Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.101697
Revised: December 7, 2024
Accepted: January 13, 2025
Published online: March 27, 2025
Processing time: 153 Days and 19 Hours
Hepatic hemangioma represents the most common benign primary hepatic neo
Herein, we report two patients with GCH coexistent with DHH successfully treated by laparoscopic microwave ablation. The two GCHs were ablated com
Thermal ablation treatment might be an effective and less invasive treatment for GCH coexistent with DHH around the hemangioma.
Core Tip: Hepatic hemangiomas are the most common benign liver tumors, with giant cavernous hemangioma (GCH) often requiring intervention. This report discusses two patients with GCH coexistent with diffuse hepatic hemangiomatosis (DHH), treated successfully with laparoscopic microwave ablation. Both GCHs were completely ablated, showing sig
- Citation: Xu F, Kong J, Dong SY, Xu L, Wang SH, Sun WB, Gao J. Laparoscopic microwave ablation for giant cavernous hemangioma coexistent with diffuse hepatic hemangiomatosis: Two case reports. World J Gastrointest Surg 2025; 17(3): 101697
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/101697.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.101697
Hepatic hemangioma is the most frequently encountered solid benign liver neoplasm, with an incidence of 3% to 20% in the general population[1]. Giant cavernous hemangioma (GCH) is defined as being ≥ 5 cm and warranting therapy when it leads to continuous growth in clinical symptoms or the risk of complications[2]. Diffuse hepatic hemangiomatosis (DHH) is an uncommon disease of undetermined etiology characterized by numerous hemangiomas infiltrating and replacing the liver parenchyma[3,4]. In contrast to GCH, which presents with smooth, well-defined margins and typical imaging characteristics, DHH appears as a poorly defined lesion[5]. Isolated DHH without extrahepatic involvement is exceedingly rare in adults.
However, hemangiomatosis is frequently occurs in the liver parenchyma adjacent to a GCH[5]. The approach to managing hepatic hemangiomas varies based on the presence of associated hemangiomatosis and the extent and location of the remaining hepatic parenchyma. Surgical candidates must be selected with caution to mitigate complications, including excessive intraoperative blood loss and the risk of postoperative liver failure resulting from overestimation of functional residual liver volume due to unrecognized involvement by hemangiomatosis. Recently, thermal ablation treatment using radiofrequency (RF) ablation or microwave (MW) ablation has been investigated as a less invasive treatment for hepatic hemangioma, which has shown favorable outcomes[6,7]. However, thermal ablation has not been described in the published papers of hepatic hemangioma coexistent with DHH. We herein present two cases with GCH coexistent with DHH around the hemangioma that were treated successfully by laparoscopic MW ablation.
Case 1: In September 2022, a 63-year-old female was admitted to the hospital due to abdominal discomfort.
Case 2: In January 2024, a 54-year-old female was admitted to the hospital because regular follow-up images showed an enlarging hepatic hemangioma over the past 5 years.
Case 1: A hepatic hemangioma and multiple hepatic nodules were incidentally identified during a routine health check via abdominal ultrasound (US).
Case 2: No other specific discomfort was described.
Case 1: The patient had a documented history of hypertension.
Case 2: The patient had no previous abnormalities.
No significant personal or family history was reported by both patients.
The physical examination findings of case 1 and case 2 were within normal limits.
Laboratory tests revealed no abnormalities for both patients.
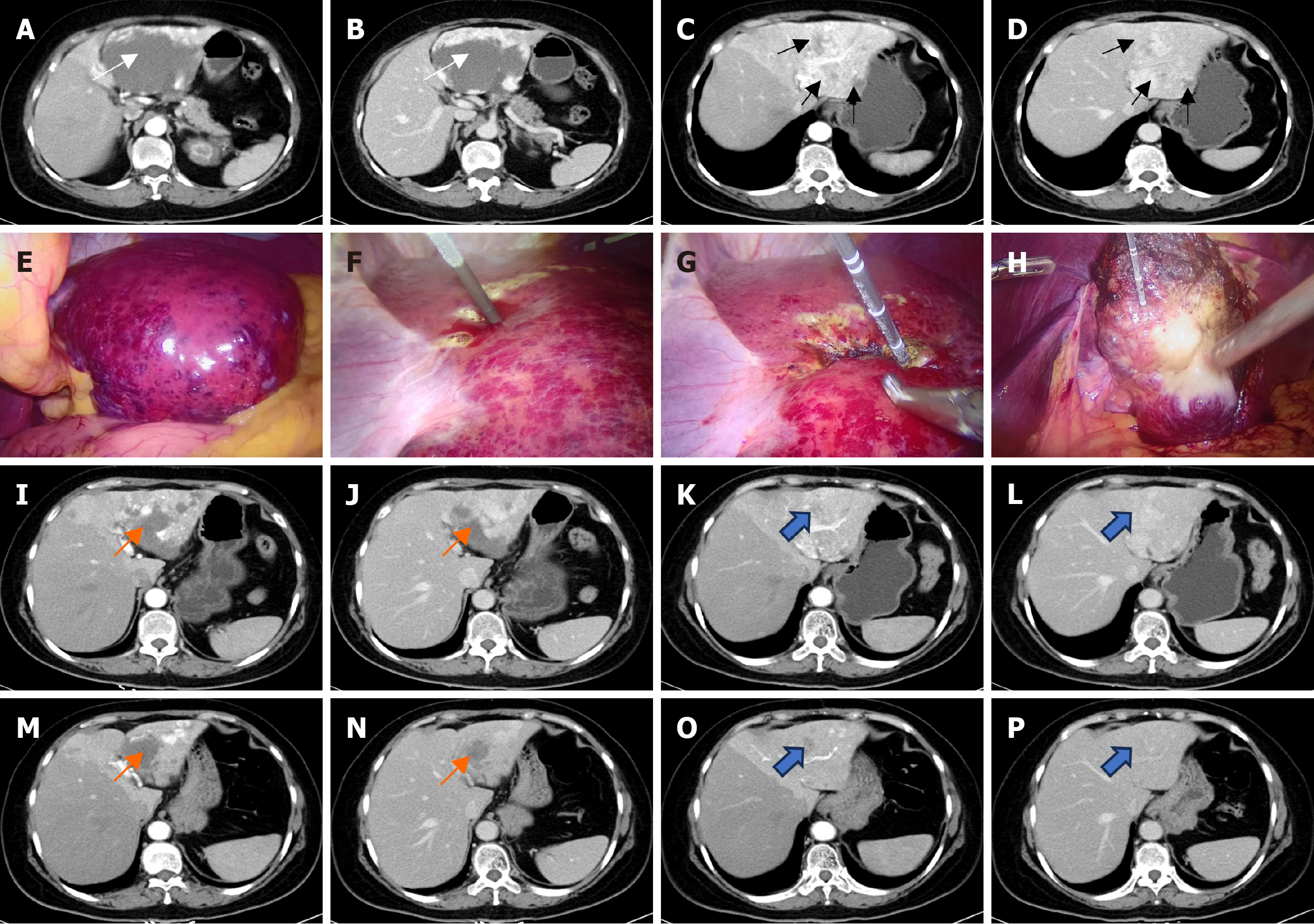
Case 1: A contrast-enhanced computed tomography (CT) scan demonstrated peripheral enhancement of the GCH (7.4 cm × 9.9 cm) and heterogeneous enhancement of the adjacent liver parenchyma affected by hemangiomatous lesions (Figure 1A-D).
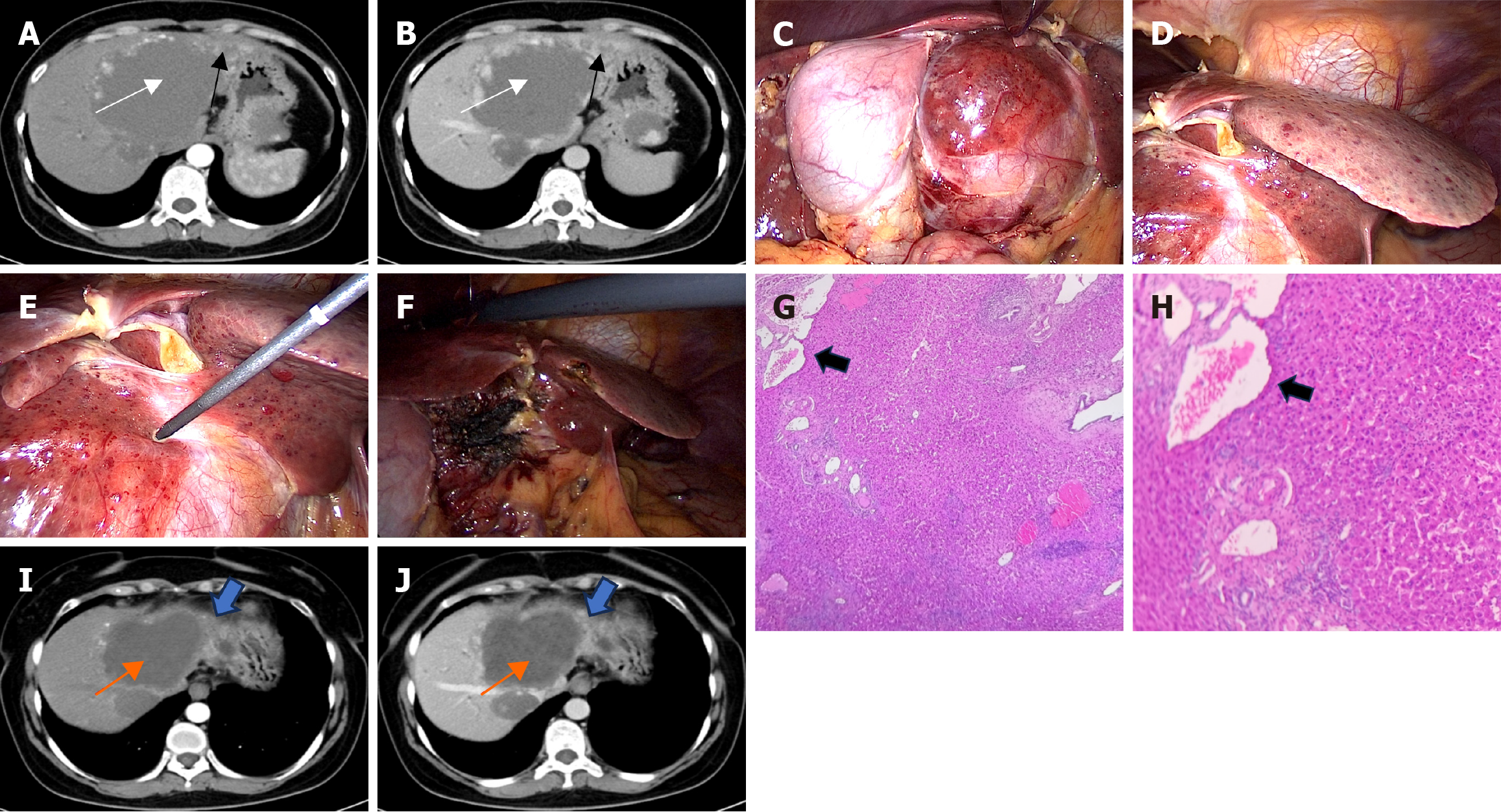
Case 2: Contrast-enhanced CT demonstrated a GCH (10.7 cm × 8.2 cm) and multiple small hemangiomatous lesions adjacent to the hemangioma in the liver (Figure 2A and B).
Based on imaging findings and clinical features, both patients in the study were diagnosed with GCH coexistent with DHH.
Following multidisciplinary team discussions, laparoscopic MW ablation was planned and executed to target the hemangioma. Two hepatobiliary surgeons, each with over a decade of experience in percutaneous and laparoscopic image-guided MW ablation of giant hemangiomas, performed the procedures (Figure 1E-H). Tumor coagulation was achieved using an ECO-100Al8 internally cooled MW antenna and a water-cooled MW ablation system (Yigao Medical, Nanjing, China), with the power output set at 150 W and a frequency of 2450 MHz. Details of the MW ablation process have been previously described[1]. Intraoperative US monitored tissue responses, with hyperechoic areas indicating adequately ablated tissue due to gas release from heating. DHH lesions were left intact to preserve as much normal liver parenchyma as possible. The ablation procedure lasted 56 minutes, with a total operative time of 125 minutes, including 20 sessions of ablations at different tumor sites. Intraoperative blood loss, quantified through drained blood, was measured at 10 mL.
Two hepatobiliary surgeons, each with over a decade of experience in percutaneous and laparoscopic image-guided MW ablation of giant hemangiomas, performed the procedures as previously described (Figure 2C-F). In the operative field, multiple small hemorrhagic blood-filled honeycomb areas from 2-3 mm up to 3 cm in diameter were scattered adjacent to the GCH throughout the entire left lobe (Figure 2D). To further obtain a definitive diagnosis, a liver biopsy was con
The patient was discharged on postoperative day 3 without significant complications, such as intrahepatic hematoma, and was in good health at the latest follow-up. Three months and 18 months after ablation, contrast-enhanced CT scans showed that the GCH was completely ablated and remarkably smaller. Moreover, a reduction in the size of DHH was noted (Figure 1I-P). No recurrence or delayed complications were identified, and the patient’s subjective health status and quality of life were assessed as good to excellent at the final follow-up.
The patient was discharged on postoperative day 5 without significant complications, such as intrahepatic hematoma, and was in good health at the latest follow-up. Four months after ablation, contrast-enhanced CT scans showed that the hepatic hemangioma was completely ablated and remarkably smaller. Moreover, a reduction in the size of DHH was noted too (Figure 2I and J). Subsequently, we will continue to monitor the patient’s follow-up one year after ablation. No recurrence or delayed complications were identified, and the patient’s subjective health status and quality of life were assessed as good to excellent at the final follow-up.
Hepatic hemangiomas are the most common benign liver tumors incidentally detected in most patients. DHH is a rare disease characterized by ill-defined tissue with a similar histological presentation to typical cavernous hemangiomas, replacing the hepatic parenchyma[5,8,9]. Histological features of DHH include irregularly dilated non-anastomotic vascular spaces lined with flat endothelial cells, infiltration of the native hepatic parenchyma without any encapsulation, and the latter two features distinguishing it from a cavernous hemangioma. However, hemangiomatosis is not rare in the liver parenchyma adjacent to a GCH[5]. Jhaveri et al[5] evaluated 41 patients who had undergone CT or magnetic resonance imaging (MRI) with reported GCH. They found 42 GCHs identified in 41 patients, and hemangiomatosis was present in 18 of 41 patients (44%). The extent of liver tissue involved by hemangiomatosis was variable but was confined to the same lobe as the GCH in the majority of patients (13/18).
As previously explained, enucleation is preferred when feasible for symptomatic GCH[10]. However, for GCH coexistent with DHH around the hemangioma, surgical candidates must be carefully selected to avoid surgical complications related to excessive blood loss from oozing due to the deroofing of the areas of hemangiomatosis after the enucleation of a GCH[5]. These cases can be better managed by extensive lobectomy, as the GCH may have small hemangiomatous lesions around the hemangioma, and large surgical margins are needed. When the tumor exceeds a certain volume in patients with limited hepatic reserve, liver resection is precluded. Liver transplantation should be considered for nonresectable benign hepatic neoplasms in patients with imminent life-threatening complications, an underlying liver disease, or the presence of severe symptoms[11]. In current published literature, there have been a total of 6 reported cases of GCH with DHH undergoing surgical intervention. In these 6 cases, 5 GCHs with DHH around the hemangioma underwent enucleation (one case)[3], extensive lobectomy (three cases)[12-14], and liver transplantation (one case)[11] respectively. Only one giant hepatic hemangioma was accompanied by DHH distributed throughout the liver, which received liver transplantation (Table 1)[15]. In fact, liver transplantation should be considered for hepatic hemangioma with DHH distributed throughout the entire liver. For hepatic hemangioma with DHH around the he
| Year | Clinical features | The largest GCH location | The largest GCH size, cm | DHH distribution | Treatment | Operation time, minutes | Intraoperation blood loss, mL | Postoperative length of stay, days | Follow up | Prognosis |
| 2000 | Post-prandial epigastric discomfort | Right lobe | 17 × 14 × 9 | Locally | Right hepatectomy | NA | NA | 14 | 9 months | No recurrence |
| 2014 | Epigastric pain and abdominal fullness | Right lobe | 20 × 14 × 8.5 | Locally | Extensive right hepatectomy | 190 | 845 | 9 | NA | NA |
| 2018 | Abdominal pain, hepatomegaly | Central portion of the liver | 16 | Locally | Liver transplantation | 575 | 1100 | 11 | 1.5 year | No recurrence |
| 2020 | NA | Segment IVb | 7.8 | Locally | Left hepatectomy | NA | NA | 12 | 1 year | No recurrence |
| 2021 | Abdominal pain and distension | Segment IV | 32 × 23 × 20 | Throughout the liver | Liver transplantation | 660 | 3000 | 16 | 6 months | No recurrence |
| 2022 | Right upper quadrant fullness | Left lobe | 11 × 8 × 11.3 | Locally | Enucleation | NA | NA | 5 | 6 months | No recurrence |
In recent years, thermal ablation techniques, including RF ablation and MW ablation, have gained prominence in the treatment of hepatic hemangiomas due to advantages, such as minimal invasiveness, high efficacy, safety, rapid recovery, and broad applicability[1]. RF and MW ablation utilize heat generated by high-frequency alternating current or electromagnetic waves to disrupt endothelial cell-lined vascular structures, promoting thrombosis, inducing necrotic coagu
Herein, we report two patients with GCH coexistent with DHH successfully treated by laparoscopic MW ablation. The two patients presented without severe complications during hospitalization and were in generally good condition, thus qualifying for thermal ablation. Protruding hemangiomas and superficial hemangiomas are indications for laparoscopic thermal ablation[18]. Therefore, a laparoscopic MW ablation procedure was employed in our two cases. Due to DHH within the liver parenchyma, it was left in situ without ablation to preserve liver parenchyma. It is worth noting that, due to the perivenous hemangioma in case 2, even though the tumor is larger, we aimed to minimize the ablation time and the number of ablation sessions in order to protect the surrounding normal tissue.
To the best of current knowledge, this report is the first to investigate the safety and efficacy of thermal ablation for GCH coexisting with DHH. In the study, both patients underwent successful MW ablation. Complete ablation of the hemangiomas was achieved, and the ablated zones demonstrated significant atrophy. Notably, follow-up imaging revealed a gradual reduction and eventual diminishment of DHH. Compared to surgical therapies described in previous literature, MW ablation was associated with reduced intraoperative bleeding and a shorter postoperative hospital stay, although no statistical comparisons were performed. Both patients were discharged shortly after the procedure without any symptoms or complications. The postoperative course was uneventful, with no recurrence of liver lesions detected during follow-up. It is important to note that this report specifically addresses cases involving thermal ablation for DHH located in proximity to GCH. Further clinical studies are needed to confirm the efficacy of thermal ablation for GCH combined with diffusely distributed DHH throughout the liver.
In conclusion, we reported two patients with GCH coexistent with DHH successfully treated by laparoscopic MW ablation. Thermal ablation treatment may be an effective and less invasive treatment for GCH coexistent with DHH around the hemangioma.
The authors would like to thank each of the investigators who contributed to this article.
| 1. | Xu L, Wu S, Kong J, Ke S, Yin T, Guo S, Ning C, Wang X, Li S, Ding J, Li A, Kong X, Wang Q, Xu Y, Gao J, Sun W. Thermal ablation of hepatic hemangioma: A multi-center experience with long-term outcomes. Eur J Radiol. 2023;164:110842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Li X, An C, Liu F, Cheng Z, Han Z, Yu X, Dong L, Yu J, Liang P. The value of 3D visualization operative planning system in ultrasound-guided percutaneous microwave ablation for large hepatic hemangiomas: a clinical comparative study. BMC Cancer. 2019;19:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Bhardwaj N, Parkhi M, Kumar M, Kaman L, Mitra S. Adult diffuse hepatic hemangiomatosis. Autops Case Rep. 2022;12:e2021401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Kim JD, Chang UI, Yang JM. Clinical challenges and images in GI. Diffuse hepatic hemangiomatosis involving the entire liver. Gastroenterology. 2008;134:1830, 2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Jhaveri KS, Vlachou PA, Guindi M, Fischer S, Khalili K, Cleary SP, Ayyappan AP. Association of hepatic hemangiomatosis with giant cavernous hemangioma in the adult population: prevalence, imaging appearance, and relevance. AJR Am J Roentgenol. 2011;196:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Yan L, Li B, Wen T, Wang W, Xu M, Wei Y, Yang J. Comparison of laparoscopic radiofrequency ablation versus open resection in the treatment of symptomatic-enlarging hepatic hemangiomas: a prospective study. Surg Endosc. 2016;30:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Wen SQ, Wan M, Len KM, Hu QH, Xie XY, Wu Q, Liao GQ. Safety and Efficacy of Laparoscopic Radiofrequency Ablation for Hepatic Hemangiomas: A Multicenter Retrospective Study. Ann Hepatol. 2018;17:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Vilgrain V, Boulos L, Vullierme MP, Denys A, Terris B, Menu Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics. 2000;20:379-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 258] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Rao R, Naidu J, Muhammad Nawawi KN, Wong ZQ, Ngiu CS, Mohammed F, Raja Ali RA, Yaacob NY. Diffuse hepatic haemangiomatosis: A case report and review of literature. Med J Malaysia. 2018;73:436-438. [PubMed] |
| 10. | Lerner SM, Hiatt JR, Salamandra J, Chen PW, Farmer DG, Ghobrial RM, Busuttil RW. Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg. 2004;139:818-21; discussion 821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Lee JH, Yoon CJ, Kim YH, Han HS, Cho JY, Kim H, Jang ES, Kim JW, Jeong SH. Living-donor liver transplantation for giant hepatic hemangioma with diffuse hemangiomatosis in an adult: a case report. Clin Mol Hepatol. 2018;24:163-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ohkura Y, Hashimoto M, Lee S, Sasaki K, Matsuda M, Watanabe G. Right hepatectomy for giant cavernous hemangioma with diffuse hemangiomatosis around Glisson's capsule. World J Gastroenterol. 2014;20:8312-8316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Moon WS, Yu HC, Lee JM, Kang MJ. Diffuse hepatic hemangiomatosis in an adult. J Korean Med Sci. 2000;15:471-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ota T, Kamiyama T, Kato T, Hanamoto T, Hirose K, Otsuka N, Matsuoka S, Taketomi A. A rare case of cavernous hemangioma accompanied with diffuse hepatic hemangiomatosis. Surg Case Rep. 2020;6:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Shankar S, Rammohan A, Kulaseharan VH, Kanagavelu R, Reddy MS, Rela M. Liver Transplantation for Rapidly Progressive Giant Hepatic Hemangioma With Diffuse Hemangiomatosis. Exp Clin Transplant. 2021;19:1106-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1375] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 17. | Kong J, Gao R, Wu S, Shi Y, Yin T, Guo S, Xin Z, Li A, Kong X, Ma D, Zhai B, Sun W, Gao J. Safety and efficacy of microwave versus radiofrequency ablation for large hepatic hemangioma: a multicenter retrospective study with propensity score matching. Eur Radiol. 2022;32:3309-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Huang S, Yu J, Liang P, Yu X, Cheng Z, Han Z, Li Q. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. 2014;83:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Tashi S, Gogna A, Leong S, Venkatanarasimha N, Chandramohan S. Vascular complications related to image-guided percutaneous thermal ablation of hepatic tumors. Diagn Interv Radiol. 2023;29:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/