Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.113571
Revised: September 19, 2025
Accepted: November 4, 2025
Published online: December 27, 2025
Processing time: 110 Days and 4.5 Hours
Obesity is a major global health concern associated with increased morbidity and mortality. Sleeve gastrectomy is an effective bariatric surgery; however, the im
To evaluate the effect of RGV on weight loss and body mass index (BMI) changes in patients undergoing sleeve gastrectomy.
This retrospective study included 49 patients. Preoperative and postoperative body weight and BMI (at 6 months and 12 months after surgery) were recorded. RGV was calculated using the ellipsoid formula based on the specimen’s length, width, and thickness measurements. Statistical analyses included parametric tests, repeated-measures one-way analysis of variance, Bonferroni post-hoc tests, and Pearson correlation analysis.
Both body weight and BMI significantly decreased over time (weight: F = 951.34, P < 0.01, η2 = 0.95; BMI: F = 345.97, P < 0.01, η2 = 0.88). A positive and statistically significant correlation was found between preoperative body weight and RGV (r = 0.285, P < 0.05). However, no significant associations were identified between RGV and weight or BMI at 6 months and 12 months.
RGV correlated with preoperative weight but not with postoperative outcomes, indicating that weight loss after sleeve gastrectomy is a multifactorial process and influenced by hormonal, metabolic, and lifestyle factors.
Core Tip: This study investigated the effect of resected gastric volume on weight loss after sleeve gastrectomy. The findings revealed that although resected gastric volume correlated with preoperative body weight, it did not predict postoperative weight loss, supporting the concept that weight reduction after sleeve gastrectomy is influenced by multifactorial mechanisms beyond gastric volume alone.
- Citation: Öndeş B, Gökdere OG, Kanat BH. Effect of resected gastric volume on weight loss after sleeve gastrectomy: A retrospective clinical study. World J Gastrointest Surg 2025; 17(12): 113571
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/113571.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.113571
Obesity is a multifactorial metabolic disease with a rapidly increasing prevalence worldwide and represents a major public health burden by elevating the risk of diabetes, cardiovascular disease, cancer, and premature mortality[1]. According to World Health Organization data, between 1990 and 2022, the prevalence of obesity more than doubled among adults, increased more than four-fold among children and adolescents, and reached alarming levels of morbid obesity. This dramatic rise demonstrates that obesity is no longer confined to high-income countries but has become a global public health crisis, increasingly affecting developing nations as well[2]. Bariatric surgery is considered one of the most effective methods for achieving long-term weight loss and metabolic improvement, particularly in patients with morbid obesity[3].
Sleeve gastrectomy is a relatively simple and safe bariatric surgical procedure that, by avoiding intestinal bypass, carries a lower risk of malabsorptive complications while maintaining high efficacy. Consequently, it has become one of the most frequently performed bariatric procedures worldwide[4]. During the operation, approximately 75% of the stomach is resected, leaving a narrow tubular gastric remnant. This leads to mechanical restriction of food intake, with weight reduction further supported by hormonal effects such as decreased ghrelin secretion[5].
The direct impact of resected gastric volume (RGV) on postoperative weight loss after sleeve gastrectomy remains unclear. While some studies have reported no significant association, others have described positive short-term correlations[6,7]. Several systematic reviews and clinical studies have demonstrated significant correlations between RGV and percentage excess weight loss (EWL)%, suggesting that variations in RGV may account for up to 26% of the differences in weight loss outcomes[8]. In contrast, other prospective studies and meta-analyses have failed to confirm these associations, with heterogeneity in patient cohorts, measurement techniques, and follow-up durations contributing to inconsistent findings[9]. Additional variability may also arise from differences in surgical technique, measurement methods (e.g., computed tomography volumetry vs specimen-based formulas), postoperative follow-up duration, and patient-related lifestyle factors.
The present study retrospectively evaluated the relationship between RGV and postoperative weight loss in patients undergoing sleeve gastrectomy, to assess its clinical significance and contribute to the resolution of conflicting findings in the literature.
This retrospective study analyzed the medical records of 49 patients who underwent sleeve gastrectomy at our clinic between January 2021 and December 2023. The study was approved by the local ethics committee, approval No. E-30785963-020-286154 and carried out in accordance with the principles of the Declaration of Helsinki.
A total of 55 patients aged 18-65 years who had undergone laparoscopic sleeve gastrectomy (LSG) for morbid obesity were initially evaluated. Patients with a history of previous bariatric surgery, malignancy, advanced organ failure, in
Demographic data (age, sex), preoperative body weight and body mass index (BMI), and postoperative weight and BMI at 6 months and 12 months were recorded.
During surgery, the resected gastric specimen was measured for length (L), width (W), and thickness (T). The RGV was then estimated using the ellipsoid formula: V ≈ π/6 × L × W × T. This mathematical approach, originally proposed by D’Ugo et al[6] and widely adopted in the literature, was applied to estimate RGV. All gastric-specimen measurements (L, W, and T) were performed by a pathologist using a standardized protocol. Intra-observer consistency was assessed and found to be high. Any doubtful measurements were re-evaluated to ensure accuracy.
All data were analyzed using SPSS for Windows, version 22 (IBM Corp., Armonk, NY, United States). The assumptions for applying parametric or nonparametric tests were assessed first. Normality of distribution was assessed using the Kolmogorov-Smirnov test and by examining skewness and kurtosis values. For independent two-group comparisons, the independent samples t-test was applied; for comparisons among more than two groups, one-way analysis of variance (ANOVA) was performed, followed by Bonferroni post-hoc tests for multiple comparisons. Correlations between numerical variables were evaluated using Pearson’s correlation coefficient. A P value < 0.05 was considered statistically significant.
A total of 49 patients were included in the study; of these, 30 (61.2%) were male and 19 (38.8%) were female. The mean RGV was 162.98 ± 84.94 cm3 in male patients and 210.54 ± 84.99 cm3 in female patients. No statistically significant difference in RGV was observed between sexes (t = -1.91; P = 0.06) (Table 1).
| Sex | n | mean ± SD | t | P value |
| Male | 30 | 162.98 ± 84.94 | -1.91 | 0.06 |
| Female | 19 | 210.54 ± 84.99 |
Significant reductions were observed in both body weight and BMI when comparing preoperative values with those at 6 months and 12 months postoperatively.
Weight: The mean preoperative body weight was 128.0 ± 23.6 kg, which decreased to 95.7 ± SD kg at 6 months and 83.6 ± SD kg at 12 months. Repeated-measures ANOVA demonstrated a highly significant effect of time on weight reduction (F = 951.34; P < 0.01; partial η² = 0.95). Bonferroni post-hoc analysis confirmed that body weight at each time point differed significantly (P < 0.05).
BMI: The mean preoperative BMI was 45.1 ± 5.7 kg/m2, which decreased to 33.7 ± 5.5 kg/m2 at 6 months and 29.5 ± 6.0 kg/m2 at 12 months. Repeated-measures ANOVA demonstrated a significant time-dependent effect on BMI reduction (F = 345.97; P < 0.01; partial η2 = 0.88).
The mean percentage total weight loss was 25.3% ± SD at 6 months and 34.7% ± SD at 12 months. The mean EWL was 56.7% ± SD at 6 months and 77.6% ± SD at 12 months. The results demonstrate a rapid weight loss during the first 6 months, followed by a slower but continuous decline between 6 months and 12 months, with approximately three-quarters of EWL at 12 months (Table 2).
| Time point | Body weight (kg) | BMI | TWL (%) | EWL (%) | F value | P value | Partial η2 | Bonferroni |
| Preoperative | 127.38 ± 23.8 | 44.95 ± 5.67 | 0.00 | 0.00 | Weight: 951.34; BMI: 345.97 | < 0.01 | Weight: 0.95; BMI: 0.88 | 1 > 2, 3/2 > 3 |
| 6 months | 95.36 ± 19.77 | 33.69 ± 5.44 | 24.84 ± 9.07 | 57.45 ± 20.27 | - | - | - | - |
| 12 months | 83.61 ± 19.45 | 29.5 ± 6.02 | 34.45 ± 10.21 | 80.0 ± 24.33 | - | - | - | - |
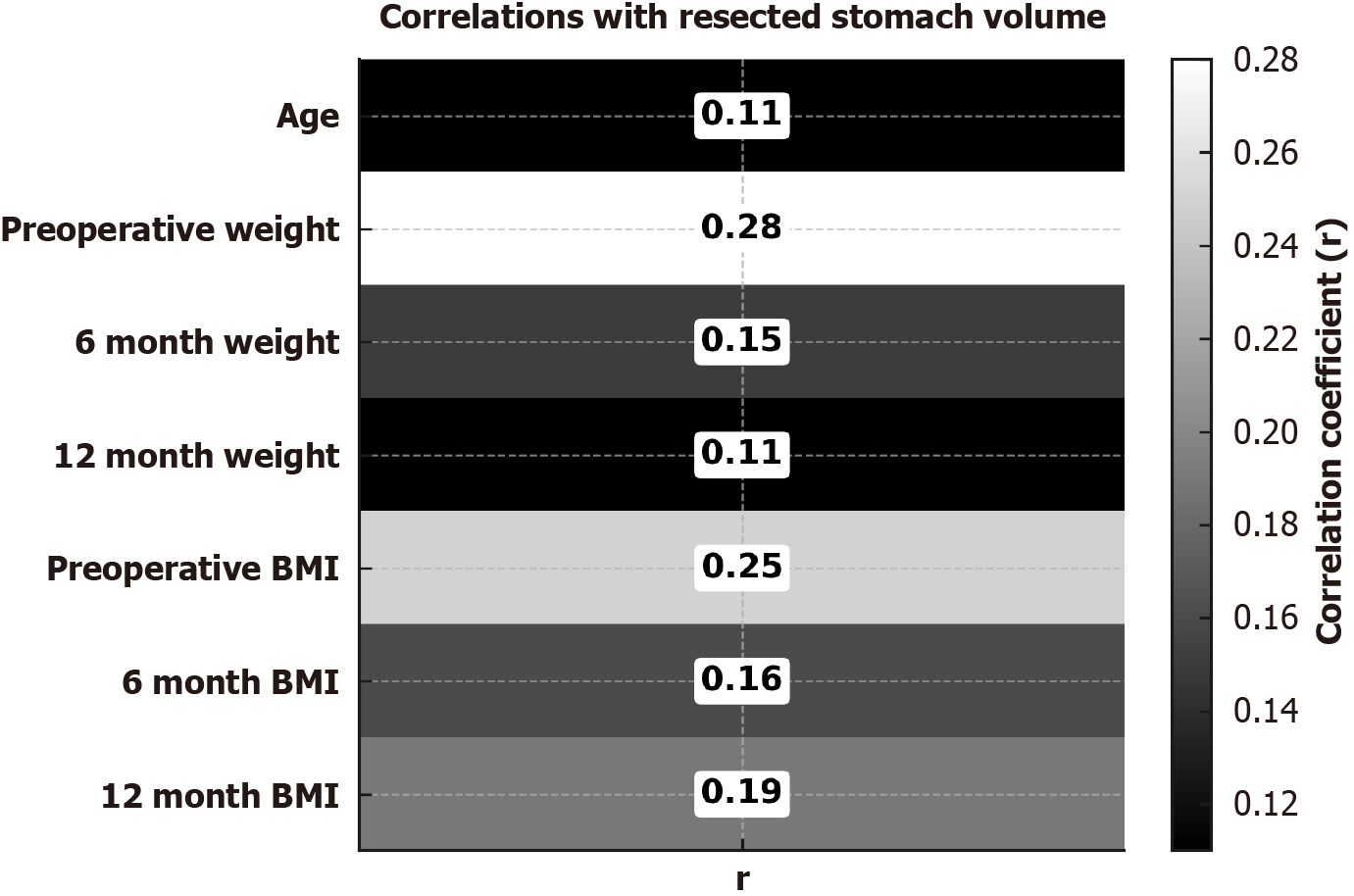
Pearson correlation analysis demonstrated a weak, borderline statistically significant association between preoperative body weight and RGV (r = 0.285, P = 0.05). Patients with higher baseline body weight tended to have a greater gastric volume resected. However, this correlation was weak (r < 0.3).
No significant correlations were found between RGV and body weight at 6 months (r = 0.15, P = 0.31) or 12 months (r = 0.11, P = 0.47). Similarly, no significant associations were found with BMI at baseline (r = 0.25, P = 0.09), 6 months (r = 0.16, P = 0.27), or 12 months (r = 0.19, P = 0.20). These findings indicate that RGV is not an independent determinant of postoperative changes in neither weight nor BMI.
Age was not significantly correlated with RGV (r = 0.11, P = 0.44), indicating that age is not a determinant of gastric volume.
Taken together, these results indicate that RGV shows only a weak association with preoperative body weight and has no significant impact on postoperative weight loss or BMI reduction. Instead, postoperative outcomes are more likely determined by residual gastric volume, hormonal and metabolic adaptations, and lifestyle-related factors (Figure 1).
In the current literature, LSG has been recognized as a strong alternative to gastric bypass, primarily owing to its technical simplicity, lower complication profile, and effective weight loss outcomes. Several studies have reported that LSG has become the most commonly performed bariatric procedure worldwide, positioning it as a practical “gold-standard” surgical approach in contemporary practice[10-12]. Furthermore, recent data from the American Society for Metabolic and Bariatric Surgery indicate that LSG not only achieves substantial weight reduction but also ensures durable long-term outcomes, making it a robust option comparable to gastric bypass in terms of efficacy[13].
In this retrospective study, we evaluated the relationship between RGV and weight loss in patients undergoing sleeve gastrectomy. Although RGV showed a statistically significant positive correlation with preoperative body weight, it did not independently affect the magnitude of postoperative weight loss. Moreover, although significant reductions in body weight and BMI were observed at 6 months and 12 months postoperatively, these changes were not found to be associated with RGV.
In the literature, conflicting results have been reported regarding the relationship between RGV and postoperative weight loss. Bekheit et al[14] demonstrated that although RGV was associated with baseline BMI, it was not an independent predictor of postoperative weight loss. Conversely, in a large cohort study, Kizilkaya et al[15] suggested that the ratio of RGV to the number of staplers used was correlated with EWL and might serve as a reproducible predictor of surgical success. Similarly, Tartaglia et al[16] reported that residual gastric area, measured using gastrointestinal imaging, could be associated with postoperative weight loss.
In our study, a weak yet statistically significant positive correlation was identified between preoperative body weight and RGV (r = 0.285; P = 0.05). However, no significant correlation was observed between RGV and either body weight or BMI at 6 months and 12 months postoperatively. These findings suggest that while RGV may be associated with baseline body weight, its role in predicting long-term weight loss appears limited. The discrepancies observed across the literature may be attributed to heterogeneity in patient populations, variations in surgical technique, differences in postoperative dietary compliance and lifestyle factors, and inconsistencies in measurement methodologies.
The findings of this study indicate that weight loss after sleeve gastrectomy is not determined solely by RGV but rather by multiple mechanisms that act in concert. These include hormonal changes - particularly a reduction in fasting ghrelin levels accompanied by increased postprandial secretion of glucagon-like peptide-1 and peptide YY - as well as increased energy expenditure, alterations in gastric emptying rate, and patient adherence to dietary and physical activity recommendations. According to evidence from systematic reviews and meta-analyses, sleeve gastrectomy leads to a significant decrease in fasting ghrelin concentrations along with notable increases in postprandial glucagon-like peptide-1 and peptide YY levels[17].
In our study, although the mean RGV was higher in female patients compared to males, this difference did not reach statistical significance. This finding may reflect sex-specific differences in body composition, variations in surgical technique, or individual anatomical characteristics. Similar results have been reported in the literature. Hider et al[18] demonstrated that one-year weight loss outcomes and complication risks after bariatric surgery were statistically comparable between male and female patients, suggesting that sex is not a decisive factor in surgical success.
The strengths of our study include the use of a standardized formula for measuring RGV in all patients and the consistent documentation of follow-up data. However, the main limitation of this study lies in its relatively small sample size and retrospective design, both of which may limit generalizability of the results. Nevertheless, our data provide an important basis for future large-scale prospective studies.
This retrospective study demonstrated that while RGV after sleeve gastrectomy was positively associated with preoperative weight, it did not independently affect postoperative weight loss nor BMI changes. The findings indicate that weight loss is a multifactorial process influenced not only by gastric volume but also by hormonal alterations, metabolic adaptations, dietary adherence, and physical activity. In clinical practice, the limited association between RGV and postoperative weight loss suggests that surgical success should not be evaluated solely based on gastric volume. Patient education, support for lifestyle modifications, and multidisciplinary follow-up during the postoperative period are critical for achieving sustained long-term weight control.
The authors would like to thank all the patients who participated in this study and the clinical staff involved in their care. We also acknowledge the contributions of our colleagues in data collection and follow-up.
| 1. | GBD 2021 Adolescent BMI Collaborators. Global, regional, and national prevalence of child and adolescent overweight and obesity, 1990-2021, with forecasts to 2050: a forecasting study for the Global Burden of Disease Study 2021. Lancet. 2025;405:785-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 164] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 2024;403:1027-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 934] [Cited by in RCA: 1136] [Article Influence: 568.0] [Reference Citation Analysis (0)] |
| 3. | Courcoulas AP, Daigle CR, Arterburn DE. Long term outcomes of metabolic/bariatric surgery in adults. BMJ. 2023;383:e071027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 4. | Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 580] [Cited by in RCA: 566] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 5. | Weiner RA, Weiner S, Pomhoff I, Jacobi C, Makarewicz W, Weigand G. Laparoscopic sleeve gastrectomy--influence of sleeve size and resected gastric volume. Obes Surg. 2007;17:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | D'Ugo S, Bellato V, Bianciardi E, Gentileschi P. Impact of Resected Gastric Volume on Postoperative Weight Loss after Laparoscopic Sleeve Gastrectomy. Gastroenterol Res Pract. 2019;2019:3742075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Salman MA, Elshazli M, Shaaban M, Esmat MM, Salman A, Ibrahim HMM, Tourky M, Helal A, Mahmoud AA, Aljarad F, Saadawy AMI, Shaaban HE, Mansour D. Correlation Between Preoperative Gastric Volume and Weight Loss After Laparoscopic Sleeve Gastrectomy. Int J Gen Med. 2021;14:8135-8140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Singla V, Aggarwal S, Aggarwal S, Gupta M, Singh D. Correlation of weight loss with residual gastric volume on computerized tomography in patients undergoing sleeve gastrectomy: A systematic review. Clin Obes. 2020;10:e12394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Winter K, Falk GE, Alderson JW, Quinn KR, Helmer SD, Brown NM. Sleeve Gastrectomy: Does the Amount of Stomach Removed Matter? Obes Surg. 2023;33:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 576] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 11. | Nienhuijs SW, de Zoete JP, Berende CA, de Hingh IH, Smulders JF. Evaluation of laparoscopic sleeve gastrectomy on weight loss and co-morbidity. Int J Surg. 2010;8:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Peterli R, Wölnerhanssen BK, Vetter D, Nett P, Gass M, Borbély Y, Peters T, Schiesser M, Schultes B, Beglinger C, Drewe J, Bueter M. Laparoscopic Sleeve Gastrectomy Versus Roux-Y-Gastric Bypass for Morbid Obesity-3-Year Outcomes of the Prospective Randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS). Ann Surg. 2017;265:466-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 13. | American Society for Metabolic and Bariatric Surgery. Bariatric surgery more effective and durable than new obesity drugs and lifestyle intervention. Jun 11, 2024. [cited 6 September 2025]. Available from: https://medicalxpress.com/news/2024-06-bariatric-surgery-effective-durable-obesity.html. |
| 14. | Bekheit M, Abdel-Baki TN, Gamal M, Abdel-Salam W, Samir M, ElKayal E, Katri K. Influence of the Resected Gastric Volume on the Weight Loss After Laparoscopic Sleeve Gastrectomy. Obes Surg. 2016;26:1505-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Kizilkaya MC, Basaran C, Kalafat UM, Mutlu AU, Saracoglu C, Aytac E. Resected gastric volume/number of staplers fired ratio as a tool in predicting complication and midterm results in sleeve gastrectomy. Sci Rep. 2024;14:30769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Tartaglia N, Pavone G, Germano MP, Russo G, Pacilli M, Ambrosi A. Relationship between residual gastric area and weight loss after sleeve gastrectomy: A Cohort study. Ann Med Surg (Lond). 2022;73:103177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | McCarty TR, Jirapinyo P, Thompson CC. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-analysis. Ann Surg. 2020;272:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Hider AM, Bonham A, Carlin A, Finks J, Ghaferi A, Varban O, Ehlers AP. Association of Sex Differences on Weight Loss and Complications Following Bariatric Surgery. J Surg Res. 2024;299:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/