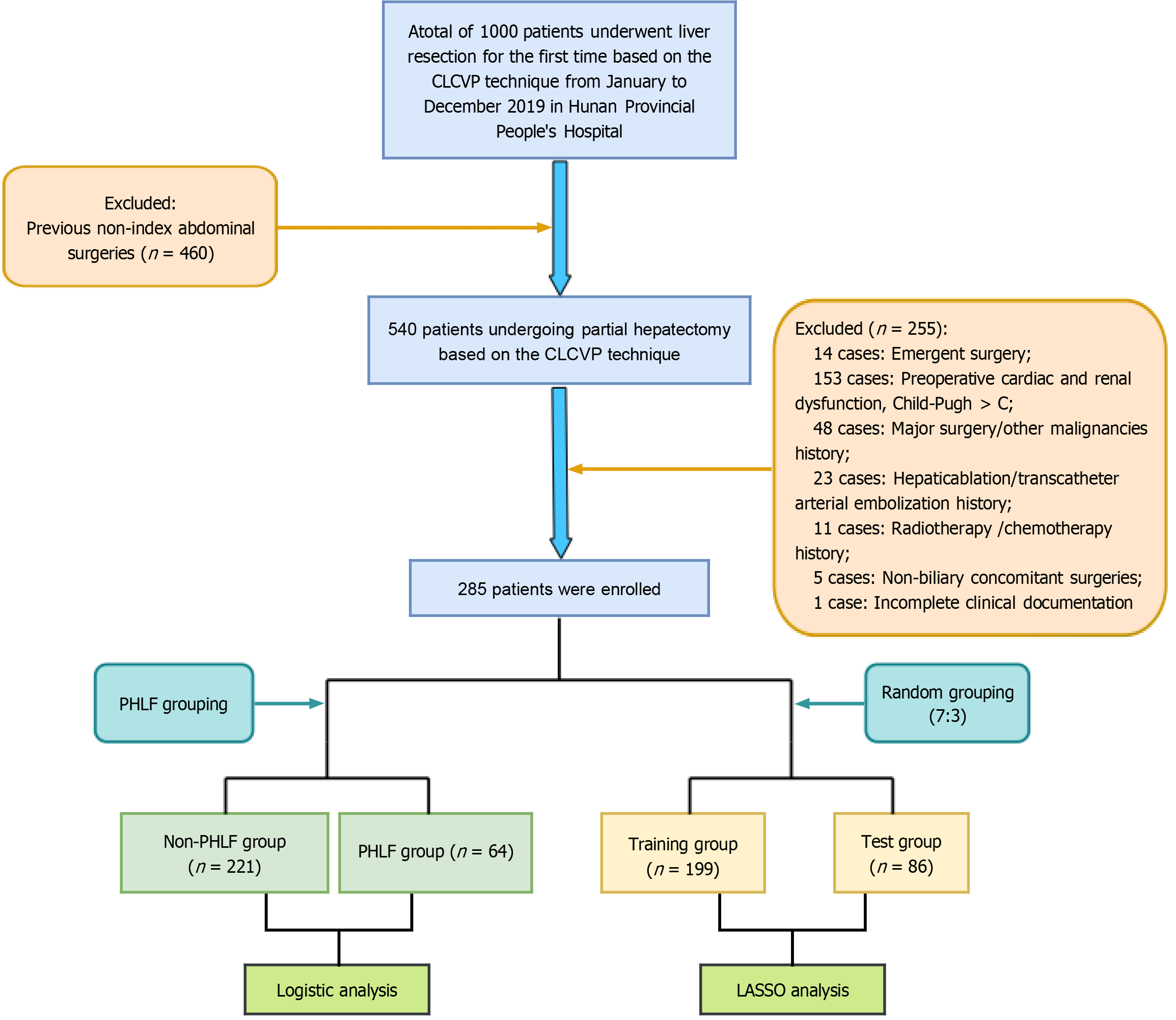
The flowchart of the study is shown in Figure 1. Of the 1000 patients initially screened, 460 were excluded due to a history of prior abdominal surgeries, thus leaving 540 patients undergoing partial hepatectomy based on the CLCVP technique. Then 255 patients were excluded due to emergent surgery (n = 14), preoperative cardiac, renal, or liver failure (Child-Pugh > C) (n = 153), history of major surgery or other malignancies (n = 48), history of hepatic ablation or transcatheter arterial embolization (n = 23), radiotherapy or chemotherapy history (n = 11), non-biliary concomitant surgeries (n = 5), or incomplete clinical documentation (n = 1). Finally, 285 patients were enrolled. This study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee of Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University) (IRB approval No. [2024]-273).
Preoperative risk factors: The following potential preoperative risk factors were evaluated: (1) General information: Age, height, weight, gender, history of hepatitis, liver cirrhosis, past medical history, etc.; (2) Lab Indicators: The last preoperative liver function, kidney function, heart function, complete blood count, blood lipids, myocardial enzymes, etc.; and (3) The results of re-examinations on or after the 5th postoperative day (if not checked on the 5th day, the 4th day’s results were taken; if the patient was discharged within 4 days postoperatively, the last re-examination results were taken);
Intraoperative risk factors: The following anesthesia and surgery-related variables were evaluated: Reason for liver resection, extent of resection, duration of surgery, extubation time, blood gas analysis before and after liver resection, blood loss, fluid intake, etc.
Outcome measure: PHLF was the outcome measure of this study, which was defined as total bilirubin (TBIL) and INR levels on or after the 5th postoperative day higher than preoperative levels or TBIL > 50 mmol/L (if the patient was discharged within 4 days postoperatively, liver function and coagulation function on the 4th day were taken; if the patient was discharged within 4 days postoperatively and no liver function or coagulation function data was available on or after the 4th day, the presence of postoperative liver failure was determined based on follow-up and outpatient reviews).
All statistical analyses were performed with SPSS software (version 25.0), the statistical software packages R (http://www.R-project.org, The R Foundation), and Free Statistics software versions 2.0. Normally distributed quantitative data are expressed as the mean ± SD, and comparisons between the two groups were performed using independent samples t-tests. Non-normally distributed quantitative data are expressed as median and interquartile range, and comparisons were performed using the Mann-Whitney U test. Qualitative data are displayed as n (%), and comparisons were made using the χ2 test or Fisher’s exact test. Variables with P < 0.05 from (1); (2), and (3) were subjected to univariate logistic regression analysis, and variables significantly associated with the incidence of PHLF (P < 0.05) in the univariate logistic regression analysis were included in the multivariate regression analysis. The least absolute shrinkage and selection operator (LASSO) regression analysis was employed to identify predictor variables and construct a nomogram-based risk prediction model for PHLF. All statistical tests were two-sided, and a P-value of less than 0.05 was considered statistically significant.
There were 1000 patients who underwent their first hepatectomy with hepatic inflow occlusion combined with CLCVP at Hunan Provincial People’s Hospital between January and December 2019. Of these 1000 patients, 715 met the exclusion criteria and 285 were ultimately included in the analysis, as shown in Figure 1. The incidence of PHLF was 22.4% (64/285). A total of 141 (49.5) patients were male, and the median (interquartile range) age of patients was 54.0 (48.0-61.0) years (Table 1).
| Variable | Total (n = 285) | Non-PHLF (n = 221) | PHLF (n = 64) | P value |
| Preoperative risk factors | ||||
| Gender | - | - | - | 0.008 |
| Male | 141 (49.5) | 100 (45.2) | 41 (64.1) | - |
| Female | 144 (50.5) | 121 (54.8) | 23 (35.9) | - |
| Age (years) | 54.0 (48.0-61.0) | 54.0 (47.0-61.0) | 55.0 (49.0-62.0) | 0.264 |
| BMI (kg/m2), mean ± SD | 22.5 ± 2.8 | 22.3 ± 2.8 | 22.9 ± 3.0 | 0.197 |
| LOS (d), mean ± SD | 18.1 ± 7.5 | 16.6 ± 5.9 | 23.2 ± 9.8 | < 0.001 |
| History of hepatitis | - | - | - | 0.046 |
| No | 198 (69.5) | 160 (72.4) | 38 (59.4) | - |
| Yes | 87 (30.5) | 61 (27.6) | 26 (40.6) | - |
| Cirrhosis | - | - | - | < 0.001 |
| No | 203 (71.2) | 174 (78.7) | 29 (45.3) | - |
| Yes | 82 (28.8) | 47 (21.3) | 35 (54.7) | - |
| Jaundice | - | - | - | 0.001 |
| No | 254 (89.1) | 204 (92.3) | 50 (78.1) | - |
| Yes | 31 (10.9) | 17 (7.7) | 14 (21.9) | |
| Ascites | - | - | - | 0.263 |
| No | 252 (89.7) | 197 (90.8) | 55 (85.9) | - |
| Yes | 29 (10.3) | 20 (9.2) | 9 (14.1) | - |
| Child-Pugh score | - | - | - | < 0.001 |
| 5 | 211 (74.0) | 174 (78.7) | 37 (57.8) | - |
| 6 | 45 (15.8) | 34 (15.4) | 11 (17.2) | - |
| 7 | 21 (7.4) | 9 (4.1) | 12 (18.8) | - |
| 8 | 6 (2.1) | 3 (1.4) | 3 (4.7) | - |
| 9 | 2 (0.7) | 1 (0.5) | 1 (1.6) | - |
| Cause of liver resection | - | - | - | < 0.001 |
| Tumor | 122 (42.8) | 81 (36.7) | 41 (64.1) | - |
| Other | 163(57.2) | 140 (63.3) | 23(35.9) | |
| TC (mmol/L) | 4.4 ± 1.1 | 4.4 ± 1.0 | 4.2 ± 1.4 | 0.299 |
| LDL-C (mmol/L) | 2.6 (2.1-3.2) | 2.6 (2.1-3.2) | 2.4 (2.1-3.0) | 0.206 |
| TG (mmol/L) | 1.2 (0.8-1.8) | 1.2 (0.8-1.8) | 1.0 (0.8-1.6) | 0.515 |
| Neutrophils (109/L) | 3.4 (2.6-4.6) | 3.5 (2.6-4.7) | 3.2 (2.5- 4.4) | 0.332 |
| Lymphocytes (109/L), mean ± SD | 1.6 ± 0.6 | 1.7 ± 0.6 | 1.4 ± 0.5 | 0.004 |
| Leukocytes (109/L) | 5.7 (4.6-7.1) | 5.8 (4.7-7.2) | 5.3 (4.3-6.7) | 0.059 |
| Platelets (109/L) | 200.5 (149.0-264.0) | 213.0 (163.0-264.0) | 153.0 (108.0-249.5) | < 0.001 |
| Hemoglobin (g/L) | 128.4 ± 18.4 | 128.7 ± 16.4 | 127.3 ± 24.1 | 0.596 |
| Albumin (g/L) | 40.6 ± 5.1 | 41.2 ± 4.9 | 38.4 ± 5.1 | < 0.001 |
| TBIL (μmol/L) | 14.9 (11.4-20.8) | 14.5 (11.0-20.2) | 17.0 (12.4-29.7) | 0.012 |
| DBIL (μmol/L) | 5.0 (3.7-7.7) | 4.8 (3.6-7.2) | 5.5 (4.2-12.3) | 0.005 |
| ALT (U/L) | 31.4 (19.0-56.4) | 28.8 (18.0-50.7) | 46.3 (28.1-66.5) | 0.001 |
| AST (U/L) | 28.4 (21.8-45.2) | 26.2 (20.8-39.2) | 38.8 (28.7-52.6) | < 0.001 |
| Intraoperative risk factors | ||||
| Operation mode | - | - | - | 0.611 |
| Laparoscopic | 153 (53.7) | 122 (55.2) | 31 (48.4) | - |
| Open | 119 (41.8) | 89 (40.3) | 30 (46.9) | - |
| Conversion from laparoscopic to open | 13 (4.6) | 10 (4.5) | 3 (4.7) | - |
| Extensive resection | - | - | - | 0.09 |
| No | 160 (56.1) | 130 (58.8) | 30 (46.9) | - |
| Yes | 125 (43.9) | 91 (41.2) | 34 (53.1) | - |
| Operation time (min) | 327.7 ± 154.9 | 306.2 ± 134.4 | 401.8 ± 194.4 | < 0.001 |
| Liver blockage time (min) | 41.0 (22.0-72.0) | 38.0 (21.0-63.0) | 58.0 (24.0-95.0) | 0.003 |
| Number of blocks | 3.0 (1.0-4.0) | 2.0 (1.0-4.0) | 4.0 (2.0-6.0) | 0.002 |
| Total amount volume (mL), mean ± SD | 2684.2 ± 1155.3 | 2490.7 ± 901.6 | 3352.6 ± 1610.2 | < 0.001 |
| Blood loss volume (mL) | 200.0 (100.0-400.0) | 200.0 (100.0-300.0) | 300.0 (200.0-800.0) | < 0.001 |
| Infusion of blood products | - | - | - | 0.001 |
| No | 254 (89.1) | 204 (92.3) | 50 (78.1) | - |
| Yes | 31 (10.9) | 17 (7.7) | 14 (21.9) | - |
| Urine volume (mL) | 500.0 (300.0-950.0) | 500.0 (300.0-800.0) | 700.0 (362.5-1137.5) | 0.001 |
| Infusion of albumin | - | - | - | 0.523 |
| No | 71 (24.9) | 57 (25.8) | 14 (21.9) | - |
| Yes | 214 (75.1) | 164 (74.2) | 50 (78.1) | - |
| Pre-blocking PCO2 (mmHg) | 42.0 (37.0-48.0) | 43.0 (37.0-48.0) | 42.0 (37.0-49.0) | 0.91 |
| Post-blocking PCO2 (mmHg) | 43.0 (36.0-49.0) | 43.5 (36.0-48.2) | 43.0 (37.0-50.0) | 0.535 |
| Pre-blocking potassium (mmol/L) | 3.7 (3.4-4.0) | 3.6 (3.4-3.9) | 3.7 (3.5-4.0) | 0.15 |
| Post-blocking potassium (mmol/L) | 4.0 ± 0.6 | 3.9 ± 0.6 | 4.3 ± 0.6 | < 0.001 |
| Pre-blocking glucose (mmol/L) | 5.8 (5.2-6.8) | 5.8 (5.2-6.7) | 6.2 (5.4-7.3) | 0.25 |
| Post-blocking glucose (mmol/L) | 9.2 (7.0-11.1) | 8.9 (7.0-11.0) | 9.3 (7.0-11.0) | 0.768 |
| Pre-blocking lactic acid (mmol/L) | 0.8 (0.6-1.2) | 0.8 (0.6-1.2) | 1.0 (0.7-1.4) | 0.019 |
| Post-blocking lactic acid (mmol/L) | 1.6 (1.1-2.3) | 1.4 (1.0-2.0) | 2.0 (1.3-3.0) | < 0.001 |
We performed logistic regression analysis of the included variables. The univariate regression analysis revealed that among the potential preoperative risk factors, the following 12 variables were significantly associated with PHLF (P < 0.05): Preoperative serum albumin level (odds ratio [OR] = 0.89, 95 confidence interval [CI]: 0.84-0.95, P < 0.001), lymphocyte count (OR = 0.45, 95%CI: 0.26-0.78, P = 0.004), platelet count (OR = 0.99, 95%CI: 0.99-1, P = 0.001), prothrombin time (OR = 1.35, 95%CI: 1.09-1.67, P = 0.006), prothrombin activity level (OR = 0.99, 95%CI: 0.98-1, P = 0.043), TBIL (OR = 1.02, 95%CI: 1.01-1.03, P = 0.001), direct bilirubin (DBIL) (OR = 1.03, 95%CI: 1.01-1.05, P = 0.002), gender (OR = 2.16, 95%CI: 1.21-3.83, P = 0.009) history of viral hepatitis (OR = 1.79, 95%CI: 1.01-3.2, P = 0.048), jaundice (OR = 3.36, 95%CI: 1.55-7.27, P = 0.002), causes of liver resection (cancer or others) (OR = 0.32, 95%CI: 0.18-0.58, P < 0.001), and cirrhosis (OR = 4.47, 95%CI: 2.48-8.05, P < 0.001). Among the potential intraoperative risk factors, urinary volume (OR = 1.001, 95%CI: 1-1.001, P < 0.001), blood loss volume (OR = 1.002, 95%CI: 1.001-1.002, P < 0.001), infusion fluid volume (OR = 1.001, 95%CI: 1-1.001, P < 0.001), blood transfusion (OR = 3.36, 95%CI: 1.553-7.272, P = 0.002), operation time (OR = 1.004, 95%CI: 1.002-1.006, P < 0.001), hepatic portal occlusion time (OR = 1.014, 95%CI: 1.007-1.021, P < 0.001), number of blocks (OR = 1.255, 95%CI: 1.12-1.406, P < 0.001), post-blocking blood potassium concentration (OR = 2.222, 95%CI: 1.362-3.627, P = 0.001), and post-blocking blood lactate concentration (OR = 1.491, 95%CI: 1.164-1.908, P = 0.002) were significantly associated with PHLF (P < 0.05) (Table 2).
| Variable | Univariate logistic regression analysis | Multivariate logistic regression analysis | ||
| OR, 95%CI | P value | OR, 95%CI | P value | |
| Preoperative serum albumin level | 0.89 (0.84-0.95) | < 0.001 | 0.93 (0.87-1) | 0.036 |
| Lymphocytes (109/L) | 0.45 (0.26-0.78) | 0.004 | 0.81 (0.41-1.61) | 0.55 |
| PLT (109/L) | 0.99 (0.99-1) | 0.001 | 1 (0.99-1) | 0.212 |
| Prothrombin time (s) | 1.35 (1.09-1.67) | 0.006 | 1.19 (0.8-1.77) | 0.389 |
| Prothrombin activity level (%) | 0.99 (0.98-1) | 0.043 | 1 (0.98-1.02) | 0.822 |
| TBIL (μmol/L) | 1.02 (1.01-1.03) | 0.001 | 1 (0.96-1.05) | 0.917 |
| DBIL (μmol/L) | 1.03 (1.01-1.05) | 0.002 | 1.01 (0.95-1.09) | 0.697 |
| Causes of liver resection, (cancer or others) | 0.32 (0.18-0.58) | < 0.001 | 0.39 (0.16-0.94) | 0.037 |
| Gender | 2.16 (1.21-3.83) | 0.009 | 1.55 (0.75-3.19) | 0.238 |
| History of viral hepatitis | 1.79 (1.01-3.2) | 0.048 | 0.72 (0.29-1.82) | 0.492 |
| Cirrhosis | 4.47 (2.48-8.05) | < 0.001 | 3.1 (1.56-6.17) | 0.001 |
| Jaundice | 3.36 (1.55-7.27) | 0.002 | 0.98 (0.28-3.42) | 0.972 |
The multiple logistic analysis showed that serum albumin level (OR = 0.93, 95%CI: 0.87-1, P = 0.036), causes of liver resection (cancer or others) (OR = 0.39, 95%CI: 0.16-0.94, P = 0.037), and cirrhosis (OR = 3.1, 95%CI: 1.56-6.17, P = 0.001) were independent preoperative risk factors for PHLF (P < 0.05) while only post-blocking blood potassium concentration (OR = 1.82, 95%CI: 1.05-3.17, P = 0.033) was an independent intraoperative risk factor for PHLF (P < 0.05) (Table 3).
| Variable | Univariate logistic regression analysis | Multivariate logistic regression analysis | ||
| OR, 95%CI | P value | OR, 95%CI | P value | |
| Urinary production (mL) | 1.001 (1-1.001) | < 0.001 | 1 (1-1) | 0.593 |
| Blood loss volume (mL) | 1.002 (1.001-1.002) | < 0.001 | 1 (1-1) | 0.567 |
| Infusion fluid volume (mL) | 1.001 (1-1.001) | < 0.001 | 1 (1-1) | 0.148 |
| Blood transfusion | 3.36 (1.553-7.272) | 0.002 | 0.94 (0.25-3.56) | 0.93 |
| Operation time (min) | 1.004 (1.002-1.006) | < 0.001 | 1 (1-1) | 0.954 |
| Hepatic portal occlusion time (min) | 1.014 (1.007-1.021) | < 0.001 | 1.01 (0.97-1.04) | 0.641 |
| Number of blocks | 1.255 (1.12-1.406) | < 0.001 | 0.93 (0.55-1.57) | 0.787 |
| Post-blocking blood potassium (mmol/L) | 2.222 (1.362-3.627) | 0.001 | 1.82 (1.05-3.17) | 0.033 |
| Post-blocking blood lactate (mmol/L) | 1.491 (1.164-1.908) | 0.002 | 1.08 (0.8-1.46) | 0.629 |
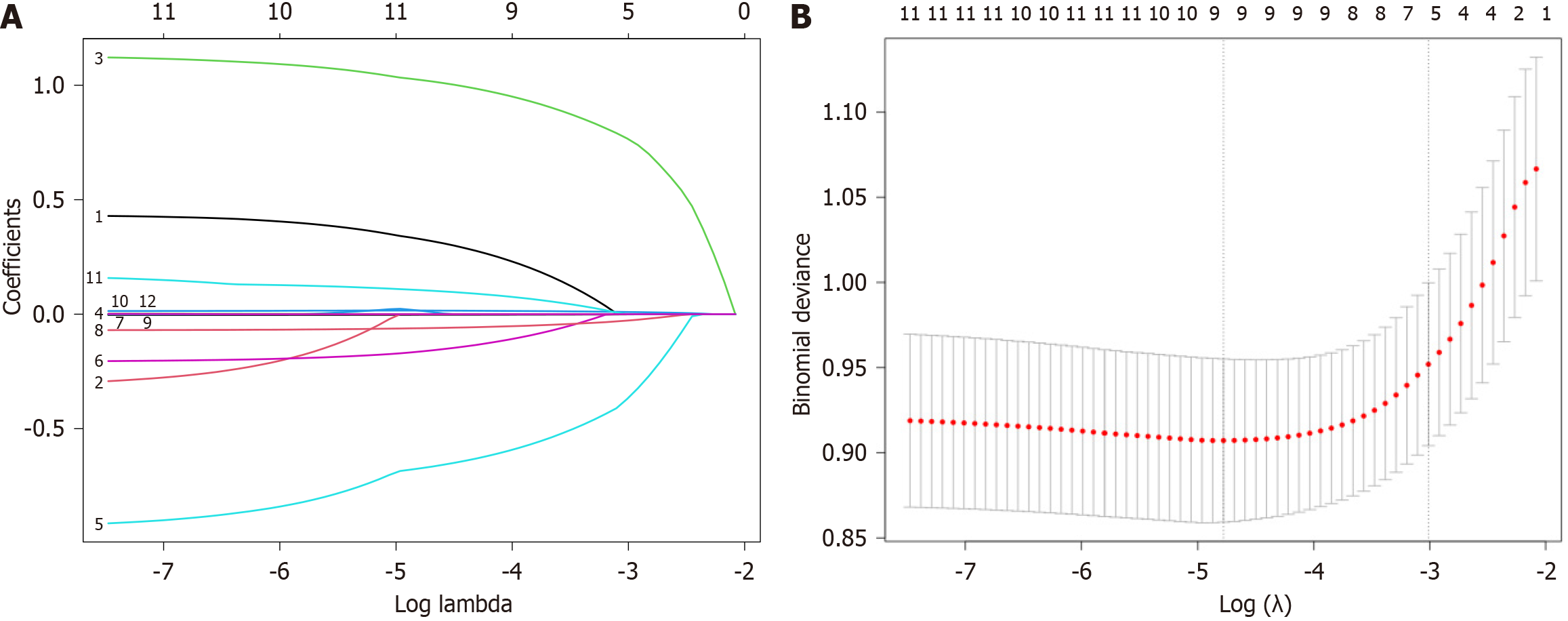
Eligible patients were randomly divided into training and validation groups in a 7:3 ratio. We used the LASSO algorithm to further refine a set of 12 preoperative variables from the univariate regression analysis (Table 4). Finally, they were reduced to five potential predictors: Preoperative serum albumin level, DBIL, platelet count, causes of liver resection (cancer or others), and cirrhosis. The LASSO coefficient trajectory path diagram and the cross-validation curve are showed in Figure 2 and Table 5.
| Variable | Total (n = 285) | Training group (n = 199) | Validation group (n = 85) | P value |
| PHLF (%) | - | - | - | 0.504 |
| No | 221 | 157 (78.9) | 64 (75.3) | - |
| Yes | 64 | 42 (21.1) | 21 (24.7) | - |
| Preoperative risk factors | ||||
| Gender | - | - | - | 0.98 |
| Male | 141 (49.5) | 101 (50.8) | 43 (50.6) | - |
| Female | 144 (50.5) | 98 (49.2) | 42 (49.4) | - |
| Age (years) | 54.0 (48.0-61.0) | 54.0 (48.0-61.0) | 55.0 (49.0-62.0) | 0.262 |
| BMI (kg/m2), mean ± SD | 22.5 ± 2.8 | 22.4 ± 2.8 | 22.6 ± 2.9 | 0.551 |
| LOS (d), mean ± SD | 18.1 ± 7.5 | 17.7 ± 7.0 | 19.0 ± 8.5 | 0.175 |
| History of hepatitis | - | - | - | 0.266 |
| No | 198 (69.5) | 142 (71.4) | 55 (64.7) | - |
| Yes | 87 (30.5) | 57 (28.6) | 30 (35.3) | - |
| Cirrhosis | - | - | - | 0.52 |
| No | 203 (71.2) | 140 (70.4) | 63 (74.1) | - |
| Yes | 82 (28.8) | 59 (29.6) | 22 (25.9) | - |
| Jaundice | - | - | - | 0.474 |
| No | 254 (89.1) | 179 (89.9) | 74 (87.1) | - |
| Yes | 31 (10.9) | 20 (10.1) | 11 (12.9) | - |
| Ascites | - | - | - | 0.572 |
| No | 252 (89.7) | 180 (90.5) | 75 (88.2) | - |
| Yes | 29 (10.3) | 19 (9.5) | 10 (11.8) | - |
| Child-Pugh score | - | - | 0.198 | |
| 5 | 211 (74.0) | 144 (72.4) | 66 (77.6) | - |
| 6 | 45 (15.8) | 35 (17.6) | 10 (11.8) | - |
| 7 | 21 (7.4) | 16 (8) | 5 (5.9) | - |
| 8 | 6 (2.1) | 4 (2) | 2 (2.4) | - |
| 9 | 2 (0.7) | 0 (0) | 2 (2.4) | - |
| Cause of liver resection | - | - | - | 0.041 |
| Tumor | 122 (42.8) | 77 (38.7) | 44 (51.8) | - |
| Other | 163(57.2) | 122 (61.3) | 41 (48.2) | - |
| TC (mmol/L), mean ± SD | 4.4 ± 1.1 | 4.3 ± 1.1 | 4.4 ± 1.1 | 0.625 |
| LDL-C (mmol/L) | 2.6 (2.1-3.2) | 2.5 (2.1-3.2) | 2.7 (2.1-3.0) | 0.816 |
| TG (mmol/L) | 1.2 (0.8-1.8) | 1.2 (0.8-1.7) | 1.1 (0.8-1.9) | 0.941 |
| Neutrophils (109/L) | 3.4 (2.6-4.6) | 3.5 (2.6-4.6) | 3.4 (2.6-4.6) | 0.698 |
| Lymphocytes (109/L) | 1.6 ± 0.6 | 1.6 ± 0.6 | 1.6 ± 0.5 | 0.447 |
| Leukocytes (109/L) | 5.7 (4.6-7.1) | 5.7 (4.7-7.2) | 5.6 (4.5-7.0) | 0.738 |
| Platelets (109/L) | 200.5 (149.0-264.0) | 199.0 (149.0-259.0) | 206.0 (156.0-279.0) | 0.587 |
| Hemoglobin (g/L) | 128.4 ± 18.4 | 127.8 ± 18.1 | 129.9 ± 19.1 | 0.382 |
| Albumin (g/L) | 40.6 ± 5.1 | 40.4 ± 5.2 | 41.0 ± 4.8 | 0.394 |
| TBIL (μmol/L) | 14.9 (11.4-20.8) | 14.8 (10.9-22.4) | 15.2 (11.9-19.8) | 0.873 |
| DBIL (μmol/L) | 5.0 (3.7-7.7) | 5.1 (3.7-7.8) | 4.8 (4.0-7.6) | 0.969 |
| ALT (U/L) | 31.4 (19.0-56.4) | 31.5 (19.6-59.0) | 30.0 (18.5-46.3) | 0.230 |
| AST (U/L) | 28.4 (21.8-45.2) | 28.9 (21.6-46.0) | 28.1 (22.0-44.6) | 0.905 |
| Intraoperative risk factors | ||||
| Operation mode | - | - | - | 0.378 |
| Laparoscopic | 153 (53.7) | 107 (53.8) | 46 (54.1) | - |
| Open | 119 (41.8) | 85 (42.7) | 33 (38.8) | - |
| Conversion from laparoscopic to open | 13 (4.6) | 7 (3.5) | 6 (7.1) | - |
| Extensive resection | - | - | - | 0.416 |
| No | 160 (56.1) | 109 (54.8) | 51 (60) | - |
| Yes | 125 (43.9) | 90 (45.2) | 34 (40) | - |
| Operation length (min), mean ± SD | 327.7 ± 154.9 | 322.1 ± 155.8 | 340.5 ± 153.7 | 0.360 |
| Liver blockage time (min) | 41.0 (22.0-72.0) | 40.0 (18.2-68.0) | 43.0 (28.0-75.0) | 0.294 |
| Number of blocks | 3.0 (1.0-4.0) | 3.0 (1.0-4.0) | 3.0 (2.0-5.0) | 0.520 |
| Total amount volume (mL), mean ± SD | 2684.2 ± 1155.3 | 2696.7 ± 1134.0 | 2647.2 ± 1214.2 | 0.742 |
| Blood loss volume (mL) | 200.0 (100.0-400.0) | 200.0 (100.0-400.0) | 200.0 (150.0-400.0) | 0.102 |
| Infusion of blood products | - | - | - | 0.344 |
| No | 254 (89.1) | 175 (87.9) | 78 (91.8) | - |
| Yes | 31 (10.9) | 24 (12.1) | 7 (8.2) | - |
| Urine volume (mL) | 500.0 (300.0-950.0) | 500.0 (300.0-912.5) | 500.0 (300.0-1000.0) | 0.995 |
| Infusion of albumin | - | - | - | 0.752 |
| No | 71 (24.9) | 48 (24.1) | 22 (25.9) | - |
| Yes | 214 (75.1) | 151 (75.9) | 63 (74.1) | - |
| Pre-blocking PCO2 (mmHg) | 42.0 (37.0-48.0) | 42.0 (37.0-49.0) | 42.0 (35.0-47.0) | 0.480 |
| Post-blocking PCO2 (mmHg) | 43.0 (36.0-49.0) | 43.0 (36.0-49.0) | 44.0 (36.0-49.5) | 0.805 |
| Pre-blocking potassium (mmol/L) | 3.7 (3.4-4.0) | 3.6 (3.3-3.9) | 3.7 (3.5-4.0) | 0.072 |
| Post-blocking potassium (mmol/L), mean ± SD | 4.0 ± 0.6 | 4.0 ± 0.6 | 4.1 ± 0.7 | 0.082 |
| Pre-blocking glucose (mmol/L) | 5.8 (5.2-6.8) | 5.8 (5.2-6.8) | 5.8 (5.2*6.8) | 0.876 |
| Post-blocking glucose (mmol/L) | 9.2 (7.0-11.1) | 8.9 (7.0-10.8) | 9.5 (7.5*11.4) | 0.181 |
| Pre-blocking lactic acid (mmol/L) | 0.8 (0.6-1.2) | 0.8 (0.6-1.2) | 0.8 (0.7-1.2) | 0.744 |
| Post-blocking lactic acid (mmol/L) | 1.6 (1.1-2.3) | 1.5 (1.0-2.2) | 1.6 (1.2-2.5) | 0.284 |
| Number | Variable, name | Coefficient | Lambda, type | Lambda, P value |
| 0 | Intercept | -0.63 | Lambda1se | 0.049 |
| 1 | Gender | - | Lambda1se | 0.049 |
| 2 | History of viral hepatitis | - | Lambda1se | 0.049 |
| 3 | Cirrhosis | 0.768 | Lambda1se | 0.049 |
| 4 | Jaundice | - | Lambda1se | 0.049 |
| 5 | Causes of liver resection (cancer or others) | -0.37 | Lambda1se | 0.049 |
| 6 | Lymphocyte count (109/L) | - | Lambda1se | 0.049 |
| 7 | PLT (109/L) | 0 | Lambda1se | 0.049 |
| 8 | Preoperative serum albumin level | -0.028 | Lambda1se | 0.049 |
| 9 | TBIL (μmol/L) | - | Lambda1se | 0.049 |
| 10 | DBIL (μmol/L) | 0.01 | Lambda1se | 0.049 |
| 11 | Prothrombin time (second) | - | Lambda1se | 0.049 |
| 12 | Prothrombin activity level (%) | - | Lambda1se | 0.049 |
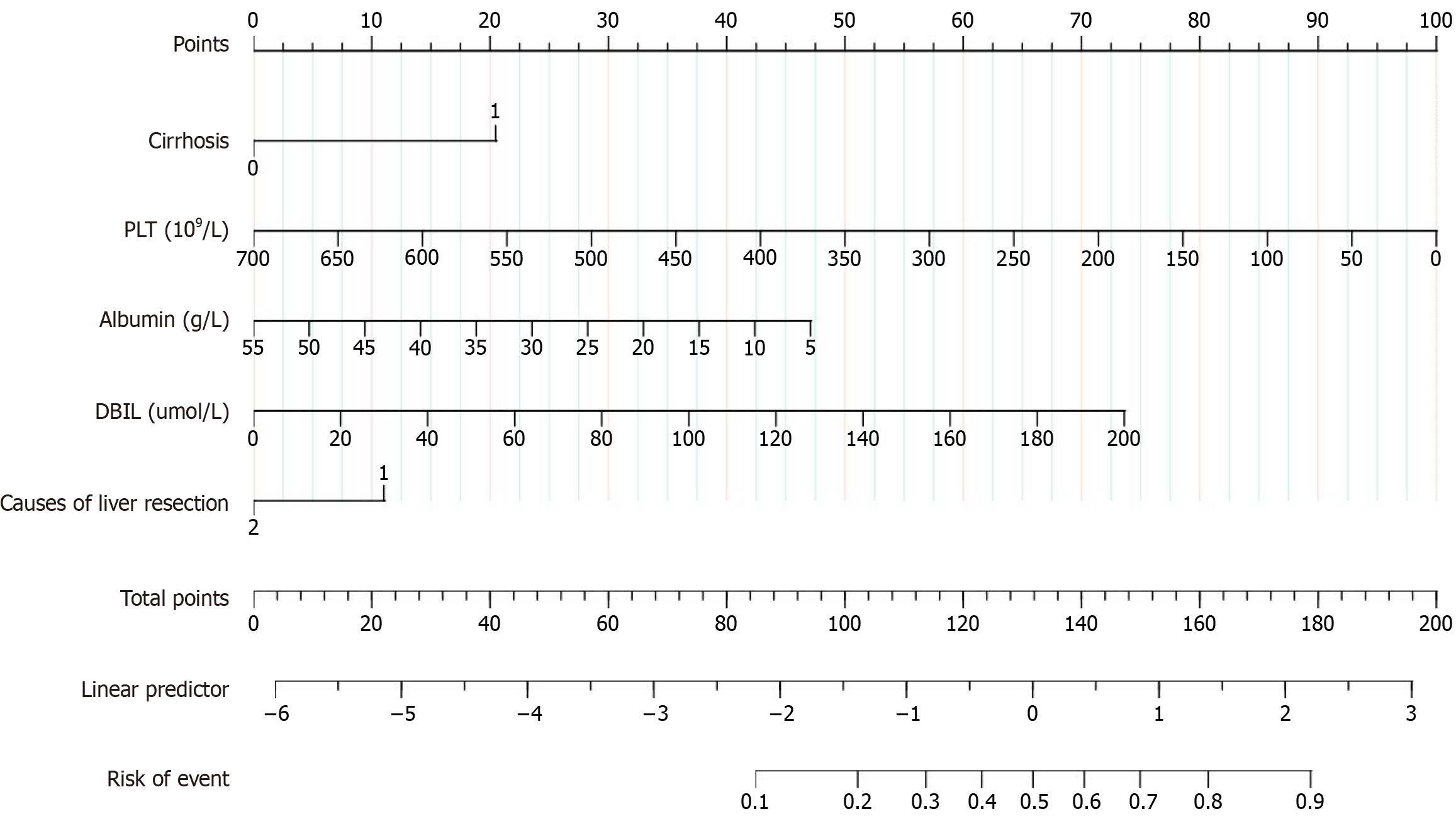
We selected the five preoperative variables including preoperative serum albumin level, DBIL, platelet count, causes of liver resection (cancer or others), and cirrhosis based on LASSO regression analysis to develop a nomogram (Figure 3). Using the nomogram, the scoring is as follows: 47.5 points for preoperative serum albumin level of 5 g/L, 50 points for platelet count of 350 × 109/L, 21 points for cirrhosis, 11 points for causes of liver resection (cancer or others), and 15 points for DBIL level of 40 μmol/L, resulting in a total score of 200, which corresponds to an event risk value of 0.9.
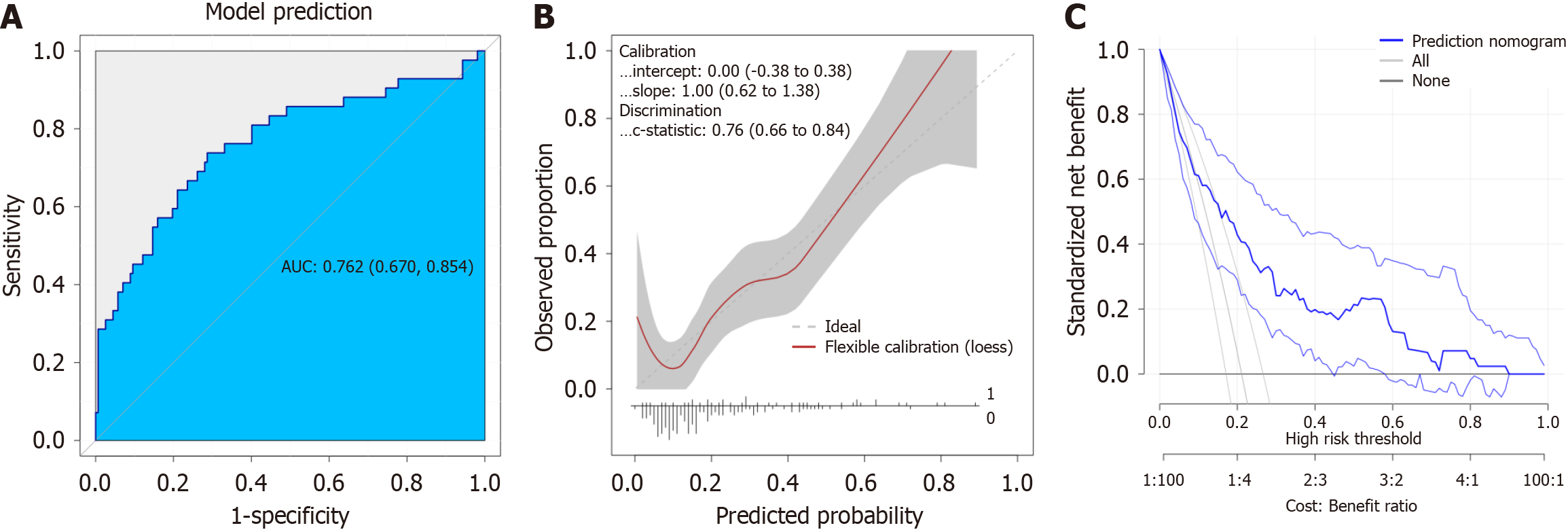
We performed a detailed sensitivity and specificity analysis of the model, with the findings visually represented in box plots (Figure 4A). Furthermore, the calibration curve demonstrated a strong alignment between the predicted and actual values for PHLF, as illustrated in Figure 4B. In addition, the decision curve analysis validated the clinical utility of the nomogram, revealing a favorable net benefit across various threshold probabilities, which underscores its practical applicability in clinical settings (Figure 4C).
The incidence of liver cancer and severe intrahepatic bile duct stones is relatively high in China. The patients with liver cancer in China account for about 45% of the world’s cases, and the incidence of intrahepatic bile duct stones accounts for about 16.1%[12], most of which require liver resection. The main postoperative risk of hepatectomy is PHLF. According to previous reports in the literature, PHLF is closely related to prognosis and is the most important cause of perioperative mortality[13]. PHLF has not yet had a uniform definition. Balzan et al[14] proposed the “50-50” standard - the prothrombin index < 50% and serum total bilirubin > 50 μmol/L. However, this standard has a high specificity but low sensitivity and did not consider other clinical indicators, limiting its clinical application. In 2011, the International Liver Surgery Research Group proposed a new definition and severity grading criteria for PHLF, which is defined as impairment of postoperative liver synthesis, secretion, and detoxification functions, mainly using total bilirubin and INR as the evaluation criteria[3]. Using the International Liver Surgery Research Group criteria, in this study, out of 285 patients undergoing liver resection, 64 developed PHLF, with an incidence of 22.4%, which is generally consistent with the incidence reported in the literature[4,5].
Hepatoportal block is an important method to reduce intraoperative bleeding and improve the success rate of liver resection, but excessive liver blockage time can cause aggravated ischemia-reperfusion injury and increase the risk of PHLF[15,16]. Due to the abundant blood supply in the liver, the portal block alone cannot prevent the bleeding of the hepatic vein, and the filled intrahepatic vein will also increase the difficulty of resecting liver parenchyma. Therefore, the combined application of CLCVP technique to maintain low CVP and collapse the vena cava and its branches, can effectively reduce wound bleeding, improve the surgical field, conducive to the free of liver parenchyma, and indirectly reduce the total blocking time. CLCVP is an anesthetic technique that uses anesthesia-related drugs and techniques to maintain the CVP at 5 cm H2O and below, while ensuring the systolic blood pressure above 90 mmHg or the average arterial pressure above 60 mmHg to reduce intraoperative bleeding. However, the effect of intraoperative application of CLCVP on the occurrence of PHLF remains unclear. Wang et al[17] found that CLCVP is not only easy to achieve in anesthesia management, but also helps to reduce blood loss during liver resection, shorten hospital stay, and have no obvious effect on liver and kidney function. A study of the best CVP in liver resection in 97 hepatocellular carcinoma patients found that the intraoperative blood loss also decreased simultaneously as CVP decreased[18]. However, some studies have pointed out that excessively low CVP maintenance level should be avoided to decrease the risk of tissue malperfusion and PHLF, especially in elderly or prolonged portal blockade patients[19]. In this study, we found that for patients treated with the CLCVP technique, neither the liver clamping time nor the blood loss was a risk factor for PHLF. This may be related to the fact that the application of CLCVP reduces bleeding and accelerates the surgical process. However, the serum potassium level after clamping was a significant risk factor for PHLF (P < 0.05). This indicates that when applying CLCVP, attention must be paid to electrolyte changes, especially the change in serum potassium, and intraoperative intervention should be carried out promptly to reduce the incidence of PHLF.
In addition to intraoperative factors, the occurrence of PHLF is also closely related to patients’ preoperative basic factors and liver conditions. Previous studies have shown that the preoperative risk factors for PHLF cover patients’ own factors (such as age, gender, and body mass index)[20], as well as factors related to impaired liver reserve function (such as cholestasis, decreased secretory function, liver cirrhosis, and platelet abnormalities)[3,21,22]. In China, the incidence of liver cancer and hepatobiliary stones is relatively high. Most patients have different degrees and types of liver cirrhosis when undergoing surgery. A large number of studies have confirmed that hypoalbuminemia can have a serious impact on the success rate and prognosis of hepatectomy[23] and a retrospective study involving 268 patients who underwent hepatectomy found that the preoperative albumin level is an important indicator for predicting liver failure after hepatectomy.
In this study, through multivariate logistic regression analysis, it was found that based on the CLCVP technique, liver cirrhosis is a significant risk factor for PHLF in patients undergoing hepatectomy (P = 0.001). Therefore, for patients with liver cirrhosis, performing hepatectomy requires more thorough preoperative preparation, more refined intraoperative anesthesia management, and more meticulous postoperative management. In our study, it was found that preoperative albumin is also an independent risk factor for PHLF in patients undergoing hepatectomy with the application of the CLCVP technique (P = 0.036), and it has a negative linear correlation with the occurrence of PHLF. Therefore, before performing hepatectomy, close attention should be paid to the patient’s albumin level. For patients with hypoproteinemia, reasonable supplementation of human albumin during the perioperative period may have a positive effect in improving the success rate of the surgery and reducing the incidence of postoperative complications. Moreover, in our research, patients with liver cancer as the indication for surgery emerged as a notable risk factor for PHLF (P = 0.037). When compared with patients undergoing surgery for other causes, those with liver cancer exhibit a markedly more rapid decline in liver function, possess poor hepatic reserve capacity, and necessitate a more extensive scope of liver resection[24].
In a retrospective analysis of 285 patients undergoing hepatectomy with CLCVP, cirrhosis, preoperative albumin level, causes of liver resection (cancer or others), DBIL and platelet count were independent risk factors and predictors of PHLF in hepatectomy patients under CLCVP, and this model has a good ability to predict PHLF in hepatectomy patients under CLCVP. Cirrhosis, liver cancer, low preoperative albumin, and high preoperative DBIL can lead to an increased likelihood of PHLF. This suggests that the risk of PHLF is higher in patients with cirrhosis using the CLCVP technique. Before surgery, reasonably increasing albumin levels and reducing DBIL levels may have a positive impact in reducing the risk of PHLF. In this study, we found that for patients treated with the CLCVP technique, neither the liver clamping time nor the blood loss was a risk factor for PHLF. This may be related to the fact that the application of CLCVP reduces bleeding and accelerates the surgical process.
| 1. | Meyer J, Balaphas A, Combescure C, Morel P, Gonelle-Gispert C, Bühler L. Systematic review and meta-analysis of thrombocytopenia as a predictor of post-hepatectomy liver failure. HPB (Oxford). 2019;21:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | van Mierlo KM, Schaap FG, Dejong CH, Olde Damink SW. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J Hepatol. 2016;65:1217-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (1)] |
| 4. | Jaeck D, Bachellier P, Oussoultzoglou E, Weber JC, Wolf P. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10:S58-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, Belghiti J. Prospective validation of the "fifty-fifty" criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Martin AN, Kerwin MJ, Turrentine FE, Bauer TW, Adams RB, Stukenborg GJ, Zaydfudim VM. Blood transfusion is an independent predictor of morbidity and mortality after hepatectomy. J Surg Res. 2016;206:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Johnson M, Mannar R, Wu AV. Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg. 1998;85:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Pan YX, Wang JC, Lu XY, Chen JB, He W, Xu L, Zhang YJ, Chen MS, Lai RC, Zhou ZG. Advantages of Controlled Low Central Venous Pressure in Laparoscopic Hepatectomy for Hepatocellular Carcinoma - A Randomized Clinical Trial. HPB. 2019;21:S224. [DOI] [Full Text] |
| 10. | Wang F, Sun D, Zhang N, Chen Z. The efficacy and safety of controlled low central venous pressure for liver resection: a systematic review and meta-analysis. Gland Surg. 2020;9:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Li Z, Sun YM, Wu FX, Yang LQ, Lu ZJ, Yu WF. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol. 2014;20:303-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56657] [Article Influence: 7082.1] [Reference Citation Analysis (134)] |
| 13. | Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 14. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 839] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 15. | Doi S, Yasuda S, Hokuto D, Kamitani N, Matsuo Y, Sakata T, Nishiwada S, Nagai M, Nakamura K, Terai T, Kohara Y, Sho M. Impact of the Prolonged Intermittent Pringle Maneuver on Post-Hepatectomy Liver Failure: Comparison of Open and Laparoscopic Approaches. World J Surg. 2023;47:3328-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Morino K, Seo S, Yoh T, Toda R, Yoshino K, Nishio T, Yamamoto G, Ishii T, Taura K, Hatano E. Impact of the Intermittent Pringle Maneuver for Predicting Post-hepatectomy Liver Failure: A Cohort Study of 597 Consecutive Patients. World J Surg. 2023;47:1058-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 169] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Lin CX, Guo Y, Lau WY, Zhang GY, Huang YT, He WZ, Lai EC. Optimal central venous pressure during partial hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Liu Z, Yang X, Yang H, Ling Z, Li Y, Wu W, Shi F, Ji F. Controlled low central venous pressure maintenance level during laparoscopic hepatectomy negatively associated with PHLF incidence: a retrospective propensity score matching study. Surg Endosc. 2025;39:1101-1113. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Fang T, Long G, Wang D, Liu X, Xiao L, Mi X, Su W, Zhou L, Zhou L. A Nomogram Based on Preoperative Inflammatory Indices and ICG-R15 for Prediction of Liver Failure After Hepatectomy in HCC Patients. Front Oncol. 2021;11:667496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Mehrabi A, Golriz M, Khajeh E, Ghamarnejad O, Probst P, Fonouni H, Mohammadi S, Weiss KH, Büchler MW. Meta-analysis of the prognostic role of perioperative platelet count in posthepatectomy liver failure and mortality. Br J Surg. 2018;105:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Gu J, Zhang E, Liang B, Zhang Z, Chen X, Xiong M, Huang Z. Liver Collagen Contents Are Closely Associated with the Severity of Cirrhosis and Posthepatectomy Liver Failure in Patients with Hepatocellular Carcinoma and Child-Pugh Grade A Liver Function. Ann Surg Oncol. 2021;28:4227-4235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Morise Z, Aldrighetti L, Belli G, Ratti F, Cheung TT, Lo CM, Tanaka S, Kubo S, Okamura Y, Uesaka K, Monden K, Sadamori H, Hashida K, Kawamoto K, Gotohda N, Chen K, Kanazawa A, Takeda Y, Ohmura Y, Ueno M, Ogura T, Suh KS, Kato Y, Sugioka A, Belli A, Nitta H, Yasunaga M, Cherqui D, Halim NA, Laurent A, Kaneko H, Otsuka Y, Kim KH, Cho HD, Lin CC, Ome Y, Seyama Y, Troisi RI, Berardi G, Rotellar F, Wilson GC, Geller DA, Soubrane O, Yoh T, Kaizu T, Kumamoto Y, Han HS, Ekmekcigil E, Dagher I, Fuks D, Gayet B, Buell JF, Ciria R, Briceno J, O'Rourke N, Lewin J, Edwin B, Shinoda M, Abe Y, Hilal MA, Alzoubi M, Tanabe M, Wakabayashi G. An International Retrospective Observational Study of Liver Functional Deterioration after Repeat Liver Resection for Patients with Hepatocellular Carcinoma. Cancers (Basel). 2022;14:2598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/