Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.100951
Revised: December 4, 2024
Accepted: January 10, 2025
Published online: March 27, 2025
Processing time: 177 Days and 5.1 Hours
Hepatocellular carcinoma (HCC) is a prevalent malignancy in China, primarily diagnosed at advanced stages, which limits treatment options and increases mortality rates. Conversion therapy, which includes systemic and locoregional treatments, aims to render unresectable tumors resectable. Nonetheless, research is scant on the risks of disease progression during the temporary cessation of targeted drugs and immune checkpoint inhibitors before surgery.
This report describes a 58-year-old male with HCC who developed lung meta
Our study highlights the complex challenges in managing advanced HCC and emphasizes the critical need for ongoing research to refine treatment strategies and improve patient outcomes.
Core Tip: In recent years, targeted therapies, immunotherapies with immune checkpoint inhibitors, and localized inter
- Citation: Wang CD, Liu RD, Liu MJ, Song J. Lung metastasis following temporary discontinuation of lenvatinib and tislelizumab in hepatocellular carcinoma: A case report. World J Gastrointest Surg 2025; 17(3): 100951
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/100951.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.100951
Liver cancer is the fourth most common malignant tumor in China[1], with hepatocellular carcinoma (HCC) constituting over 80% of primary liver cancer cases[2]. A comprehensive review of the treatment landscape for HCC in China indicates that a majority of patients are diagnosed at intermediate to advanced stages [China Liver Cancer stage IIb, IIIa, and IIIb, encompassing a proportion of patients with Barcelona Clinic Liver Cancer (BCLC) stage B and all patients with BCLC stage C][3]. This late-stage diagnosis typically precludes surgical options, contributing significantly to the high mortality rates associated with HCC.
In this context, the advent of conversion therapy has transformed the treatment paradigm for patients with inter
Despite these advancements, a critical gap persists in our understanding of the risks associated with discontinuing targeted drugs and immune checkpoint inhibitors (ICIs) during the pre-surgical waiting period. We present the first case of lung metastasis occurring during a cessation period of lenvatinib, which was planned prior to surgical resection. This case reveals the urgent need for further research into optimal management strategies during this critical phase of treat
A 58-year-old man was referred to our hospital due to being diagnosed with chronic hepatitis type B and HCC in October 2023.
The patient was incidentally diagnosed with chronic hepatitis B virus infection during a routine examination. Subsequent ultrasonography at a local hospital revealed a large tumor at the transition zone between the left and right lobes of the liver.
The patient had no significant medical history prior to this diagnosis.
The patient denied any familial history of HCC.
Physical examination revealed no symptoms of jaundice, vascular spiders, palmar erythema, or other abnormal signs.
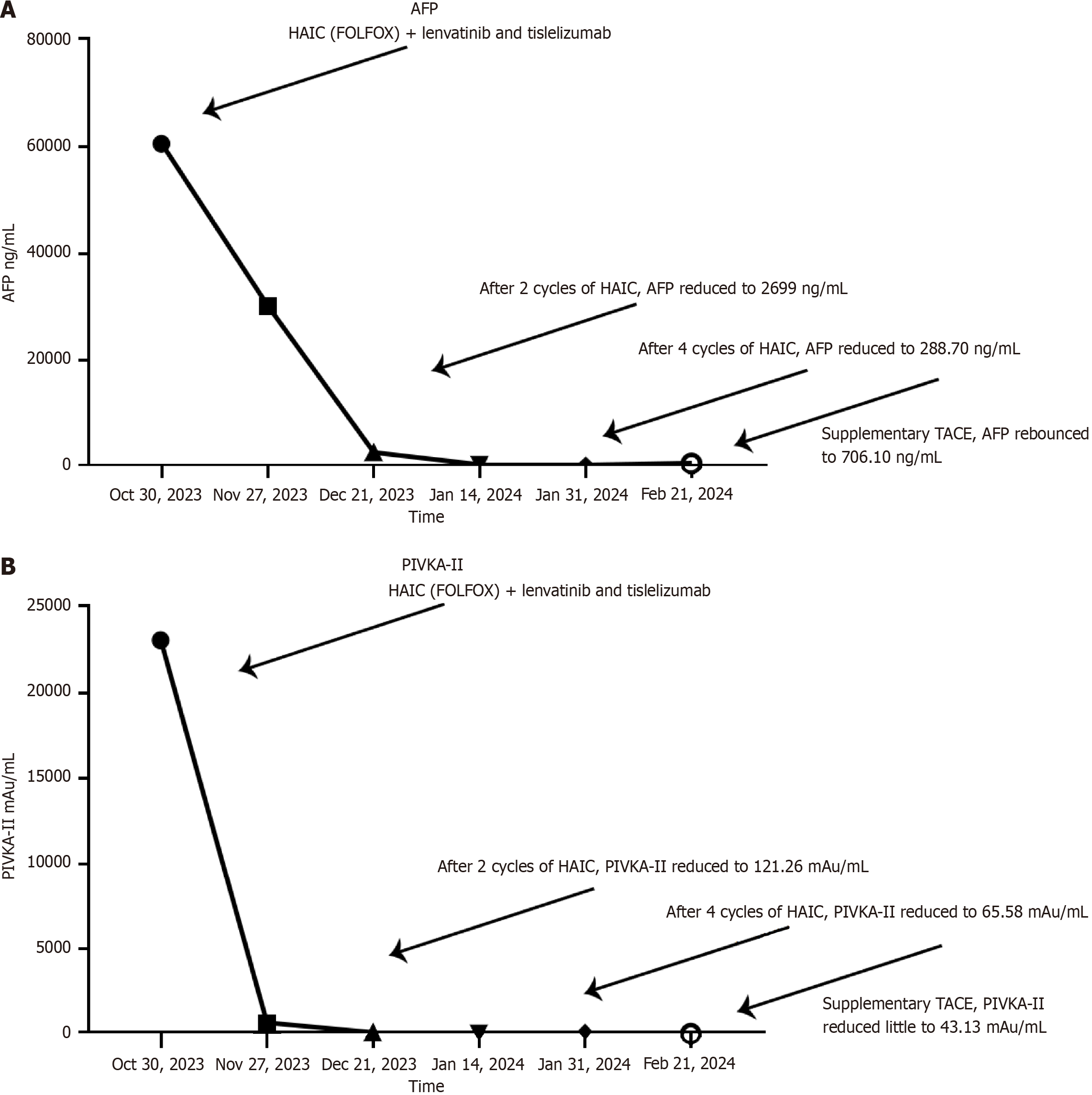
Initial laboratory tests showed the following results: Alanine aminotransferase 28 U/L, aspartate aminotransferase 78 U/L, total bilirubin albumin 16.0 μmol/L, and prothrombin time 12.6 s. Tumor biomarkers were notably elevated with alpha-fetoprotein (AFP) at 60500 ng/mL and Prothrombin Induced by Vitamin K Absence or Antagonism Type II (PIVKA-II) at 23099.79 mAu/mL. Quantitative HBV-DNA testing indicated a viral load of 1.11 × 102 IU/mL.
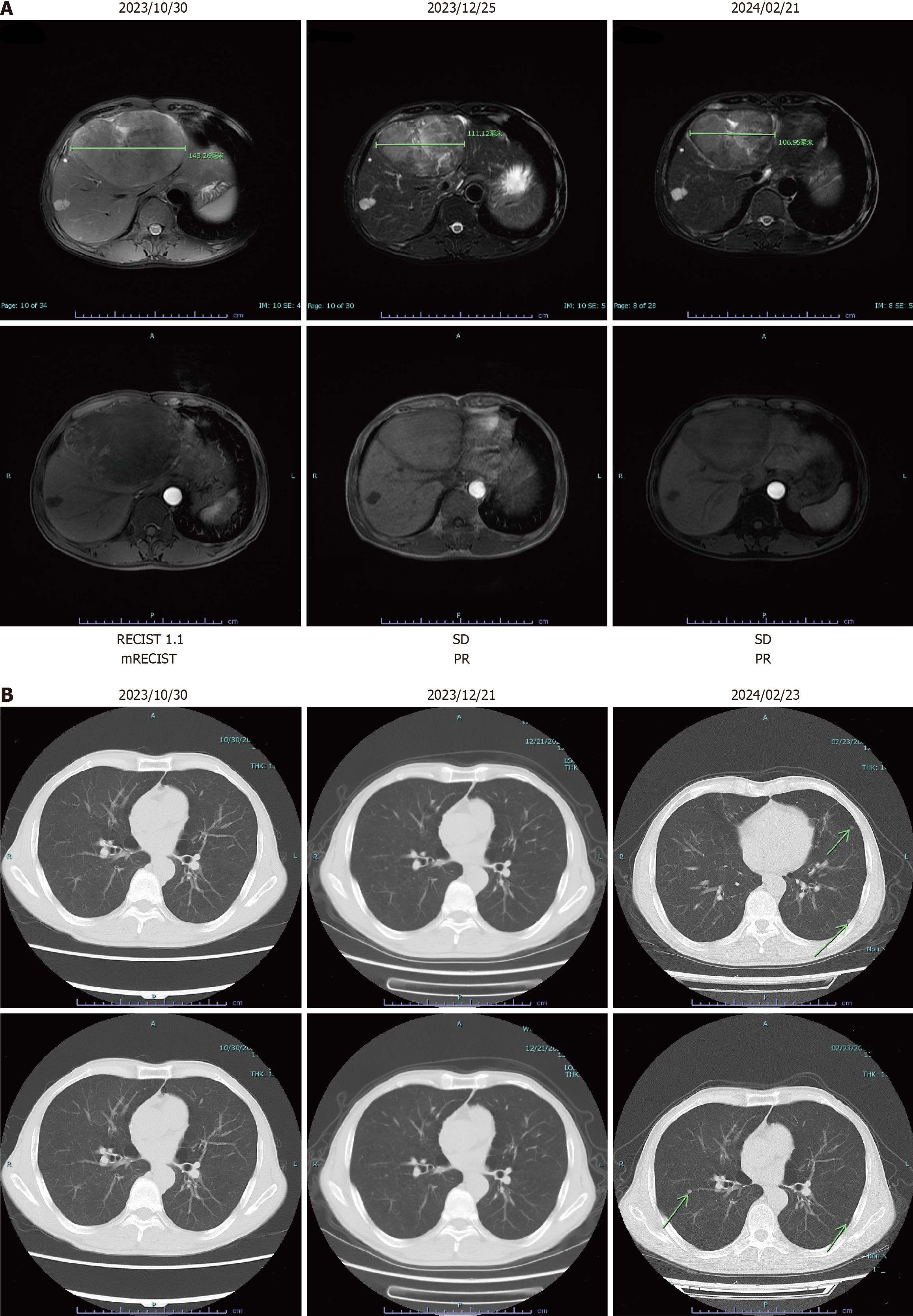
During the first admission, magnetic resonance imaging showed a large mass located at the transition zone between the left and right lobes of the liver, measuring approximately 14 cm (Figure 1).
The conclusive diagnosis for the case was HCC with no pulmonary metastasis (Figure 2).
Considering the extensive tumor burden, surgical resection was deemed potentially unsuitable for the patient. Consequently, a multimodal therapeutic approach was employed. The patient received HAIC followed by treatment with tyrosine kinase inhibitors (TKIs) and ICIs. The HAIC regimen included oxaliplatin at a dose of 85 mg/m2, leucovorin at 400 mg/m2, and intra-arterial 5-fluorouracil at 400 mg/m2 on day 1.
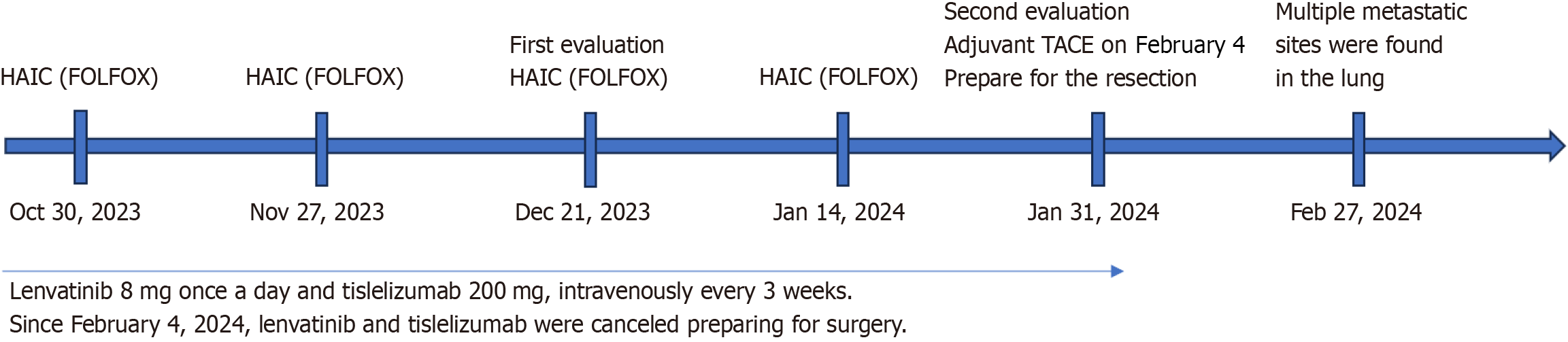
This was followed by a repeated infusion of 5-fluorouracil at a dose of 2400 mg/m2 on days 2–3, with a three-week interval between each treatment cycle. The treatment strategy involved evaluating the patient after every two cycles of HAIC. Initially, on October 30, 2023, the FOLFOX regimen was first administered to the patient. Following nearly three days of infusion chemotherapy, the patient was proposed to commence daily lenvatinib (8 mg) and intravenous tislelizumab 200 mg, an anti-PD-1 monoclonal antibody, every three weeks (Figure 3). Additionally, considering the HBV burden, the patient was prescribed daily antiviral therapy with tenofovir amibufenamide (25 mg). The primary adverse reactions during HAIC treatment included abdominal pain and nausea.
Since February 4, 2024, after four cycles of the planned HAIC, TKIs, and ICIs, TACE was integrated into the adjuvant therapy regimen instead of HAIC to further enhance treatment efficacy. Concurrently, lenvatinib and tislelizumab were temporarily halted in preparation for the upcoming surgical intervention.
After four cycles of therapy, a response evaluation was conducted. Notably, there was a decrease in PIVKA-II levels to 65.58 mAu/mL and AFP levels to 288.70 ng/mL (refer to Figure 4). Radiologic imaging showed partial response (PR) in the lesions (Figure 2A). Based on these improvements in tumor biomarkers and the modified Response Evaluation Criteria in Solid Tumors criteria, the patient was considered potentially eligible for surgical resection due to well-restricted tumor activity. Since February 04, 2024, TACE replaced HAIC in the adjuvant therapy regimen to further enhance treatment effectiveness. Concurrently, lenvatinib and tislelizumab were temporarily suspended in preparation for surgery.
On February 21, 2024, the patient returned to Tongji Hospital for surgical resection. Upon arrival, a comprehensive preoperative assessment was initiated to evaluate his suitability for surgery. Unfortunately, computed tomography imaging revealed multiple metastatic sites in the lungs (Figure 1B), necessitating the cancellation of the planned ope
As previously noted, most HCC patients are diagnosed at intermediate or advanced stages[3], where direct surgical intervention may offer better outcomes than non-surgical approaches in a small, carefully selected subset of patients[10]. However, the high recurrence rate post-surgery indicates that surgery often fails to provide a curative outcome for late-stage patients[11]. Meanwhile, advancements in systemic and locoregional therapies have improved response rates among those with unresectable or advanced HCC[5]. These therapies have enabled tumor downstaging to the point where surgical resection becomes feasible, a strategy known as conversion therapy.
Recent systemic therapies combining TKIs and ICIs have shown favorable effects. Zhu et al[12] reported that among 63 patients initially diagnosed with unresectable liver cancer, 15.9% achieved conversion to resection through combined PD-1 inhibitors and TKI therapy. Disappointing results from monotherapy have steered the trend towards integrating local and systemic therapies in managing advanced HCC. In a randomized controlled trial comparing the efficacy of HAIC and sorafenib vs sorafenib alone in patients with HCC and portal vein invasion, 12.8% of patients in the combination therapy group experienced downstaging post-treatment and underwent radical surgical resection[13]. This reveals the potential of combining HAIC (FOLFOX) with TKIs and PD-1 inhibitors to achieve superior tumor shrinkage and extend survival, making this combination a promising approach for improving outcomes in HCC management.
In this case study, we report on a patient where conversion therapy nearly succeeded using a combined treatment regimen of HAIC with FOLFOX, lenvatinib, and tislelizumab. While this approach effectively restricted tumor growth, lung metastasis unexpectedly developed after the cessation of lenvatinib and tislelizumab in preparation for radical surgery. Recent findings suggest a strong correlation between the duration of targeted therapy in melanoma and reduced risk of disease progression following drug discontinuation[14]. However, the relationship between cessation of targeted therapies and the development of new metastatic sites in HCC remains unclear. Lung metastasis might be part of the natural progression of HCC. This case highlights the urgent need for a revised management strategy that considers the timing of drug discontinuation and addresses the risk of recurrence or disease progression. Currently, there is no con
In conclusion, our case report of a 58-year-old male with HCC who developed lung metastases after the temporary discontinuation of lenvatinib and tislelizumab underscores the complexities in managing advanced HCC. It highlights the challenges in managing advanced HCC and emphasizes the need for ongoing research to optimize treatment strategies and improve patient outcomes.
The authors would like to thank our patient for allowing his case to be presented.
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13326] [Article Influence: 1332.6] [Reference Citation Analysis (4)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56694] [Article Influence: 7086.8] [Reference Citation Analysis (135)] |
| 3. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 1016] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 4. | Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, Zhao M, Bi X, Li G, Bai X, Ji Y, Xu L, Zhu XD, Bai D, Chen Y, Chen Y, Dai C, Guo R, Guo W, Hao C, Huang T, Huang Z, Li D, Li G, Li T, Li X, Li G, Liang X, Liu J, Liu F, Lu S, Lu Z, Lv W, Mao Y, Shao G, Shi Y, Song T, Tan G, Tang Y, Tao K, Wan C, Wang G, Wang L, Wang S, Wen T, Xing B, Xiang B, Yan S, Yang D, Yin G, Yin T, Yin Z, Yu Z, Zhang B, Zhang J, Zhang S, Zhang T, Zhang Y, Zhang Y, Zhang A, Zhao H, Zhou L, Zhang W, Zhu Z, Qin S, Shen F, Cai X, Teng G, Cai J, Chen M, Li Q, Liu L, Wang W, Liang T, Dong J, Chen X, Wang X, Zheng S, Fan J; Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11:227-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 5. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 924] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 6. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5325] [Article Influence: 887.5] [Reference Citation Analysis (29)] |
| 7. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 1051] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 8. | Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, Shao G, Zhang Y, Xu L, Yin T, Liu J, Ren Z, Xiong J, Mao X, Zhang L, Yang J, Li L, Chen X, Wang Z, Gu K, Chen X, Pan Z, Ma K, Zhou X, Yu Z, Li E, Yin G, Zhang X, Wang S, Wang Q. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res. 2021;27:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 427] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 9. | Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol. 2022;40:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 10. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 387] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 11. | Xia YX, Zhang F, Li XC, Kong LB, Zhang H, Li DH, Cheng F, Pu LY, Zhang CY, Qian XF, Wang P, Wang K, Wu ZS, Lyu L, Rao JH, Wu XF, Yao AH, Shao WY, Fan Y, You W, Dai XZ, Qin JJ, Li MY, Zhu Q, Wang XH. [Surgical treatment of primary liver cancer:a report of 10 966 cases]. Zhonghua Wai Ke Za Zhi. 2021;59:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 12. | Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, Chen L, Shi WK, Li ML, Zhu JJ, Tan CJ, Tang ZY, Zhou J, Fan J, Sun HC. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer. 2021;10:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 13. | He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019;5:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (1)] |
| 14. | Lee J, Ahmed T, Maurichi A, Di Guardo L, Stagno AM, Warburton L, Taylor AM, Livingstone E, Rehman S, Khattak A, Kahler KC, Vanella V, Atkinson V, Millward M, Schadendorf D, Johnson DB, Ascierto PA, Hauschild A, Lo SN, Long GV, Menzies AM, Carlino MS. BRAF inhibitor cessation prior to disease progression in metastatic melanoma: Long-term outcomes. Eur J Cancer. 2023;179:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/