INTRODUCTION
Colorectal cancer (CRC) is a significant contributor to cancer-related morbidity and mortality worldwide. In 2020, approximately 1.9 million new cases and around 0.93 million deaths from CRC were reported globally. Projections indicate that by 2040, these figures could escalate to 3.2 million new cases and 1.6 million deaths annually[1]. The incidence of CRC exhibits considerable variation across different geographical regions, a disparity attributed to diverse dietary habits and lifestyle factors[2-4]. Key risk factors include age, genetic predisposition, dietary choices, physical inactivity, and smoking, underscoring the multifactorial nature of this malignancy[5,6].
Despite advancements in surgical techniques and adjunct therapies such as chemotherapy and radiotherapy, overall survival rates for CRC have improved only modestly, particularly in advanced stages of the disease[7-9]. This situation highlights the urgent need for a deeper understanding of the pathophysiological mechanisms that underpin CRC development and progression. Investigating these mechanisms will not only aid in identifying novel therapeutic targets but will also enhance early prevention strategies, potentially improving patient outcomes.
Nitidine chloride (NC), a bioactive benzophenanthridine alkaloid sourced from various plants in the Rutaceae family, has garnered attention for its diverse pharmacological properties[10,11]. While primarily noted for its antimalarial and antibacterial effects[12,13], NC also shows significant potential in oncology. Promising anticancer effects of NC have been observed in several malignancies, including breast cancer[14], liver cancer[15], and leukemia[16], where it primarily induces apoptosis and inhibits cell proliferation. However, the investigation of NC’s efficacy in CRC remains limited. Preliminary studies suggest that NC may impede CRC cell growth[17]; nonetheless, comprehensive research is necessary to clarify its therapeutic potential and mechanisms of action in this specific context. This knowledge gap emphasizes the need for further exploration of NC’s applicability in CRC treatment, with the aim of enhancing therapeutic outcomes and establishing a foundation for clinical application.
The stearoyl-coenzyme A desaturase (SCD) gene, located on chromosome 10q24.31, encodes an enzyme crucial for the biosynthesis of monounsaturated fatty acids from saturated fatty acids[18]. This enzyme significantly influences cellular lipid metabolism, affecting membrane fluidity, signal transduction, and cell proliferation[18-20]. In the realm of oncology, SCD gene expression has been associated with various cancers, including prostate[21], breast[22], and liver cancers[23], where its overexpression correlates with enhanced tumor cell survival and resistance to apoptosis. However, the relationship between SCD expression, NC, and CRC remains poorly understood. This uncertainty highlights the necessity for thorough studies to elucidate the connection between SCD gene expression, NC, and CRC pathogenesis.
In this study, we utilized multi-center high-throughput datasets to assess the standardized mean difference (SMD) of SCD mRNA expression in CRC. To determine the relevance of SCD in CRC, we employed immunohistochemistry (IHC), Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) knockout screening, and single-cell sequencing. We calculated parameters including sensitivity, specificity, and likelihood ratios using pooled receiver operating characteristic (ROC) curves. Furthermore, we explored the molecular interactions of NC against CRC through Swiss target predictions, functional enrichment analyses, and molecular docking, with the goal of uncovering novel therapeutic strategies.
MATERIALS AND METHODS
Differential expression of SCD in CRC
Evaluation of SCD mRNA expression in CRC using high-throughput data: A comprehensive analysis was conducted to assess the differential expression of SCD mRNA in CRC tissues compared to non-CRC tissues. The data were obtained from various repositories, including the Gene Expression Omnibus (GEO), Genotype-Tissue Expression project, The Cancer Genome Atlas, ArrayExpress, and the Sequence Read Archive. The search incorporated keywords such as “colon”, “rectal”, “colorectal”, “carcinoma”, “cancer”, “malignant”, and “tumor”. Inclusion criteria required human samples with accessible SCD expression data, comprising at least three samples of both CRC tissues and corresponding non-tumor controls. Samples were excluded if they had undergone pre-treatment with drugs, radiation, or genetic modifications. Furthermore, groups with fewer than three samples, those without SCD expression data, and samples classified as metastatic or recurrent CRC were also excluded from the analysis. During data processing, datasets from identical GEO platforms were merged to create a unified matrix. The mRNA expression data were normalized and transformed to a logarithmic scale using the log2(x + 1) method, while batch effects were mitigated using the ‘limma’ and ‘sva’ packages in R software.
Examination of differential SCD protein expression in CRC using IHC: This study employed tissue microarrays consisting of 208 paired samples of CRC and adjacent non-tumor tissues to investigate SCD protein expression in CRC. The tissue microarrays were sourced from Yulin Red Cross Hospital, with patients having not received any preoperative treatments. A thorough set of clinicopathological data was collected, including age, sex, macroscopic classification, vascular invasion, nerve invasion, lymph node invasion, tumor-node-metastasis classification, survival status, and clinical stage. Ethical approval was granted by both Yulin Red Cross Hospital and the First Affiliated Hospital of Guangxi Medical University. For histological examination, 2 μm thick sections were sliced from paraffin-embedded blocks and heated at 75 °C for 20 minutes to remove paraffin. Following dewaxing, sections were treated with 3% H2O2 for 15 minutes, washed with distilled water, and phosphate-buffered saline (PBS). Immunostaining was performed using a polyclonal rabbit anti-SCD antibody, followed by PBS washes. The sections were then counterstained, dehydrated, cleared, and mounted. Adjacent non-cancerous tissue sections served as positive controls, while PBS was used as the negative control. IHC analysis was carried out on both CRC and adjacent non-cancerous tissues, integrating the collected data. Two independent pathologists, blinded to the sample origins, evaluated the stained slides. Evaluation criteria included the percentage of cells showing positive staining and the intensity of the staining. The scoring for the percentage of positively stained cells was categorized as follows: 0 for no expression, 1 for less than 10% positive cells, 2 for 10%-35% positive cells, 3 for 36%-75% positive cells, and 4 for more than 75% positive cells. Staining intensity was rated from 0 for no staining to 3 for intense brown-yellow staining[24-26]. The overall score was calculated by multiplying the staining intensity by the percentage of positively stained cells. In cases of discrepancies between the evaluations of the two pathologists, the average of their scores was used as the definitive score.
Appraisal of SCD expression in single-cell analysis of CRC samples: Single-cell RNA sequencing data from the GEO dataset GSE20097 were analyzed to explore the single-cell expression patterns of the SCD gene in CRC. The cell selection criteria specified that cells should possess between 200 and 2500 RNA features, with mitochondrial DNA content below 5%, utilizing the Seurat package for analysis. Normalization of the data was performed using the NormalizeData function. To account for batch effects introduced by varying sequencing technologies, the Harmony package was employed. Principal component analysis was conducted using the FindVariableFeatures function, with a resolution parameter set to 0.5. Dimensionality reduction was then executed using UMAP, analyzing dimensions 1 through 20. The results from the principal component analysis were visualized in a two-dimensional plot, effectively highlighting the variations in SCD expression between normal and CRC cells.
Investigating the role of SCD in regulating CRC cell proliferation: The involvement of SCD in CRC cells was assessed utilizing CRISPR knockout screening technology. The significance of SCD across various CRC cell lines was evaluated by calculating dependency scores with the CERES algorithm. A negative dependency score, indicating impaired growth post-SCD knockout, emphasizes its necessity for cell proliferation. Conversely, a positive dependency score, wherein SCD knockout results in enhanced growth, suggests a potential inhibitory role for SCD in these cell lines[27].
Evaluation of targeted strategies in CRC
Analysis of NC targets in CRC: To elucidate the mechanisms of NC in CRC treatment, this study utilized SwissTargetPrediction to identify potential targets of NC. Overexpressed genes (OEGs) in CRC were defined based on three criteria: (1) Presence in at least three separate studies; (2) A SMD greater than zero; and (3) A 95% confidence interval (CI) that excludes zero. The potential NC targets were then intersected with this set of CRC OEGs to pinpoint genes common to both groups. These intersected genes underwent Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses to further elucidate their biological functions and pathways.
Molecular docking of targeted SCD for CRC: The investigation identified potential therapeutic agents targeting SCD for CRC treatment through molecular docking between SCD and NC. The SCD crystal structure (PDB ID: 4ZYO) was sourced from the RCSB Protein Data Bank (RCSB PDB). Solvent molecules and co-crystallized ligands were removed from the SCD structure using PyMOL 2.4. Active sites of SCD were predicted using POCASA 1.1. The NC structure was retrieved from the PubChem database, with both SCD and drug structures prepared using AutoDockTools. Molecular docking was performed utilizing AutoDock Vina 1.5.7, with lower binding energy indicating a more stable interaction between NC and SCD’s active site. The visualization of the molecular docking model allowed for further analysis of this interaction.
Statistical analysis
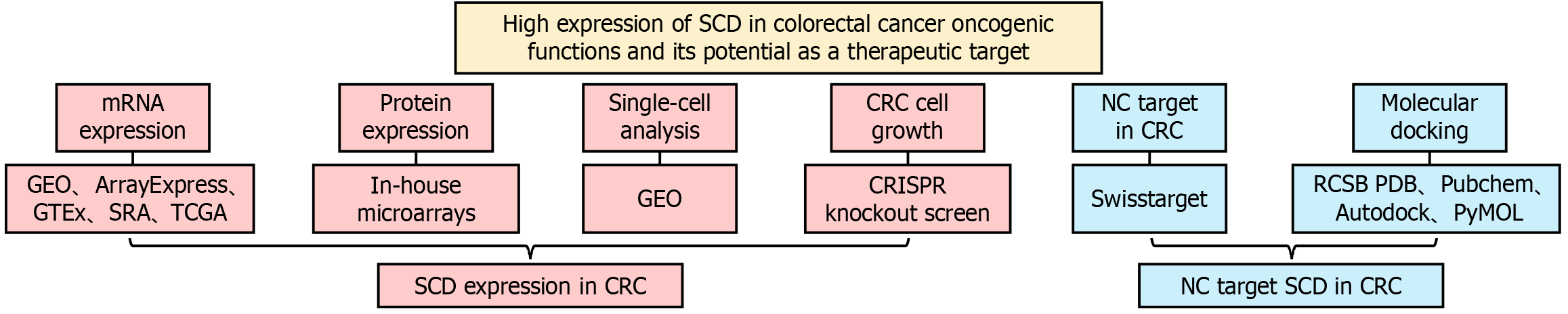
Given the inherent instability and randomness often associated with analyses based on limited or singular samples, a thorough evaluation was conducted. This evaluation incorporated mRNA microarray and sequencing data to characterize the typical expression pattern of SCD in CRC relative to non-cancerous colorectal tissue. The Wilcoxon test, conducted using the Statistical Package for the Social Sciences (SPSS) version 22.0, assessed the expression differences of SCD between CRC and non-tumor tissues. Differences were visualized using the ggplot2 package, with statistical significance established at P < 0.05. Moreover, SMD and 95%CI were calculated using STATA 12.0 software. The selection between a random effects model and a fixed effects model was guided by I² and χ2 tests, opting for the former in instances of high heterogeneity (I² > 50% and P < 0.05). Begg’s and Egger’s tests were employed to evaluate publication bias in the integrated analysis, with P > 0.05 indicating no significant bias. Additionally, the correlation between SCD protein expression and clinicopathological characteristics was examined using independent-samples t-tests or ANOVA. The design of this study is illustrated in Figure 1.
Figure 1 Overview of study design.
SCD: Stearoyl-coenzyme A desaturase; CRC: Colorectal cancer; NC: Nitidine chloride; GEO: Gene Expression Omnibus; GTEx: Genotype-Tissue Expression; SRA: Sequence Read Archive; TCGA: The Cancer Genome Atlas.
RESULTS
Increased SCD mRNA levels observed in CRC
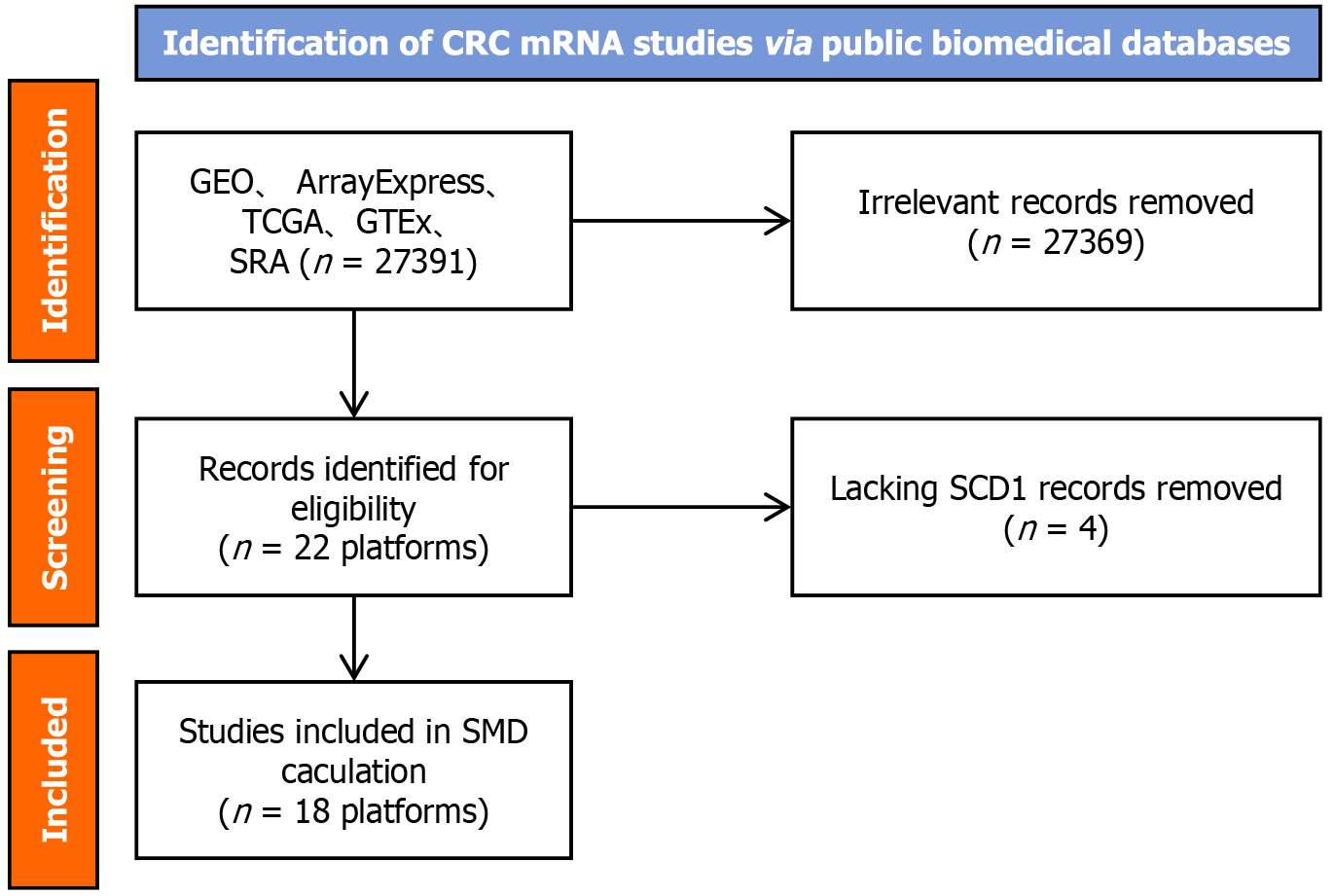
The evaluation of extensive datasets from microarray and sequencing analyses on a global scale indicated elevated SCD mRNA levels in CRC. As illustrated in Figure 2, the selection process for the SCD mRNA dataset encompassed 18 distinct platforms. This analysis included a substantial sample size of 2482 CRC cases and 1334 non-CRC cases, thereby enabling a thorough assessment of SCD expression in CRC.
Figure 2 Schematic representation of the screening procedure for the stearoyl-coenzyme A desaturase mRNA dataset.
CRC: Colorectal cancer; SCD: Stearoyl-coenzyme A desaturase; GEO: Gene Expression Omnibus; GTEx: Genotype-Tissue Expression; SRA: Sequence Read Archive; TCGA: The Cancer Genome Atlas; SMD: Standardized mean difference.
Upregulation of SCD mRNA expression in CRC
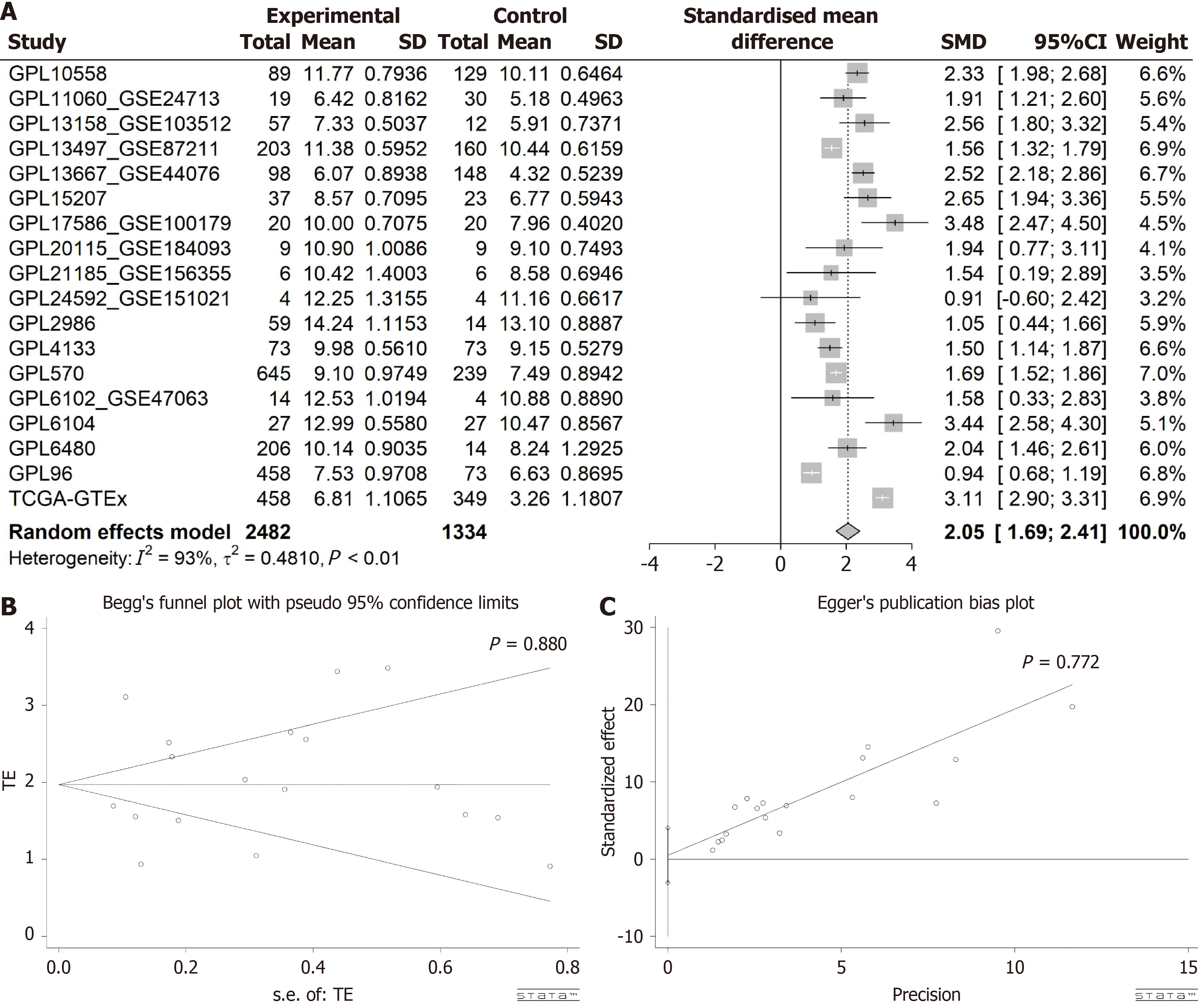
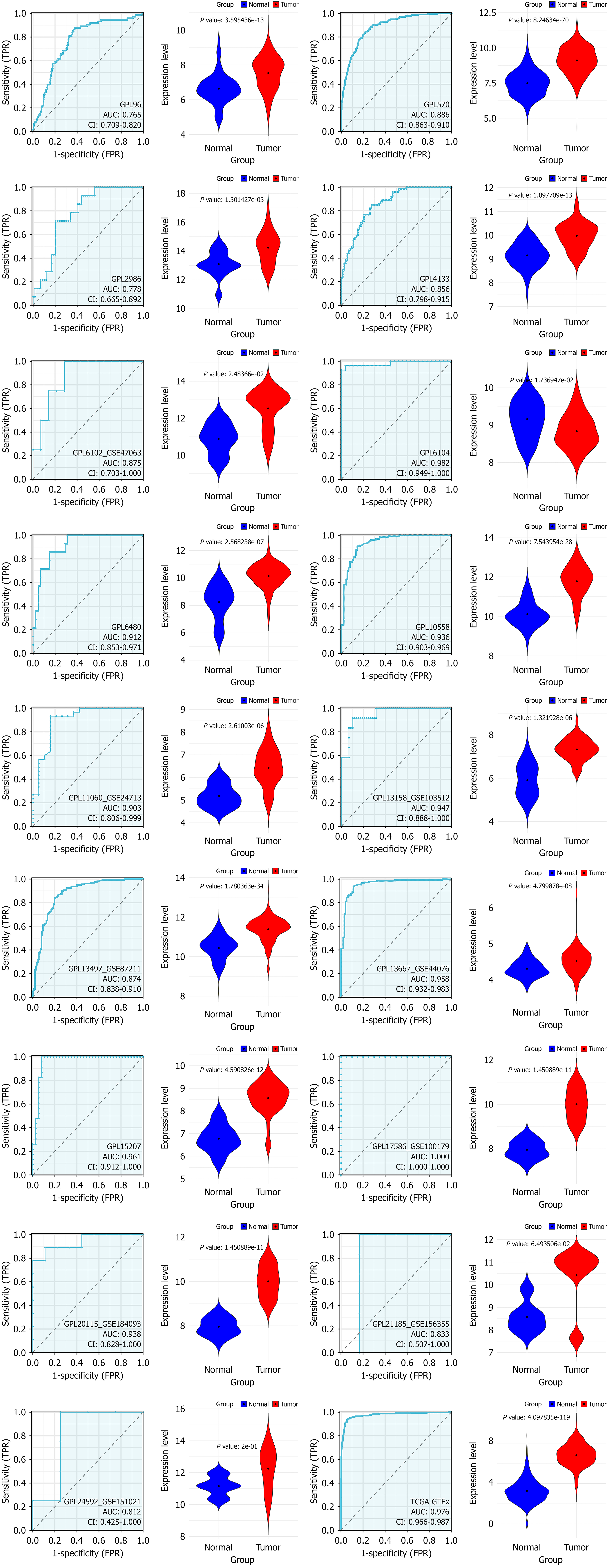
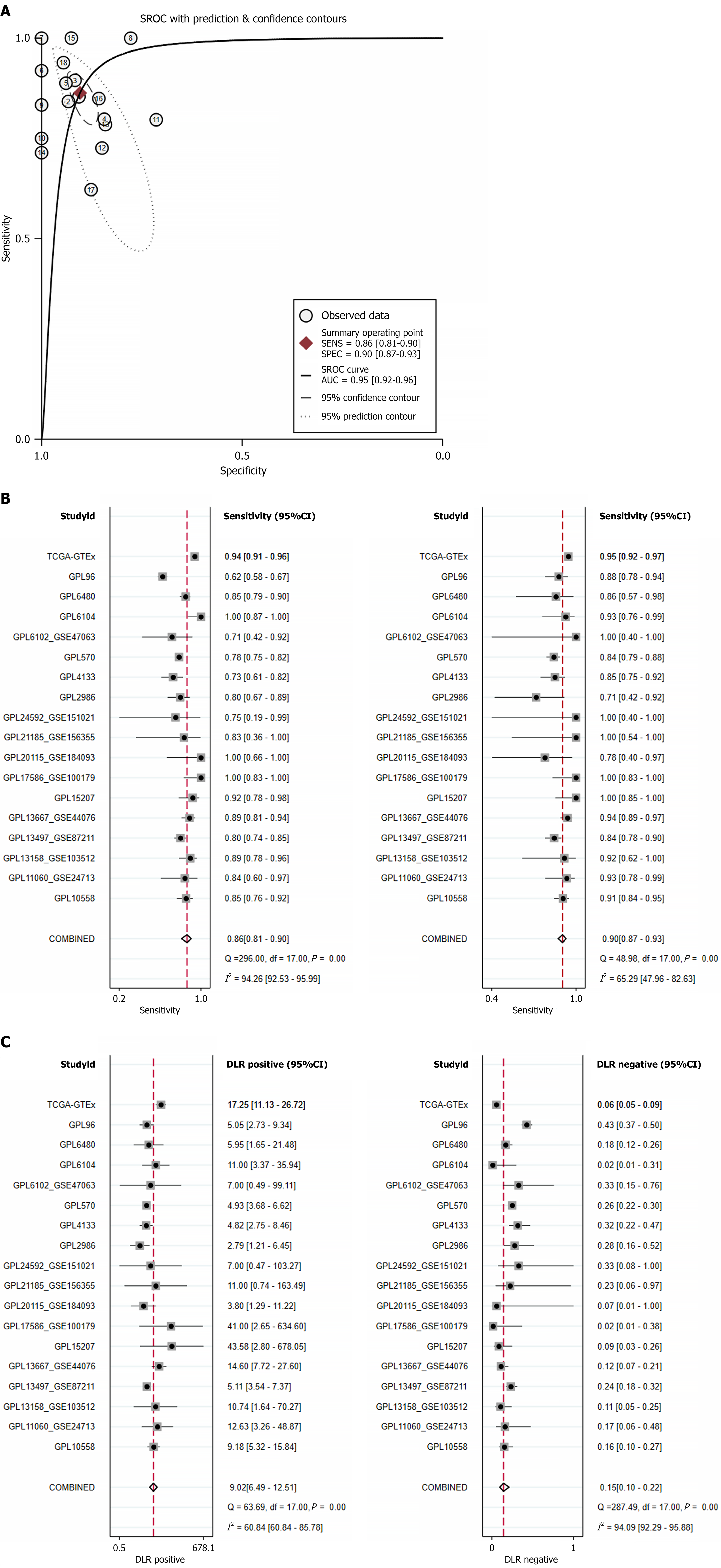
Utilizing the Wilcoxon test, a comparison of SCD expression levels between CRC and normal colorectal samples revealed a significant increase in SCD expression within CRC. Analysis, incorporating data from various sources and evaluated using a random effects model, demonstrated this elevation (SMD = 2.05, 95%CI: 1.69-2.41, I² = 93%, P < 0.01), as depicted in Figure 3A. Moreover, Begg’s and Egger’s tests indicated no evidence of publication bias (P = 0.880 and 0.772, respectively), as shown in Figure 3B and C. The increase in SCD mRNA expression was consistently confirmed across 15 different platforms, exhibiting significant differences (P < 0.05, area under the curve ≥ 0.7), illustrated in Figure 4.
Figure 3 Evaluation of stearoyl-coenzyme A desaturase expression in colorectal cancer.
A: Forest plot depicting elevated stearoyl-coenzyme A desaturase expression in colorectal cancer vs normal colorectal tissue; B and C: Outcomes of Egger’s and Begg’s tests indicating absence of publication bias. GTEx: Genotype-Tissue Expression; TCGA: The Cancer Genome Atlas; SMD: Standardized mean difference; CI: Confidence interval.
Figure 4 Violin plots illustrating differences in stearoyl-coenzyme A desaturase expression between colorectal cancer samples (red) and non-colorectal cancer samples (blue) across chosen datasets.
Statistical significance confirmed at P < 0.05 (continued). TPR: True positive rate; FPR: False positive rate.
SCD demonstrates high discriminatory potential in CRC
The ability of SCD to distinguish CRC from normal colorectal tissue was evaluated through the summary ROC curve. The results indicated an area under the curve of 0.95 (95%CI: 0.92-0.96), with a sensitivity of 0.86 (95%CI: 0.81-0.90) and specificity of 0.90 (95%CI: 0.87-0.93), as depicted in Figure 5A and B. The positive diagnostic likelihood ratio reached 9.02 (95%CI: 6.49-12.51, I² = 60.84%), while the negative diagnostic likelihood ratio was measured at 0.15 (95%CI: 0.10-0.22, I² = 94.09%), as illustrated in Figure 5C. Both the summary ROC curve and the forest plot analysis underscore the strong capability of SCD to effectively differentiate CRC from normal colorectal tissue.
Figure 5 Comprehensive assessment of stearoyl-coenzyme A desaturase expression correlation with colorectal cancer outcomes.
A: Summary receiver operating characteristic curve; B: Sensitivity and specificity; C: Positive diagnostic likelihood ratio and negative diagnostic likelihood ratio. GTEx: Genotype-Tissue Expression; TCGA: The Cancer Genome Atlas; CI: Confidence interval; SROC: Summary receiver operating characteristic curve; AUC: Area under the curve.
Comparative evaluation of SCD protein levels in CRC vs adjacent non-cancerous tissues
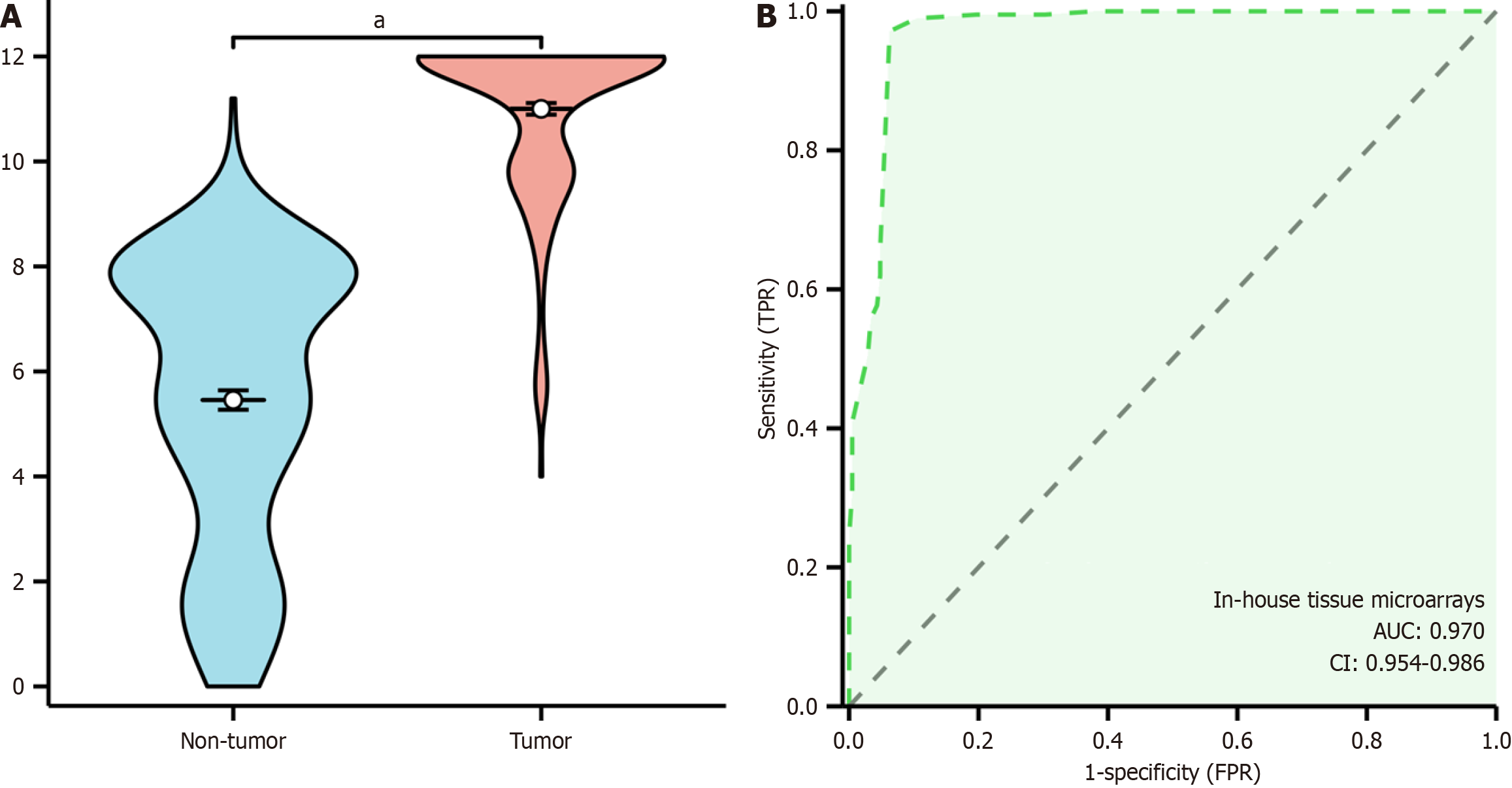
This study evaluated SCD protein expression levels in CRC compared to adjacent non-cancerous tissues. IHC revealed minimal to weak SCD protein staining in non-cancerous tissues (Figure 6A), whereas CRC tissues exhibited moderate to strong staining (Figure 6B). Quantitative assessments of SCD expression in CRC employed violin plots and ROC curves, with the ROC analysis indicating a significant elevation of SCD in CRC tissues, achieving an area under the curve of 0.962 (95%CI: 0.943-0.981), detailed in Figure 7A. This analysis confirmed a markedly higher expression of SCD protein in CRC tissues compared to adjacent non-cancerous tissues, with statistical significance (P < 0.001), as shown in Figure 7B. Additionally, the study examined the correlation between SCD protein levels and clinical-pathological characteristics of CRC patients through independent-samples t-tests and ANOVA, revealing a significant association between SCD protein expression and vascular invasion (P < 0.001).
Figure 6 Stearoyl-coenzyme A desaturase protein expression in adjacent non-tumor tissue of colorectal cancer and colorectal cancer tissue.
A: Stearoyl-coenzyme A desaturase protein expression in adjacent non-tumor tissue of colorectal cancer; B: Stearoyl-coenzyme A desaturase protein expression in colorectal cancer tissue. Left side of the image: Tissue image at 500 μm, middle side: Tissue image at 200 μm, right side: Tissue image at 50 μm.
Figure 7 Elevated expression of stearoyl-coenzyme A desaturase protein in colorectal cancer tissue.
A: Violinplot; B: Receiver operating characteristic curve. TPR: True positive rate; FPR: False positive rate; CI: Confidence interval; AUC: Area under the curve. aP < 0.001.
Elevated SCD expression in CRC in single-cell analysis
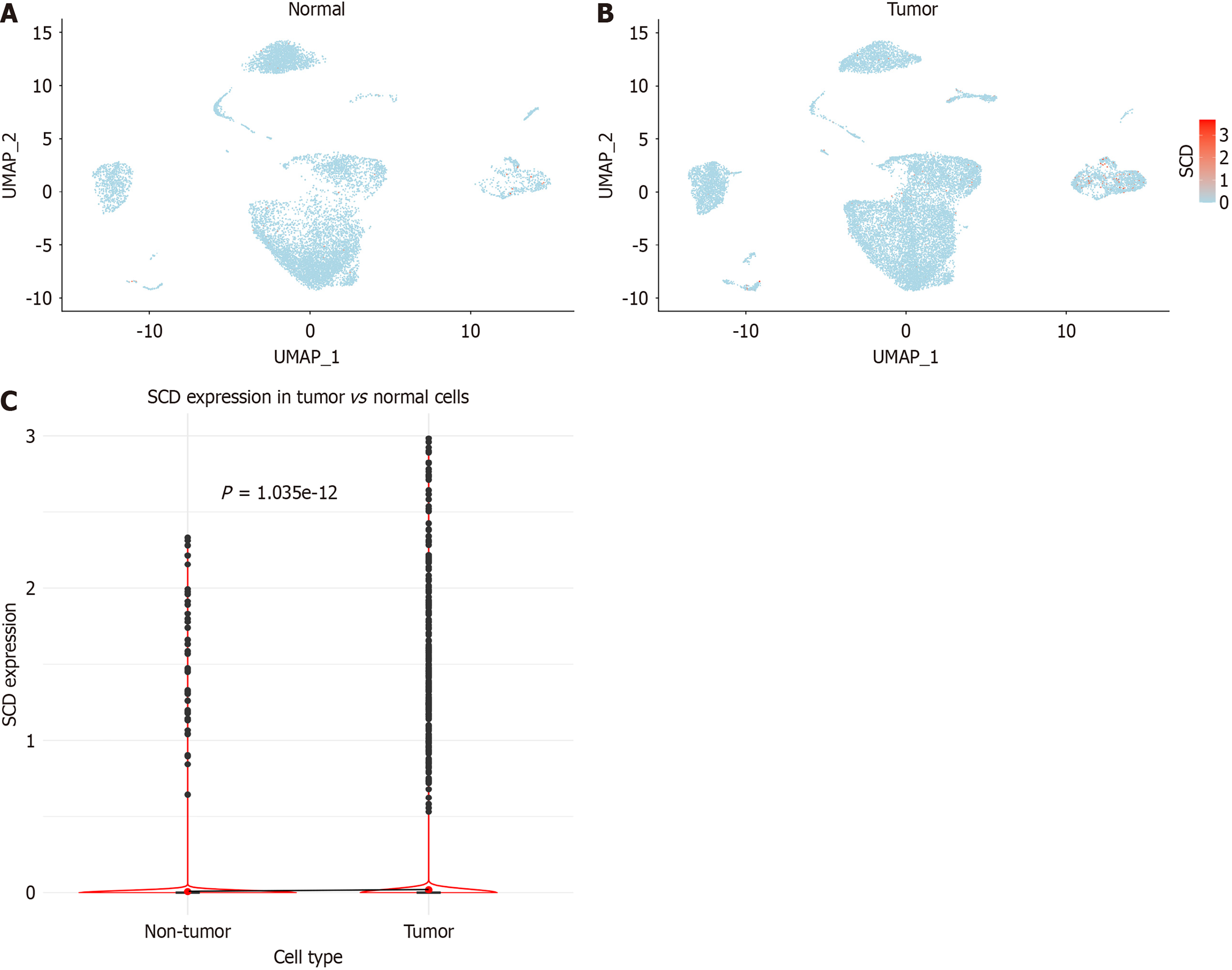
After implementing stringent quality control measures, a total of 18108 cells were isolated from CRC samples, compared to 9901 cells from normal colorectal tissues (Figure 8A and B). Further analysis indicated a significant elevation in SCD expression within single cells derived from CRC tissues, achieving statistical significance (P < 0.001), as highlighted in Figure 8C.
Figure 8 Analysis of stearoyl-coenzyme A desaturase expression in single cells.
A: Distribution within colorectal cancer samples; B: Distribution in normal colorectal samples; C: Comparative analysis of stearoyl-coenzyme A desaturase expression between colorectal cancer and normal colorectal cells. SCD: Stearoyl-coenzyme A desaturase.
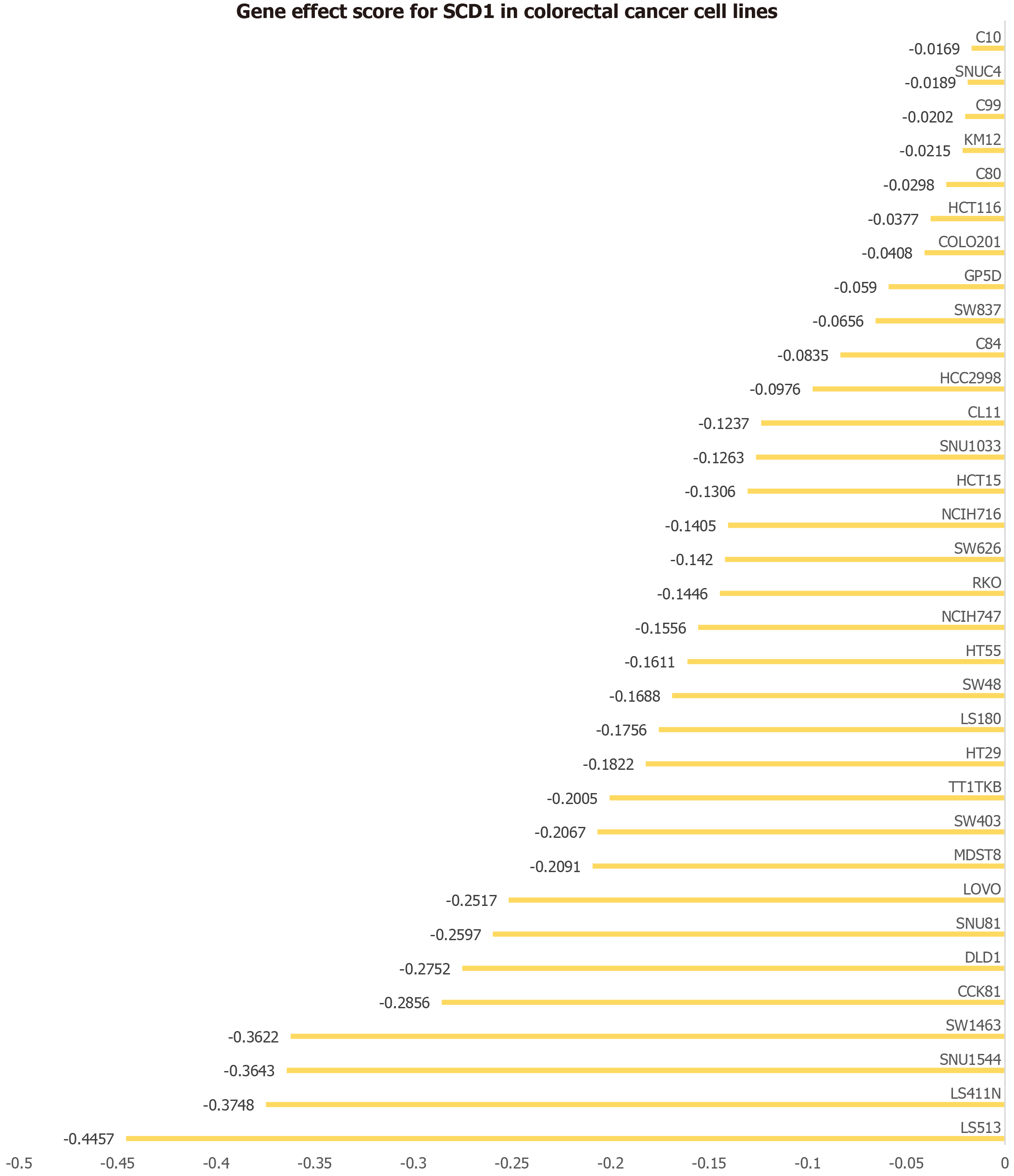
SCD-induced promotion of CRC cell proliferation
The role of SCD in CRC cell line proliferation was investigated using CRISPR knockout screening (Figure 9). The disruption of the SCD gene resulted in reduced growth rates across 33 different CRC cell lines, suggesting a potential role for SCD in promoting cell proliferation in CRC.
Figure 9 Impact of stearoyl-coenzyme A desaturase gene on 33 colorectal cancer cell lines.
The horizontal axis depicts the effect score of the stearoyl-coenzyme A desaturase gene for each cell line, while the vertical axis lists the various colorectal cancer cell lines. SCD: Stearoyl-coenzyme A desaturase.
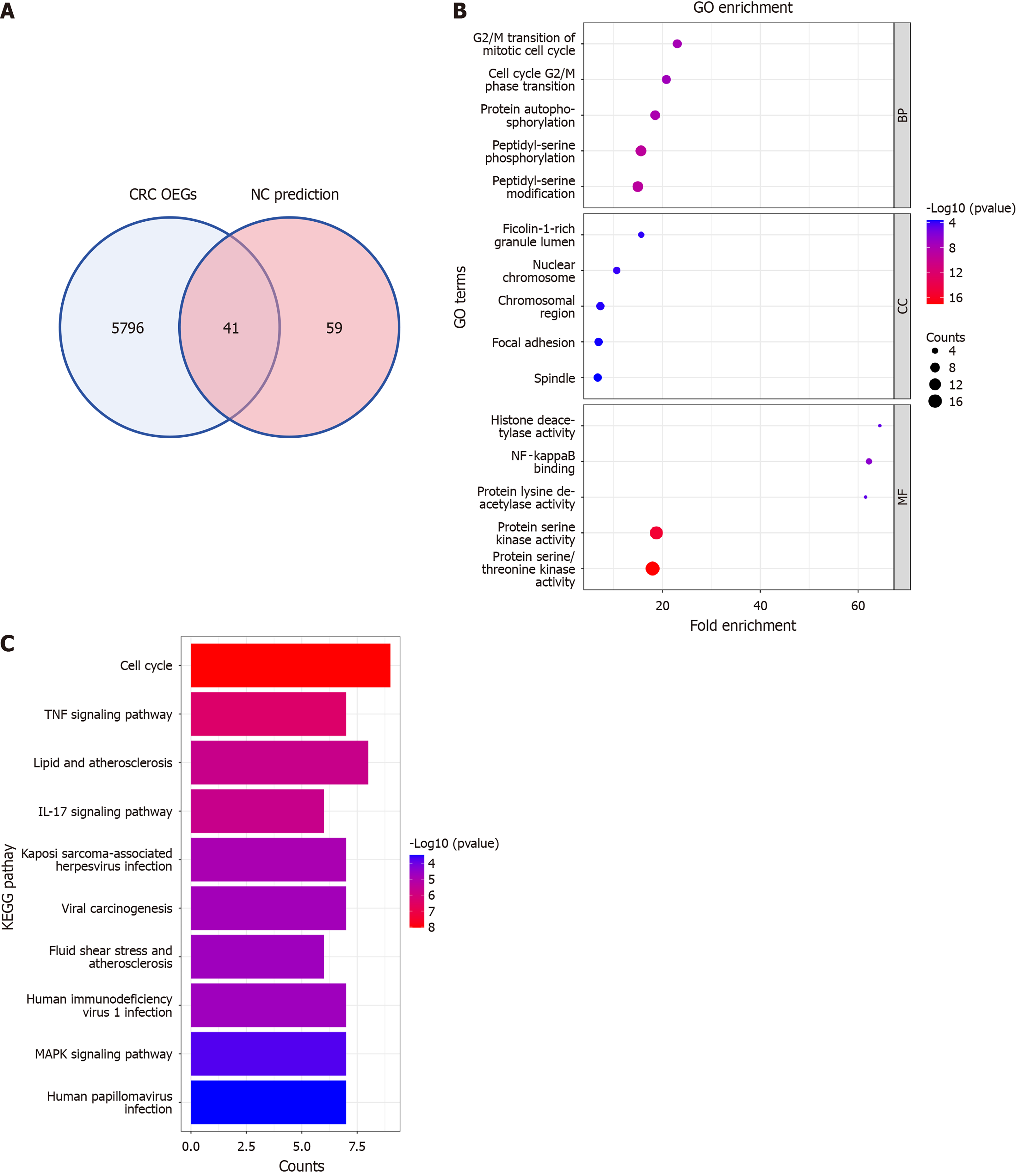
Potential mechanisms for NC to combat CRC
Investigations into the mechanisms through which NC counteracts CRC were conducted utilizing both SMD and NC target predictions. Analyses of CRC specimens identified 5837 OEGs and 100 candidate expressed genes related to these predictions, with 41 genes common to both groups. Further assessments of these shared genes involved GO and KEGG pathway enrichment analyses, presented in Figure 10A. The GO analysis revealed significant enrichments in biological process such as peptidyl-serine phosphorylation and peptidyl-serine modification; in the cell composition category, notable enrichments were observed in chromosomal regions and focal adhesions; the molecular function analysis demonstrated significant activities in protein serine kinase and protein serine/threonine kinase, as elaborated in Figure 10B. Additionally, KEGG pathway analysis highlighted critical pathways, including the cell cycle, tumor necrosis factor signaling pathway, as well as pathways associated with lipid metabolism and atherosclerosis, as shown in Figure 10C.
Figure 10 Investigating molecular mechanisms of nitidine chloride against colorectal cancer.
A: Identification of shared genes via colorectal cancer overexpressed genes and nitidine chloride target prediction overlap; B: Functional enrichment analysis of shared genes using Gene Ontology; C: Pathway analysis of shared genes using Kyoto Encyclopedia of Genes and Genomes. CRC: Colorectal cancer; OEG: Overexpressed gene; NC: Nitidine chloride; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes.
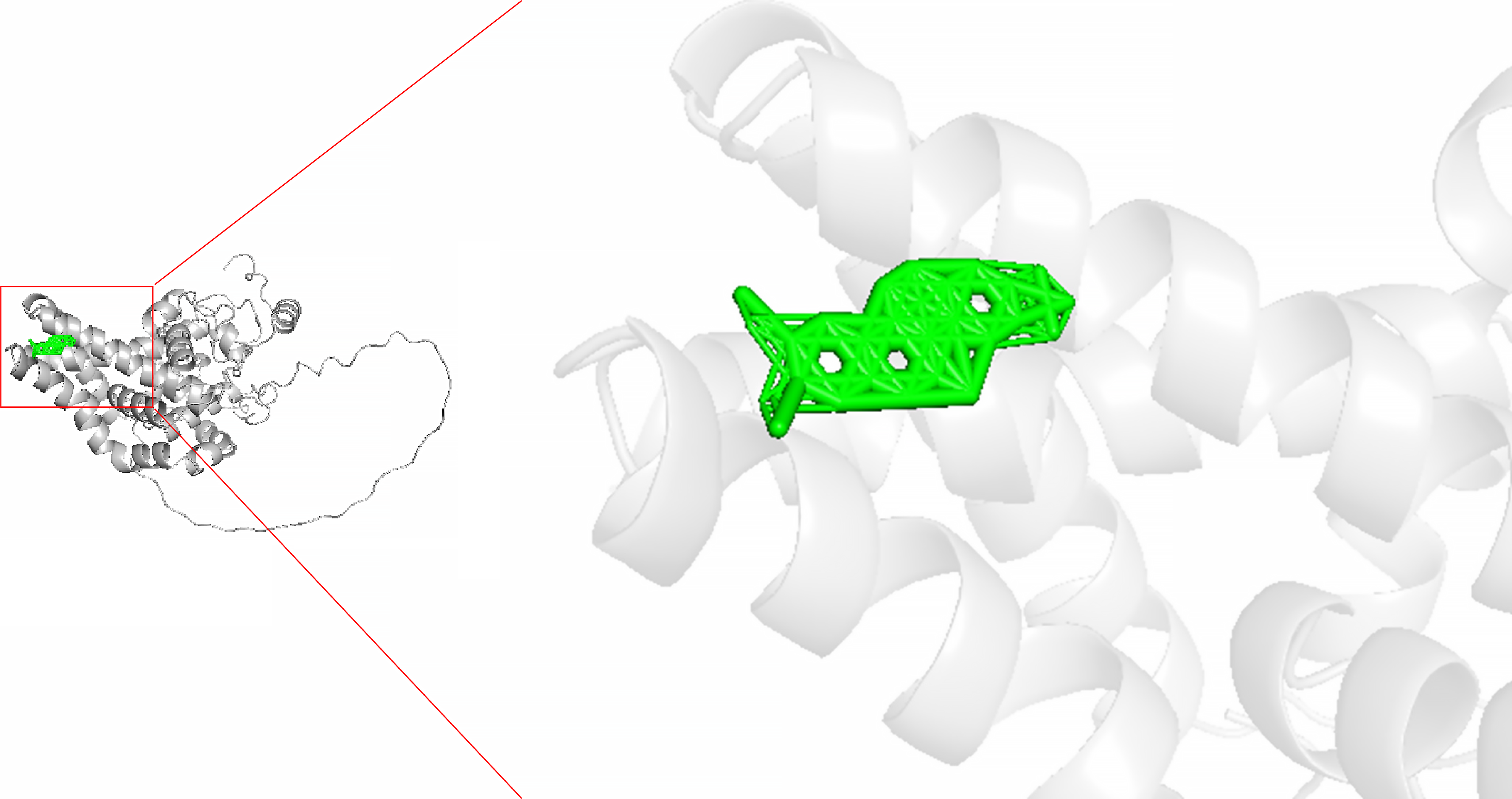
NC specifically targets SCD in CRC
Molecular docking studies were employed to assess the interactions between NC and SCD, aiming to explore the inhibitory effects of NC on SCD. The analyses indicated that NC serves as a potent inhibitor of SCD, with the results revealing the absence of hydrogen bonds between NC and SCD, as illustrated in Figure 11.
Figure 11
The molecular docking of nitidine chloride and stearoyl-coenzyme A desaturase (affinity energy: -8.8 kcal/mol).
DISCUSSION
Despite the increasing interest in the molecular underpinnings of CRC, the precise role of SCD in its pathogenesis remains uncertain. Our study addressed this significant gap in our knowledge by seeking to elucidate the role of SCD in CRC. Our findings highlight the substantial upregulation of SCD in CRC tissues compared to non-cancerous colorectal tissues, suggesting its involvement in cancer development and progression. The findings also emphasized the utility of SCD not only as a biomarker for CRC detection due to its distinct expression profiles but also as a target with therapeutic potential. The association of SCD expression with key pathological features, such as vascular invasion, indicates its role in the aggressiveness of CRC. This link suggests that SCD might influence tumour behaviour through mechanisms associated with cancer invasiveness and metastatic potential. Furthermore, the dependency of multiple CRC cell lines on SCD for growth underscores its importance in cancer cell survival and proliferation. In proposing that targeting SCD could interrupt critical cell cycle pathways, our study opens new avenues for therapeutic interventions in CRC. These insights provide a foundation for future research into targeted therapies inhibiting SCD activity, which may alter CRC treatment paradigms significantly and improve patient outcomes.
Furthermore, in this study, we have expanded the discussion on SCD beyond CRC to also include its roles in other diseases. Notably, SCD has been studied in various non-oncological conditions such as diabetes, obesity, and cardiovascular diseases, where its role in metabolic regulation is significant[28-30]. Meanwhile, in oncology, SCD has been implicated in breast cancer, pancreatic cancer, and prostate cancer, suggesting its broad relevance across cancer types[31-33]. However, these studies have often been subject to limitations, including small sample sizes, which could skew our understanding of SCD’s full biological impact. In this case, the comprehensive analysis in the present study was limited to CRC, but we sought to apply a well-designed methodology that removed other limitations. Accordingly, our analysis was conducted across 18 distinct platforms, for which we observed an elevated expression of SCD in CRC tissues compared to non-cancerous tissues. This finding was corroborated by further analyses at both the mRNA and protein levels, CRISPR knockout screening, and single-cell studies, collectively confirming the significant upregulation of SCD at the cellular level in CRC.
NC has increasingly been recognised in oncological studies for its anticancer properties. In particular, NC has shown significant efficacy in suppressing tumour growth in hepatocellular carcinoma by inducing apoptosis and inhibiting cell proliferation[34-36]. Similarly, studies in breast cancer have revealed that NC disrupts the hedgehog pathway, leading to cell cycle arrest and a reduced metastatic potential[37]. These observations provide a foundational understanding of NC’s broad-spectrum anti-tumour activity, suggesting its utility as a versatile anticancer agent. Meanwhile, we know that the enzyme SCD is critical in lipid metabolism, thus influencing the cell membrane composition and fluidity, which are essential for cell signalling and the maintenance of cellular homeostasis[38,39]. Against this background, the current findings suggest that NC may exert anticancer effects by altering SCD activity, thereby affecting cell cycle dynamics. The cell cycle comprises a complex series of events that lead to cell division and replication[40,41], and regulation of the cell cycle is crucial for maintaining cellular integrity and functioning[42]. In cancer, this regulation is often disrupted, leading to uncontrolled cell proliferation. In particular, research indicates that SCD impacts the cell cycle by modulating lipid-based signalling pathways that govern the G0/G1 transition[43,44]. The implication of these findings is significant, as they suggest that SCD could offer a potential therapeutic target for NC in the treatment of CRC. On this topic, our study adds to the growing body of evidence that NC can mediate the therapeutic effects of SCD through modulation of the cell cycle. By influencing SCD, NC likely alters lipid-based signalling pathways, leading to a disruption in cell cycle progression at critical checkpoints. This disruption is hypothesised to hinder the proliferation of CRC cells, thus contributing to the observed anticancer effects of NC. Specifically, the interaction between NC and SCD in CRC not only disrupts cell cycle progression but also points to a mechanism by which NC can be strategically used to target cancer cell growth and survival. This highlights the potential of NC as a targeted therapy for CRC, providing a promising pathway for future research and clinical applications.
In this space, molecular docking offers a computational approach whereby we can simulate the interaction between two or more molecules to predict the orientation of one molecule in relation to another when they are bound to form a stable complex[45,46]. The utility of this technique spans various fields including drug design and molecular biology, where it helps elucidate the binding affinities and interaction mechanisms of drug candidates with their respective targets[47,48]. It is especially valuable in predicting the efficacy of novel therapeutic compounds prior to experimental validation, thus potentially reducing the cost and duration of drug development[49]. In this study, molecular docking analyses demonstrated that NC exhibits a significant binding affinity to SCD, as indicated by the notably high negative binding energy values. This strong, spontaneous interaction between NC and SCD’s active site may suggest the potential of NC as a potent inhibitor, targeting key molecular pathways involved in the progression of CRC. Importantly, this predicted interaction could form the basis for the observed therapeutic efficacy of NC in our preclinical models, hinting at its potential to disrupt critical cancer cell functions. Furthermore, the theoretical underpinnings uncovered by the docking study deepen our understanding of the molecular interactions involved[50]. In particular, as a result of work to identify specific interaction sites and characterise the nature of these interactions, researchers may strategically modify NC to improve its binding efficiency and selectivity. Such insights are also instrumental in tailoring drug molecules to better target pathological pathways specifically associated with CRC, thereby improving therapeutic outcomes and minimising off-target effects[51]. Our study underscores the importance of integrating computational methods like molecular docking into the drug discovery process, providing a robust prelude to experimental drug testing[52]. By leveraging these computational insights, the development of targeted therapies for cancer treatment can be accelerated, ensuring that promising compounds like NC are efficiently optimised.
In conclusion, the overexpression of SCD in CRC tissues indicates its potential role as an oncogene in the development and progression of CRC. The molecular docking of NC with SCD provides valuable insights for CRC therapy. While our study provides valuable insights, it is important to acknowledge its limitations. Primarily, this research relies on cross-sectional data, which restricts our ability to observe the temporal dynamics of SCD expression and its impact on cancer progression and treatment response. To overcome these limitations, future research plans will encompass longitudinal studies in CRC patients. These studies aim to track SCD expression over time, offering a more comprehensive understanding of its role in cancer dynamics and its influence on therapeutic outcomes. Additionally, our study lacks direct experimental validation of the oncogenic role of SCD, which is a crucial step for confirming its potential as a treatment target. To address this, we plan to engage in detailed in vitro and in vivo studies. These experiments will be pivotal in dissecting the precise molecular mechanisms through which NC modulates the cell cycle and influences CRC pathology via SCD. Furthermore, understanding these interactions at a molecular level across diverse patient populations is vital. This will not only solidify the foundation for potential clinical applications but also pave the way for initiating preclinical and clinical trials. These trials will test the efficacy and safety of NC in targeting SCD in CRC treatment, thereby advancing our approach to developing targeted cancer therapies.