Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.99597
Revised: January 2, 2025
Accepted: January 20, 2025
Published online: March 27, 2025
Processing time: 95 Days and 1.7 Hours
Currently, very few studies have examined the analgesic effectiveness and safety of dexmedetomidine-assisted intravenous-inhalation combined general anesthesia in laparoscopic minimally invasive surgery for inguinal hernia.
To investigate the analgesic effect and safety of dexmedetomidine-assisted intravenous-inhalation combined general anesthesia in laparoscopic minimally invasive surgery for inguinal hernia.
In this retrospective study, 94 patients scheduled for laparoscopic minimally invasive surgery for inguinal hernia, admitted to Yiwu Central Hospital between May 2022 and May 2023, were divided into a control group (inhalation combined general anesthesia) and a treatment group (dexmedetomidine-assisted intrave
Baseline data, including age, hernia location, place of residence, weight, monthly income, education level, and underlying diseases, were not significantly different between the two groups, indicating comparability (P > 0.05). No significant difference was found in operation time and anesthesia time between the two groups (P > 0.05). However, the treatment group exhibited a shorter postoperative urinary catheter removal time and hospital stay than the control group (P < 0.05). Preoperatively, no significant differences were found in the visual analog scale (VAS) scores between the two groups (P > 0.05). However, at 12, 18, and 24 hours postoperatively, the treatment group had significantly lower VAS scores than the control group (P < 0.05). Although no significant differences in preoperative hemodynamic indicators were found between the two groups (P > 0.05), both groups experienced some extent of changes in postoperative HR, diastolic BP (DBP), and systolic BP (SBP). Nevertheless, the treatment group showed smaller changes in HR, DBP, and SBP than the control group (P < 0.05). Preoperative immune function indicators showed no significant differences between the two groups (P > 0.05). However, postoperatively, the treatment group demonstrated higher levels of CD3+, CD4+, and CD4+/CD8+ and lower levels of CD8+ than the control group (P < 0.05). The rates of adverse reactions were 6.38% and 23.40% in the treatment and control groups, respectively, revealing a significant difference (χ2 = 5.371, P = 0.020).
Dexmedetomidine-assisted intravenous-inhalation combined general anesthesia can promote early recovery of patients undergoing laparoscopic minimally invasive surgery for inguinal hernia. It ensures stable blood flow, improves postoperative analgesic effects, reduces postoperative pain intensity, alleviates stress response, improves immune function, facilitates anesthesia recovery, and enhances safety.
Core Tip: This study aimed to assess the analgesic effect and safety of dexmedetomidine-assisted intravenous-inhalation combined general anesthesia in laparoscopic minimally invasive surgery for inguinal hernia through systematic clinical observation and analysis. The study enrolled a total of 94 patients with inguinal hernia who were scheduled to undergo laparoscopic minimally invasive surgery. Comparative analyses were performed on the clinical outcomes between inhalation anesthesia combined with general anesthesia and dexmedetomidine-assisted intravenous-inhalation combined general anesthesia in these patients. The results revealed that dexmedetomidine-assisted intravenous-inhalation combined general anesthesia can facilitate the early recovery of patients undergoing laparoscopic minimally invasive surgery for inguinal hernia, ensure hemodynamic stability, enhance postoperative analgesic effects, alleviate stress response, and improve immune function while exhibiting a certain safety level.
- Citation: Lou QX, Xu KP. Analgesic effect and safety of dexmedetomidine-assisted intravenous-inhalation combined general anesthesia in laparoscopic minimally invasive inguinal hernia surgery. World J Gastrointest Surg 2025; 17(3): 99597
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/99597.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.99597
Inguinal hernia is a common abdominal wall disease and accounts for 85%-95% of all hernia cases. Traditional treatment methods, including hernia repair surgery and open surgery, are associated with significant postoperative pain, extensive trauma, and longer recovery period[1,2]. As medical technology advances, laparoscopic minimally invasive surgery has increasingly become the preferred treatment option for inguinal hernia[3]. However, postoperative pain remains a significant clinical issue in laparoscopic minimally invasive surgery, which affects patients’ postoperative comfort and recovery quality[4]. Currently, traditional anesthesia modalities employed in inguinal hernia surgeries present several deficiencies, including inadequate preservation of hemodynamic stability, limited postoperative analgesic efficacy, and high propensity for adverse reactions[5,6]. Consequently, an exploratory optimization endeavor regarding the anesthesia protocol for inguinal hernia surgeries is imperative. In recent years, dexmedetomidine has been widely used as an adjunct analgesic medication to improve postoperative analgesic effects and enhance surgical safety in clinical anesthesia practice. Dexmedetomidine, a selective α2-adrenergic receptor agonist[7], possesses analgesic, sedative, and anxiolytic properties. It effectively alleviates postoperative pain, promotes patient sedation, and minimizes the need for analgesic medications during and after surgery. Currently, research on the analgesic effect and safety of dexmedetomidine-assisted inhalation anesthesia in laparoscopic minimally invasive surgery for inguinal hernia is relatively scarce. Therefore, this study aimed to evaluate the analgesic effect and safety of dexmedetomidine-assisted inhalation anesthesia in laparoscopic minimally invasive surgery for inguinal hernia through systematic clinical observation and analysis.
This retrospective study included 94 patients with inguinal hernia scheduled for laparoscopic minimally invasive surgery, who were admitted to Yiwu Central Hospital between May 2022 and May 2023. The patients were allocated into a control group and a treatment group, with 47 patients in each group. The inclusion criteria were as follows: (1) Diagnosis of inguinal hernia confirmed through clinical examination and other necessary imaging studies[8]: Presence of protrusion or bulge in the inguinal region with discomfort or pain; palpable mass or bulge in the groin area, which becomes more prominent during activities such as coughing, exertion, or standing; (2) American Society of Anesthesiologists physical status classification of grades I–II[9]; (3) No history of drug allergies; (4) General surgical tolerance without severe cardiovascular, pulmonary, or other systemic diseases; (5) No contraindications, such as severe coagulation disorders, hepatic or renal insufficiency, and advanced malignant tumors; and (6) Complete clinical data. The exclusion criteria were as follows: (1) Severe cardiovascular diseases, such as severe heart failure and myocardial infarction; (2) Severe respiratory diseases, such as severe chronic obstructive pulmonary disease and respiratory failure; (3) Coagulation disorders, such as severe blood coagulation disorders or inability to adjust anticoagulant treatment; (4) Severe hepatic or renal dysfunction, such as advanced liver cirrhosis and renal failure; (5) Malignant tumors, such as advanced malignant tumors and metastatic tumors; (6) Prior or intraoperative use of other analgesic medications, or severe immunodeficiency, such as human immunodeficiency virus infection and postorgan transplant; (7) Use of other medications for treatment; and (8) Inability to contact the patients during the follow-up period due to changes in address or telephone number. Table 1 presents the baseline characteristics of the two patient groups.
| Projects | Control group (n = 47) | Treatment group (n = 47) | t/χ2 | P value | |
| Age (years) | 48.06 ± 6.58 | 48.13 ± 6.62 | 0.051 | 0.959 | |
| Hiatal hernia site (left/right) | 29/18 | 27/20 | 0.177 | 0.674 | |
| Place of residence (urban/rural) | 25/22 | 24/23 | 0.043 | 0.836 | |
| Body weight (kg) | 67.51 ± 10.68 | 68.05 ± 10.72 | 0.245 | 0.807 | |
| Monthly household income (> 2000 RMB/≤ 2000 RMB) | 24/23 | 26/21 | 0.171 | 0.679 | |
| Academic status (college and below/bachelor and above) | 21/26 | 20/27 | 0.043 | 0.835 | |
| Underlying disease (cases) | High blood pressure | 6 | 5 | 0.103 | 0.748 |
| Diabetes | 3 | 5 | 0.547 | 0.460 | |
Preoperatively, patients underwent bowel and bladder preparation. They were then transferred to the operating room and subjected to routine monitoring, including arterial pressure monitoring and establishing a vein access for intravenous administration. Various monitoring devices were connected to comprehensively monitor patients’ vital signs. Anesthesia was induced, and sedative medications, such as midazolam, were administered to alleviate anxiety and pain.
In the control group, anesthesia induction involved intravenous administration of propofol, cisatracurium besylate, and sufentanil for endotracheal intubation, followed by the inhalation of 2.5%-4.5% sevoflurane (Lunan Better Pharmaceutical Co., Ltd. H20080681, 100 mL). In the treatment group, in addition to the medications used in the control group, patients were administered dexmedetomidine at a dose of 1.0 μg/kg (Yangtze River Pharmaceutical Group, Jiangsu, China, H20183219, 2 mL/0.2 mg), prepared at a concentration of 4 μg/mL. The dose of 1 μg/kg was administered via slow intravenous infusion over a period of more than 10 minutes. The dose was adjusted based on the patient’s condition. After anesthesia induction, endotracheal intubation was performed to connect the airway to the ventilator, ensuring adequate ventilation and oxygenation of the patient. During the anesthesia procedure, vital signs [blood pressure (BP), heart rate (HR), and respiration] were monitored, and the anesthetic agent was adjusted as necessary to maintain the depth of anesthesia and stable physiological state. Once a stable anesthesia state was reached, under laparoscopic guidance, the surgeon inserted a laparoscope and surgical instruments through small incisions to perform hernia repair. After the procedure, the surgical area was examined to ensure no bleeding or residual hernia sac. The incision was sutured, dressings were applied, and the anesthetic agent was discontinued.
Surgical time, anesthesia time, postoperative urinary catheter removal time, and length of hospital stay were recorded for both groups. Pain scores in both groups were recorded before surgery and at 12, 18, and 24 hours after surgery using the visual analog scale (VAS) scoring criteria[10]. The VAS scores range from 0 to 10 points, where 0 represents no pain, < 3 indicate mild pain within the patient’s tolerance, 4-6 indicate moderate pain that affects sleep, and 7-10 indicate severe pain beyond the patient’s tolerance.
Hemodynamics: The HR, diastolic BP (DBP), systolic BP (SBP), and other indicators were measured before surgery and immediately after surgery.
Stress indicator test: In this study, 5 mL of peripheral venous blood was collected before surgery and 24 hours after surgery. The blood samples were centrifuged for 15 minutes using a KDC-2046 Low-speed centrifuge (Anhui Zhongkejia Scientific Instrument Co. Ltd.) to separate the serum. Cortisol, adrenaline, and noradrenaline levels were measured using enzyme-linked immunoassay (ELISA) kits (Shanghai Jianglai Biotechnology Co. Ltd.). The measurements were performed using an AP-960 fully automatic ELISA analyzer (Japan Kyowa Pharmaceutical Co. Ltd.), following the provided kit instructions.
Immune function: Peripheral venous blood (4 mL, anticoagulated with heparin) was extracted before and after surgery. The blood samples were centrifuged to separate the leukocyte layer. The percentages of lymphocyte subsets were calculated using a FACSCanto flow cytometer (BD Company, United States) and a three-color flow cytometry assay. Reagent kits for the detection of T lymphocyte subsets (CD3+, CD4+, CD8+, and CD4+/CD8+) were provided by Beckman Coulter (United States).
Postoperative adverse reactions, including dizziness, nausea, vomiting, dry mouth, and incision infection, were recorded and compared between the two groups. The follow-up period was from May 20, 2023, to June 20, 2023, with all patients receiving follow-up.
For quantitative data (including age, body weight, perioperative indicators, VAS scores, hemodynamic indicators, stress levels, and immune function), descriptive statistics was used to present the data as means ± SD. Comparisons at different time points were analyzed using repeated-measures analysis of variance and t-tests. Categorical data (such as hernia location, place of residence, monthly family income, educational background, underlying diseases, and postoperative adverse reactions) were presented as rates and percentages (%). The χ2 test was employed for comparison. The significance level was set at α = 0.05. Statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, United States) or a similar statistical software package. Furthermore, this study identified outliers by calculating statistical indicators such as the mean and SD of the data. Specifically, when the value of a certain data point deviated from the mean by more than three times the SD, it was identified as an outlier. Given the relatively small sample size of this study, a method of retaining outliers was adopted for comprehensive analysis to ensure data quality and accuracy of the analysis results.
No significant difference was found between the two groups in terms of age, site of inguinal hernia, place of residence, weight, monthly household income, educational status, and underlying diseases (P > 0.05; Table 1).
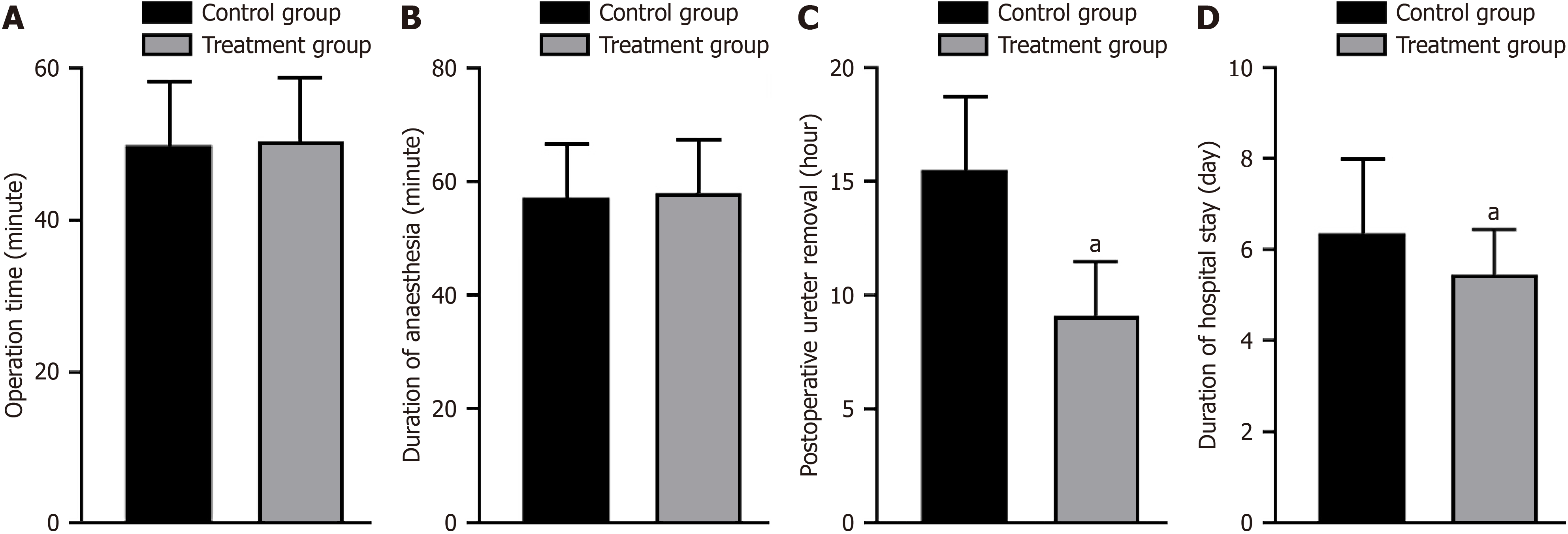
No significant difference was observed in the operative and anesthesia times between the two groups (P > 0.05). However, the treatment group had shorter postoperative urinary catheter removal time and hospital stay than the control group (P < 0.05; Figure 1).
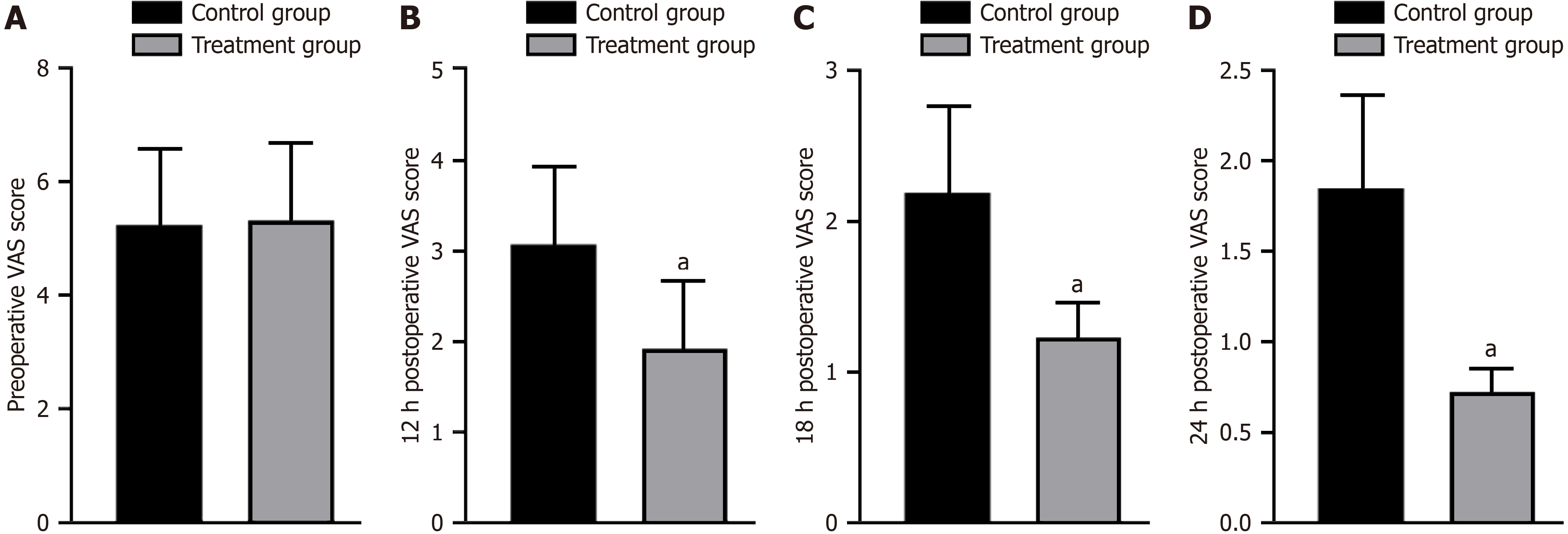
No significant difference was found in preoperative VAS scores between the two groups (P > 0.05). However, at 12, 18, and 24 h after the surgery, the treatment group exhibited significantly lower VAS scores than the control group (P < 0.05; Figure 2).
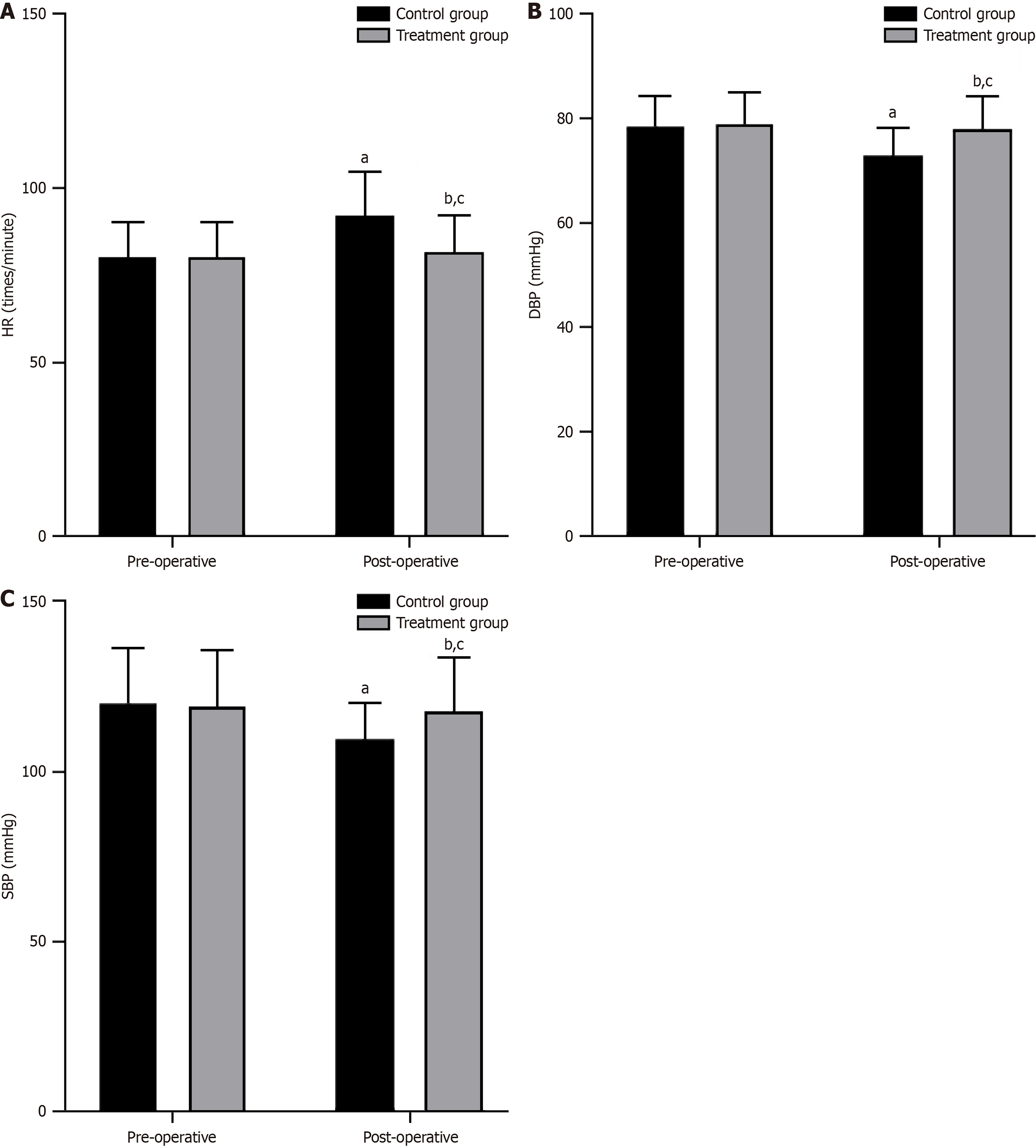
The preoperative hemodynamic indexes were not significantly different between the two groups (P > 0.05). However, after the surgery, the HR increased, and the DBP and SBP decreased in both groups. Notably, the treatment group exhibited smaller changes in HR, DBP, and SBP than the control group (P < 0.05, Figure 3).
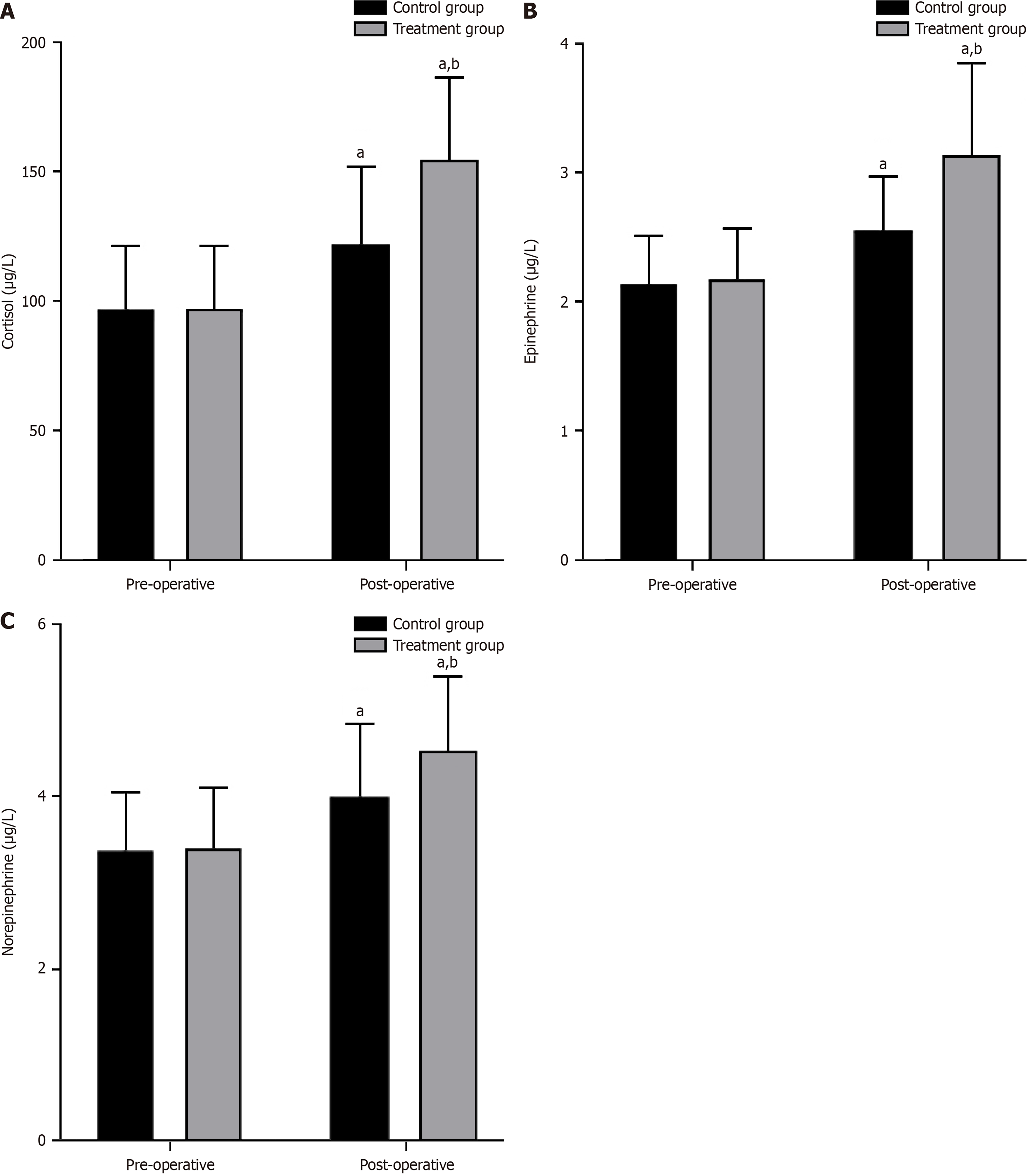
No significant differences were found in the preoperative stress index levels between the two groups (P > 0.05). However, postoperative levels of cortisol, epinephrine, and norepinephrine significantly increased in both groups compared with their preoperative levels. In addition, the treatment group exhibited higher postoperative levels of cortisol, epinephrine, and norepinephrine than the control group (P < 0.05, Figure 4).
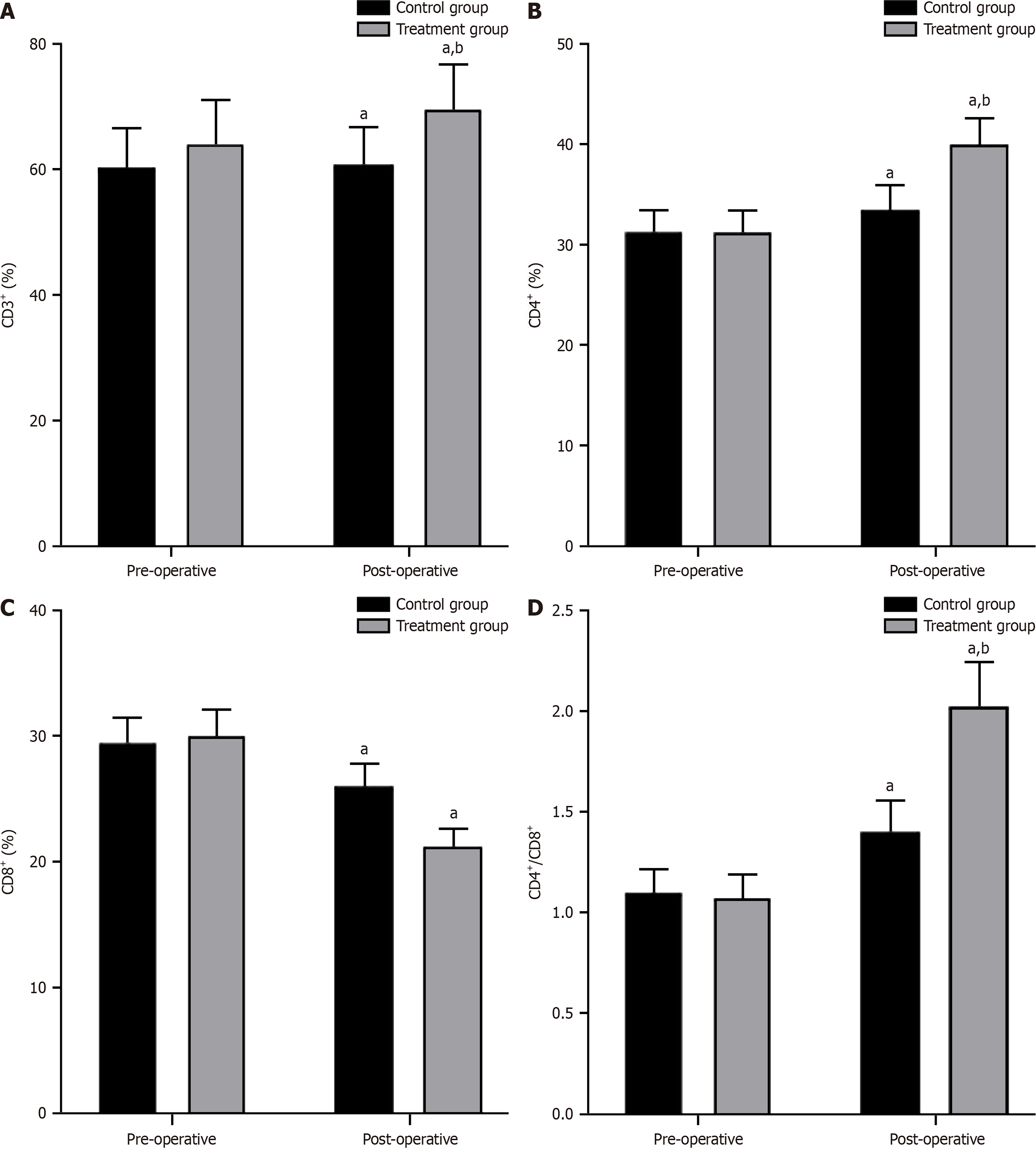
No significant differences were observed in the comparison of preoperative immune function indicators between the two groups (P > 0.05). However, both groups exhibited higher postoperative levels of CD3+, CD4+, and CD4+/CD8+ and lower levels of CD8+ (P < 0.05). The treatment group exhibited higher levels of CD3+, CD4+, and CD4+/CD8+ and lower levels of CD8+ than the control group (P < 0.05; Figure 5).
The postoperative adverse reaction rates were 6.38% and 23.40% in the treatment and control groups, respectively, demonstrating a significant difference (χ2 = 5.371, P = 0.020). Notably, these reactions were well-tolerated and resolved spontaneously within 2 days (Table 2).
| Grouping | Number of examples | Dizziness | Nausea and vomiting | Dry mouth | Infection of the incision | Incidence |
| Control group | 47 | 2 (4.26) | 3 (6.68) | 4 (7.55) | 2 (4.26) | 11 (23.40) |
| Treatment group | 47 | 0 (0.00) | 1 (2.13) | 1 (2.13) | 1 (2.13) | 3 (6.38) |
| χ2 | 5.371 | |||||
| P value | 0.020 |
Groin hernia surgery is typically performed using conventional methods, which are associated with greater trauma and higher anesthesia requirements, potentially affecting patient recovery outcomes[11]. In contrast, laparoscopic groin hernia surgery offers advantages such as minimal invasiveness and higher safety, resulting in this wide adoption in the treatment of groin hernia. Although laparoscopic surgery reduces the anesthesia depth, a certain anesthesia level is still required owing to the need for artificial pneumoperitoneum during the procedure[12]. Inappropriate sedation can lead to excessive sympathetic nervous system response during anesthesia, resulting in increased intraoperative BP and HRF, thus delaying postoperative recovery and impeding rehabilitation[13]. Therefore, selecting the appropriate anesthesia approach to maintain stable hemodynamics and minimize effects on the patient is crucial for postoperative recovery.
General anesthesia is commonly used during laparoscopic surgery[14] because of its advantages such as rapid induction, optimal oxygenation and ventilation, and alleviation of patient anxiety and tension, thereby effectively minimizing adverse reactions. Total intravenous anesthesia (TIVA) is a widely adopted anesthetic technique in laparoscopic groin hernia surgery[15]. In TIVA, dexmedetomidine is commonly used as an anesthetic agent, which is a potent sedative with a rapid onset of action[16] and reaches peak concentration shortly after administration. Furthermore, dexmedetomidine exerts dose-dependent sedative and analgesic effects and allows for precise control of anesthesia by adjusting the dosage according to the patient’s needs. Zhang et al[17] studied 102 patients aged > 65 years who had groin hernia and underwent open hernia repair surgery. They found that the addition of dexmedetomidine to ropivacaine enhanced postoperative analgesia during hospitalization, improved recovery quality, and did not affect chronic pain in older patients undergoing open hernia repair surgery. In this study involving middle-aged patients undergoing minimally invasive surgery, the treatment group had advantages regarding postoperative analgesic effects, time to removal of the urinary catheter, and length of hospital stay compared with the control group, indicating that dexmedetomidine-assisted intravenous-inhalation combined general anesthesia promotes early patient recovery. The treatment group had lower VAS scores than the control group at 12, 18, and 24 hours postoperatively, indicating significant analgesic effects of dexmedetomidine-assisted intravenous-inhalation combined general anesthesia. This may be attributed to the fact that dexmedetomidine acts as a selective α2-adrenergic receptor agonist possessing analgesic and sedative properties. It acts on the central nervous system to reduce pain transmission and elevate the pain threshold, thereby alleviating postoperative pain.
Stress indicators cortisol, epinephrine, and norepinephrine are important representative substances of the body’s stress response[18]. Among them, cortisol is one of the main adrenal cortex hormones and has functions in anti-inflammatory response, immune suppression, and stress regulation. In laparoscopic minimally invasive surgery for groin hernia, both surgical stimulation and the postoperative recovery process can elicit a stress response in the body, leading to high cortisol levels[19]. Monitoring cortisol levels can assess the stress severity intraoperatively and recovery status postoperatively[20]. Epinephrine and norepinephrine are secreted by the adrenal medulla and are primarily involved in the body’s stress response, such as increased HR and high BP[21]. In laparoscopic minimally invasive surgery for groin hernia, surgical stimulation and the use of anesthetic drugs can induce the release of these hormones[22]. Monitoring these indicators can reflect the stress level during the surgery and the body’s stress response[23]. The results regarding postoperative stress response and physiological indicators showed higher levels of cortisol, epinephrine, and norepinephrine in the treatment group than in the control group. The treatment group also exhibited smaller changes in HR, DBP, and SBP than the control group after the surgery. This finding indicates that dexmedetomidine-assisted intravenous-inhalation combined general anesthesia helps maintain the stability of blood flow in patients and reduces the effects of surgery on the circulatory system.
T lymphocytes are an important component of the body’s immune regulation[24]. Among them, CD3+ is used to evaluate the overall quantity and function of T lymphocytes[25]. CD4+ is closely associated with cellular and humoral immunity and plays an important role in regulating and coordinating immune responses[26]. CD8+ cells serve as markers for cytotoxic T lymphocytes and aid in killing infected or mutated cells[27]. The CD4+/CD8+ ratio serves as an indicator of immune balance, and changes in this ratio can be used to effectively evaluate immune function[28]. In this study, the postoperative levels of CD3+, CD4+, CD4+/CD8+, and CD8+ in the treatment group were higher than those in the control group, indicating that dexmedetomidine-assisted intravenous-inhalation combined general anesthesia can enhance overall immune function. Moreover, the incidence of postoperative adverse reactions in the treatment group (6.38%) was remarkably lower than that in the control group (23.40%). These findings highlight the enhanced postoperative safety associated with dexmedetomidine-assisted intravenous-inhalation combined general anesthesia and lower risk of adverse reactions. A potential explanation is that owing to its rapid onset of action, dexmedetomidine enables patients to rapidly achieve an anesthetic state within a short period, reaching peak concentration quickly after injection. It has a short duration of action because of fast metabolism and elimination, and patients recover quickly after discontinuing the medication, which helps reduce postoperative nausea, vomiting, and other adverse reactions.
However, this study has a few limitations. First, the sample size is relatively small, which may introduce certain selection bias. Second, the focus was only on laparoscopic minimally invasive surgery for groin hernia; thus, further research is needed for other types of minimally invasive surgeries.
Dexmedetomidine-assisted intravenous-inhalation combined general anesthesia promotes early recovery of patients after laparoscopic minimally invasive surgery for groin hernia. It ensures stable blood flow, enhances postoperative analgesic effect, reduces postoperative pain, alleviates stress response, improves immune function, facilitates anesthesia recovery, and provides greater safety.
| 1. | Solaini L, Cavaliere D, Avanzolini A, Rocco G, Ercolani G. Robotic versus laparoscopic inguinal hernia repair: an updated systematic review and meta-analysis. J Robot Surg. 2022;16:775-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | DACH-Konsensusgruppe ambulante Leistenhernienchirurgie, Niebuhr H, Köckerling F, Fortelny R, Hoffmann H, Conze J, Holzheimer RG, Koch A, Köhler G, Krones C, Kukleta J, Kuthe A, Lammers B, Lorenz R, Mayer F, Pöllath M, Reinpold W, Schwab R, Stechemesser B, Weyhe D, Wiese M, Zarras K, Meyer HJ. [Inguinal hernia operations-Always outpatient?]. Chirurgie (Heidelb). 2023;94:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Doudakmanis C, Kolla C, Bouliaris K, Efthimiou M, Koukoulis GD. Laparoscopic bilateral inguinal hernia repair: Should it be the preferred technique? World J Methodol. 2022;12:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Park JB, Chong DC, Reid JL, Edwards S, Maddern GJ. Should asymptomatic contralateral inguinal hernia be laparoscopically repaired in the adult population as benefits greatly outweigh risks? A systematic review and meta-analysis. Hernia. 2022;26:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Sarakatsianou C, Georgopoulou S, Tzovaras G, Perivoliotis K, Papadonta ME, Baloyiannis I. Hemodynamic effects of anesthesia type in patients undergoing laparoscopic transabdominal preperitoneal inguinal hernia repair under spinal vs general anesthesia. Hernia. 2019;23:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Palumbo P, Usai S, Amatucci C, Perotti B, Ruggeri L, Illuminati G, Tellan G. Inguinal hernia repair in day surgery: the role of MAC (Monitored Anesthesia Care) with remifentanil. G Chir. 2017;38:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lewis K, Alshamsi F, Carayannopoulos KL, Granholm A, Piticaru J, Al Duhailib Z, Chaudhuri D, Spatafora L, Yuan Y, Centofanti J, Spence J, Rochwerg B, Perri D, Needham DM, Holbrook A, Devlin JW, Nishida O, Honarmand K, Ergan B, Khorochkov E, Pandharipande P, Alshahrani M, Karachi T, Soth M, Shehabi Y, Møller MH, Alhazzani W; GUIDE group. Dexmedetomidine vs other sedatives in critically ill mechanically ventilated adults: a systematic review and meta-analysis of randomized trials. Intensive Care Med. 2022;48:811-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 8. | Fitzgibbons RJ Jr, Camps J, Cornet DA, Nguyen NX, Litke BS, Annibali R, Salerno GM. Laparoscopic inguinal herniorrhaphy. Results of a multicenter trial. Ann Surg. 1995;221:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 234] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Doyle DJ, Hendrix JM, Garmon EH. American Society of Anesthesiologists Classification. 2023 Aug 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 10. | Amorim AM, Simões J, Gonçalves J, Ferreira M, Ribeiro JC. The Portuguese version of the visual vertigo analog scale. Braz J Otorhinolaryngol. 2022;88 Suppl 3:S125-S129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Petersen M, Friis-Andersen H, Zinther N. Does closure of the direct hernia defect in laparoscopic inguinal herniotomy reduce the risk of recurrence and seroma formation?: a systematic review and meta-analysis. Hernia. 2023;27:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Abebe MS, Tareke AA, Alem A, Debebe W, Beyene A. Worldwide magnitude of inguinal hernia: Systematic review and meta-analysis of population-based studies. SAGE Open Med. 2022;10:20503121221139150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Chima AM, Mahmoud MA, Narayanasamy S. What Is the Role of Dexmedetomidine in Modern Anesthesia and Critical Care? Adv Anesth. 2022;40:111-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 14. | Vaghela SS, Chaurasiya MK, Prakash R, Khan MP. Ultrasound-Guided Quadratus Lumborum Block Versus Transversus Abdominis Plane Block for Laparoscopic Inguinal Hernia Repair and Appendicectomy Using Ropivacaine With Dexmedetomidine. Cureus. 2023;15:e33450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Yi X, Xu W, Li A. The Clinical Application of Remimazolam Benzenesulfonate Combined with Esketamine Intravenous Anesthesia in Endoscopic Retrograde Cholangiopancreatography. Biomed Res Int. 2022;2022:5628687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Zhao L, Lv L, Li S. Retrospective analysis of remifentanil combined with dexmedetomidine intravenous anesthesia combined with brachial plexus block on shoulder arthroscopic surgery in elderly patients. Pak J Med Sci. 2022;38:1546-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Zhang X, Zhang J, Gu W, Wu D, Shi C, Ma Z. Dexmedetomidine adjunct to ropivacaine for ultrasound-guided transversus abdominis plane block for open inguinal hernia repair in the older adults: A randomised clinical trial. J Minim Access Surg. 2024;20:187-195. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Jin W, Shen X, Jin H. Effects of Sevoflurane Laryngeal Mask Inhalation Combined with Intravenous Anesthesia on Perioperative Stress and Myocardial Injury in Elderly Patients with Acute Cholecystitis and Coronary Heart Disease. Emerg Med Int. 2022;2022:6482491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Hu H, An S, Sha T, Wu F, Jin Y, Li L, Zeng Z, Wu J, Chen Z. Association between dexmedetomidine administration and outcomes in critically ill patients with sepsis-associated acute kidney injury. J Clin Anesth. 2022;83:110960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Rehman HFU, Zaneb H, Masood S, Yousaf MS, Hayat K, Majeed KA, Zeeshan M, Ashraf S, Khan I, Khan A, Rehman H. Effect of Selenium Nanoparticles and Mannan Oligosaccharide Supplementation on Growth Performance, Stress Indicators, and Intestinal Microarchitecture of Broilers Reared under High Stocking Density. Animals (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Rendakov NL, Kaivarainen EI, Pekkoeva SN, Murzina SA, Nikerova KM. Cortisol Level and Na(+)/K(+)-ATPase Activity during Growth and Development of Juvenile Daubed Shanny Leptoclinus maculatus (Fries, 1838) in the Arctic. Dokl Biochem Biophys. 2022;507:367-369. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Ottemann Abbamonte CJ, Overton TR, Beaulieu AD, Drackley JK. In vitro addition of epinephrine, norepinephrine, and carnitine alters palmitate oxidation and esterification in isolated ovine hepatocytes. J Dairy Sci. 2023;106:3633-3640. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Fallah F, Shishehbore MR, Sheibani A. Fabrication of a novel sensor based on Cu quantum dot and SH-SiO(2) nanoparticles supported on copper-based metal organic framework (Cu QD-SH-SiO(2)@Cu-MOF) and its application for the simultaneous determination of norepinephrine, piroxicam and epinephrine. Talanta. 2023;252:123776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Kim GR, Choi JM. Current Understanding of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) Signaling in T-Cell Biology and Disease Therapy. Mol Cells. 2022;45:513-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Khan W, Augustine D, Rao RS. Inverse correlation of CD3 & vascular endothelial growth factor in incisional oral squamous cell carcinoma biopsies predicts nodal metastasis & poor survival of patients. Indian J Med Res. 2023;157:438-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Zhang H, Yao Y, Zhang X, Guan Y, Zheng F. CD4(+) T cell activation and inflammation in NASH-related fibrosis. Front Immunol. 2022;13:967410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, Wilhelm J, Li S, Song J, Li W, Sun Z, Sumer BD, Li B, Fu YX, Gao J. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun. 2022;13:4981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 28. | Murayama K, Ikegami I, Kamekura R, Sakamoto H, Yanagi M, Kamiya S, Sato T, Sato A, Shigehara K, Yamamoto M, Takahashi H, Takano KI, Ichimiya S. CD4(+)CD8(+) T follicular helper cells regulate humoral immunity in chronic inflammatory lesions. Front Immunol. 2022;13:941385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/