Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.110617
Revised: September 12, 2025
Accepted: October 17, 2025
Published online: December 27, 2025
Processing time: 146 Days and 1.3 Hours
Chronic esophagitis can progress to esophageal cancer via "inflammation-dysplasia-cancer" transformation, with nitric oxide (NO) serving as a critical mediator in this process. Traditional diagnostic methods (e.g., endoscopic biopsy) for esophageal cancer transformation have low sensitivity and require long detection time, while existing fluorescent probes lack specificity and stability for real-time NO monitoring. High-performance fluorescent probes like DAF-FM, with NO-targeting ability, show potential for visual screening and efficacy evaluation but need systematic validation in esophageal cancer models.
To validate the applicability of the fluorescent probe DAF-FM for visual screening of esophageal cancer transformation, explore the underlying mechanism of NO-regulated transformation, and evaluate the probe’s efficacy in monitoring therapeutic responses.
Laser confocal imaging and flow cytometry were used to analyze DAF-FM’s NO concentration/time-dependent fluorescence response, lysosomal targeting (via Pearson coefficient), and cytotoxicity (with cholecystokinin-8 assay) in esophageal cells. Sprague-Dawley rat esophageal cancer models (normal, esophagitis, esophageal cancer, and drug/radiotherapy intervention) were established to monitor NO dynamics and tumor volume correlation. Clinical diagnostic comparison (50 suspected patients) with endoscopic biopsy/histopathology was conducted using Kolmogorov-Smirnov test and Student’s t-test (P < 0.05). Western blot and quantitative real-time polymerase chain reaction were used to explore NO’s role in the nuclear factor-kappa B (NF-κB) pathway.
DAF-FM exhibited concentration/time-dependent fluorescence with NO (300 μM NO: 60-minute fluorescence intensity 458 ± 15 arbitrary units, P < 0.05) and specific lysosomal targeting (Pearson’s coefficient = 0.82 ± 0.03). It had low cytotoxicity (82.3% ± 4.1% cell viability at 50 μM). In rat models, DAF-FM showed that NO was correlated with tumor volume (R² = 0.87). Clinically, its sensitivity (92.5%) outperformed endoscopic biopsy (78.3%), with shorter detection time (30 minutes vs 48 hours, P < 0.05). Mechanistically, NO regulated transformation via the NF-κB pathway (Pearson’s coefficient = 0.78 ± 0.05 between DAF-FM and NF-κB).
DAF-FM is a feasible tool for visual screening of esophageal cancer transformation, enabling real-time NO monitoring, high-sensitivity diagnosis, and therapeutic efficacy evaluation. It provides a new approach for esophageal cancer diagnosis and mechanism research.
Core Tip: This study systematically evaluated the high-performance fluorescent probe DAF-FM for visual screening and efficacy assessment of esophagitis-to-cancer transformation. DAF-FM exhibited concentration-dependent and time-dependent fluorescence responses to nitric oxide (NO), targeted lysosomes specifically (Pearson coefficient = 0.82 ± 0.03), and had low cytotoxicity (82.3% ± 4.1% cell viability at 50 μM). In Sprague-Dawley rat esophagitis cancer models, DAF-FM monitored NO changes dynamically, with results positively correlated to tumor volume (R² = 0.87) post 5-fluorouracil/radiotherapy. Clinically, it outperformed endoscopic biopsy (sensitivity: 92.5% vs 78.3%) and shortened detection time (30 minutes vs 48 hours). Mechanistically, NO regulates carcinogenesis via the nuclear factor-kappa B pathway, clarifying DAF-FM’s molecular logic in reflecting transformation stages.
- Citation: Chen WH, Cai CF, Gao BZ, Hong WS, Xu YZ, Cai WJ. Visual screening and efficacy evaluation of high-performance fluorescent probe DAF-FM in esophagitis cancer transformation. World J Gastrointest Surg 2025; 17(12): 110617
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/110617.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.110617
Inflammation is a recognized feature of malignant tumors, and the immune microenvironment formed by chronic inflammation is closely related to the occurrence and development of tumors. When chronic inflammation is present for a long time, this leads to a large number of inflammatory cytokines and active mediators, which cause DNA damage, genomic instability and even inactivation of base mismatch repair enzymes, further leading to overexpression of oncogenes and inactivation of tumor suppressor genes, and ultimately to malignant transformation of cells[1,2]. In the tumor microenvi
Therefore, the in-depth study of the inflammatory-tumor transformation process of esophageal cancer and its molecular basis are of great practical significance for the discovery of new prevention and treatment strategies.
Molecular fluorescence probes have become a useful tool in biological and medical research due to their advantages of high sensitivity, easy operation and non-destructive testing[11,12]. The NO fluorescent probe 3-amino, 4-aminomethyl-2',7'-difluorofluorescein diacetate (3-Amino,4-aminomethyl-2',7'-didiacetate, DAF-FM DA) has been commercialized and widely used for intracellular NO detection[13]. It uses fluorescein as the fluorophore and the -NH2 of o-phenylenediamine as the recognition group. NO reacts with -NH2 in DAF-FM DA to produce fluorescein derivatives containing triazole which emit strong fluorescence[14]. DAF-FM DA is cellular permeable and is catalyzed by the esterase within the cell to form DAF-FM that cannot cross the cell membrane. DAF-FM itself has fluorescence. When NO is present, DAF-FM reacts with NO to produce strong fluorescence triazole fluorescein (DAF-2T) with an excitation wavelength of 491 nm and emission wavelength of 513 nm. The fluorescence intensity of the probe can be detected by fluorescence microscopy, laser confocal microscopy, flow cytometry, enzyme labeling and other instruments[15,16]. Therefore, DAF-FM DA is a relatively sensitive fluorescent probe, and the detection limit of NO at neutral pH can reach 2-5 nM[17]. The targeting NO-specific fluorescence of DAF-FM DA provides a new approach for the accurate detection of inflammation, and therefore creates a new opportunity for the early accurate detection of the malignant risk of inflammatory cancer transformation[18].
However, due to the complex and diverse pathogenesis environment, most of the probes used for the detection of inflammatory cancer transformation have poor specificity and stability, and cannot be monitored in real time; thus, the accurate and sensitive detection of esophagitis cancer transformation is limited. Based on chemical design and screening and nanoassembly strategies, we developed the NO quantitative fluorescent probe DAF-FM diacetate (DAF-FM) with high selectivity, excellent photostability and biocompatibility across the bond energy transfer ratio to accurately monitor endogenous NO during the transformation of esophagitis cancer. Through the combination of rational design and quantitative calculation, we developed a new fluorescent dye and used it to develop a series of high-performance fluorescent probes, which successfully realized the accurate detection of relevant active molecules in the transformation process of esophagitis cancer. We used the quantitative fluorescent NO probe DAF-FM to detect NO in esophageal mucosal epithelial cells and small esophageal carcinoma. On this basis, we explored the functional characteristics of the probe DAF-FM and the in vitro imaging ability of esophagitis and esophageal cancer, analyzed the expression of NO in lysozymes, statistically analyzed the above indicators detected by the probe DAF-FM, and use this probe to further reveal the physiological mechanism of the transformation process of inflammation and cancer. We summarize and elucidate the visual screening and efficacy evaluation of DAF-FM probe in the transformation of esophagitis cancer, in order that this probe becomes an important tool for exploring the transformation mechanism of esophagitis cancer, and a promising imaging tool for the diagnosis and prevention of clinically related diseases.
Lysine, glycine and ascorbic acid were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. DL-homocysteine, L-cysteine, 9,9-dioctyl-2-bromofluorene, N-acetyl-L-cysteine and pyruvaldehyde were purchased from Shanghai Maclin Biochemical Technology Co., Ltd. 4,7-dibromo2,1,3-benzothiadiazole, glutathione and NO donor salts [2 (-N,N-diethylamino)-diazene-2-sodium oxide salt hydrate] were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. Other reagents were purchased from Sinopharm Group Chemical Reagent Co., Ltd. The reagents used in the experiment were all analytically pure. The solution was prepared with secondary water. Nuclear magnetic resonance carbon and nuclear magnetic resonance hydrogen were tested using a Bruker Avance III HD 400 MHz superconducting nuclear magnetic resonance instrument. The high-resolution mass spectrometer used was an Orbitrap Fusion Lumos (manufactured by Thermo Fisher Scientific). A UV-1900 UV-VIS spectrometer produced by Shimadzu Company, Japan was used in the ultraviolet absorption spectrum experiment. The fluorescence spectrum experiment adopted the FS-5 fluorescence spectrometer produced by Edinburgh Instruments, United Kingdom. A LSM710 Laser scanning confocal microscope and a BD FACSAria III sorting flow cytometer (BD Biosciences) were also used.
We investigated the cytotoxicity of DAF-FM at different concentrations before cell imaging. By analyzing the cytotoxicity of RAW264.7 macrophages, the concentration range of DAF-FM which can be used for cell imaging was obtained. Six concentration groups (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, 50 μM), with 6 replicate wells in each group, were included to detect the cell survival rate at different concentrations. Cell level probe imaging was then performed on esophageal mucosa cells, esophageal mucosa cells treated with an inflammatory factor (lipopolysaccharide), and both NCE and TE-1 esophageal cancer cells. Three biological replicates were included for each cell, and 5 technical replicates were set for each replicate. To optimize the imaging time of the probe, in order to achieve cell imaging under the best conditions, it was necessary to investigate the change of fluorescence intensity during confocal imaging of the probe at different times (0 minute, 20 minutes, 40 minutes, 60 minutes). Each time point was detected 3 times, and the average value was used for analysis.
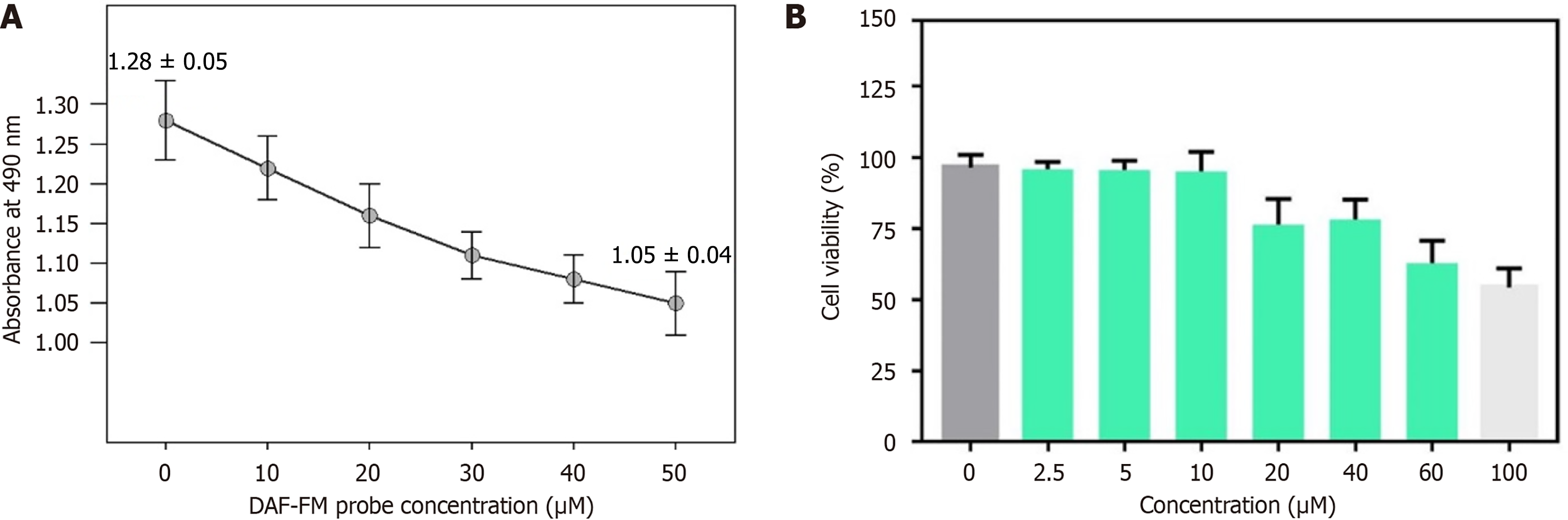
The cytotoxicity of fluorescent probes to esophageal carcinoma cells was calculated by the cholecystokinin-8 (CCK-8) method. First, esophageal cancer cells were transferred to 96-well plates (5 × 103 cells per well) and cultured for 24 hours in an incubator containing 5% CO2 and 95% air at 37 °C. The medium was then replaced with fresh DMEM containing fluorescent probes (final concentrations were 0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM, respectively). Six replicate wells were included for each group. After 24 hours, the DMEM medium was removed, and CCK-8 solution (5 mg/mL, 20 μL) was added for 4 hours, and then DMSO (200 μL) was added to dissolve and release the purple mezan compound. Finally, the absorbance at 490 nm was measured on an enzyme-labeled instrument. Cell survival rate was calculated according to the formula: Cell survival rate = (absorbance of experimental group - absorbance of blank group)/(absorbance of control group - absorbance of blank group) × 100%. The experiment was repeated 3 times, and the average value was used for statistical analysis.
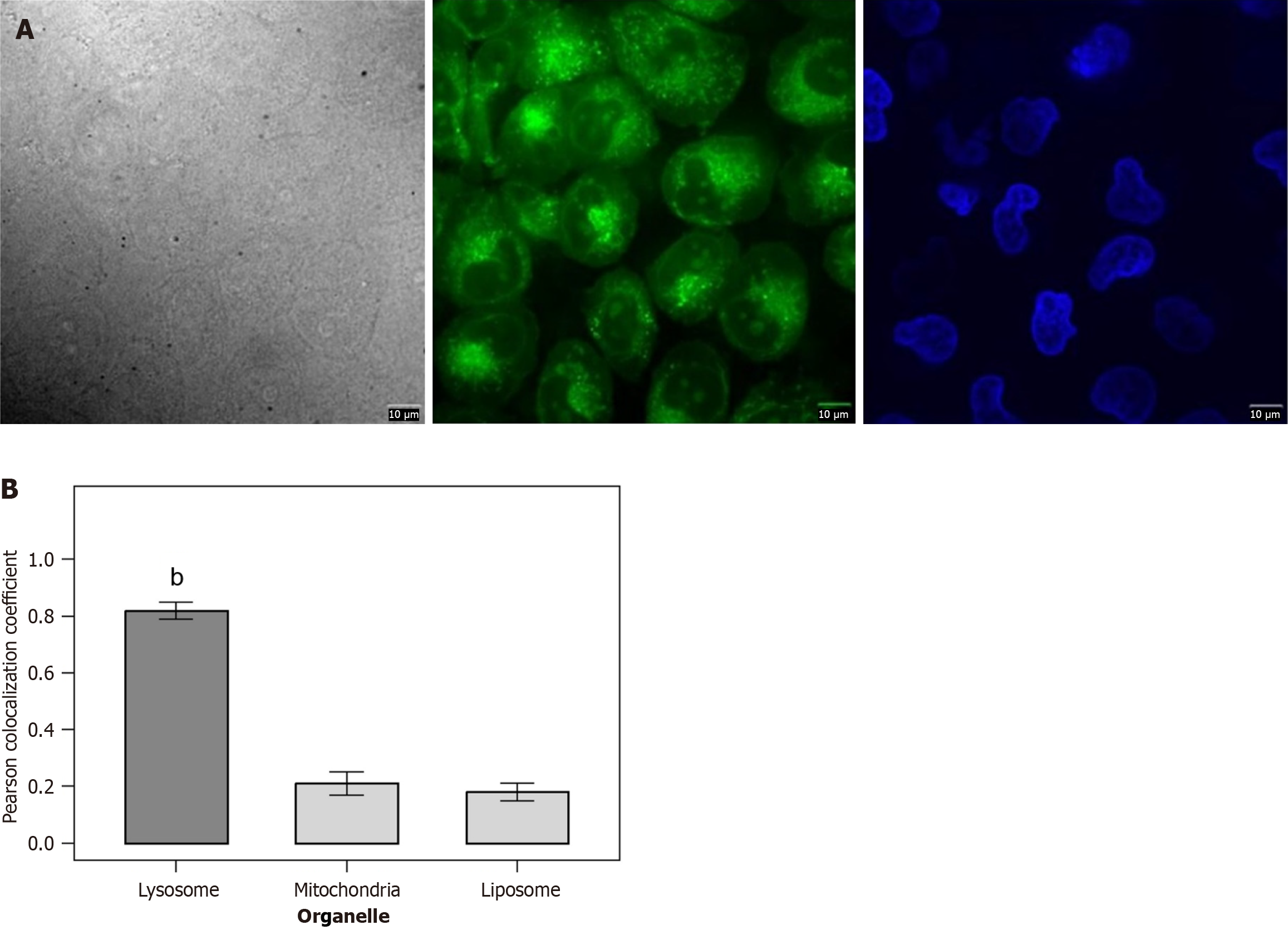
We investigated the ability of DAF-FM probe to target lysosomes. Colocalization experiments combined 5 μM probe Mito-2 or Mito-3 with 200 nM commercial dye Mito-Tracker Green or BODIPY493/593 or Lyso-Tracker Green in HeLa cells (or HepG2 or MCF-7 cells). The cells were incubated for 20 minutes, washed twice with phosphate buffer saline, and then fresh DMEM was added for laser confocal imaging. Three replicate wells were included for each cell type. Image J software was used to calculate the colocalization coefficient (Pearson correlation coefficient). Ten fields of view were randomly selected from each sample for analysis, and the average value was obtained. A colocalization coefficient > 0.7 was considered to indicate good targeting.
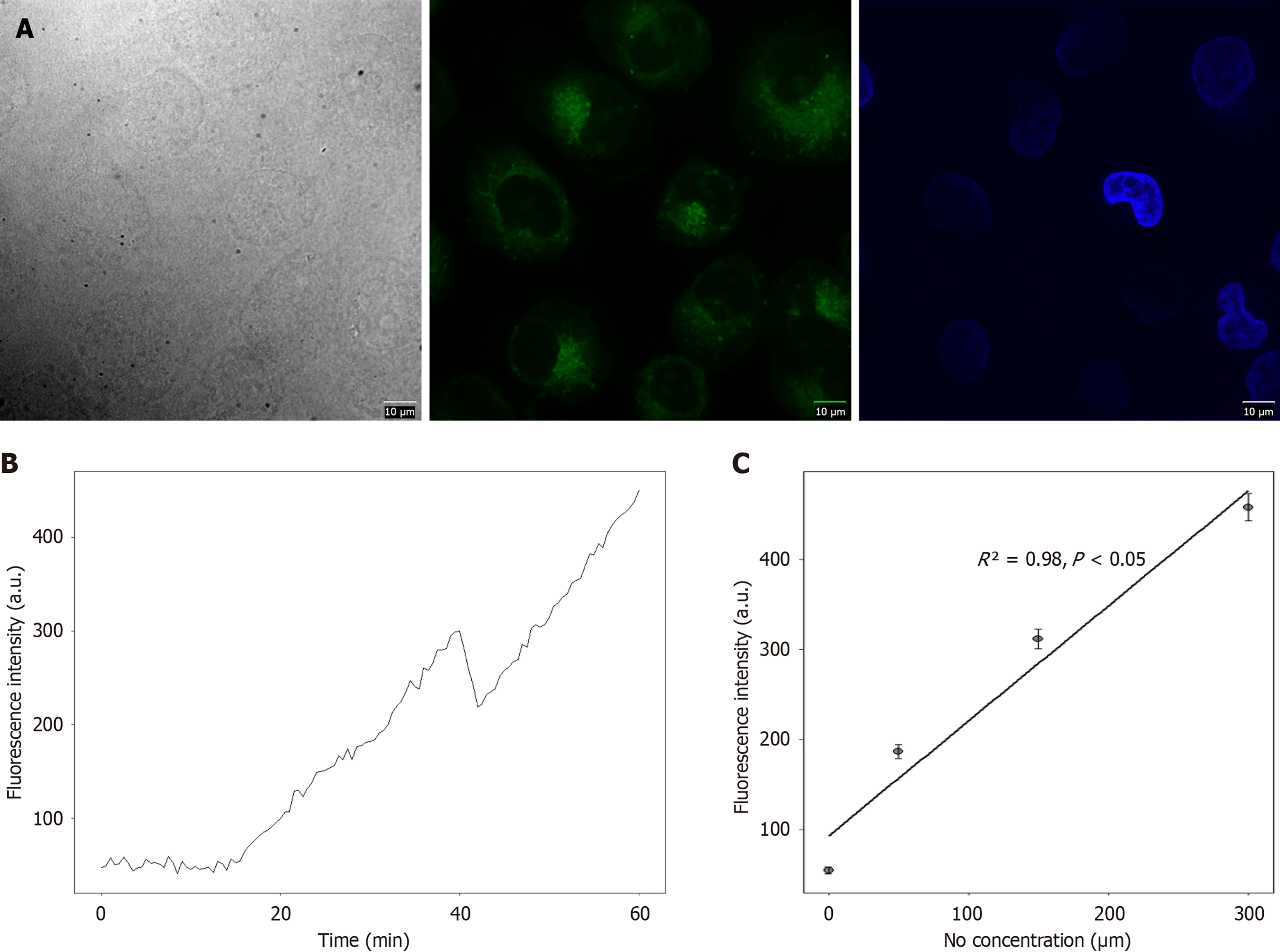
In order to explore the imaging ability of the probe on the ratio of NO in lysozymes, following incubation of the DAF-FM probe with the cells, different concentrations of NO donor DEANO (0 μM, 50 μM, 150 μM, 300 μM) were added, the cells were incubated for 1 hour, and cell imaging was performed. Three replicate wells were included for each concentration group, and Image J software was used to quantitatively analyze the fluorescence intensity. Flow cytometry proved that the probe could quantitatively detect exogenous NO. The experiment included the above-mentioned 4 DEANO concentration groups, 1 × 104 cells were detected in each group, and the experiment was repeated 3 times to draw a fluorescence intensity-concentration standard curve (R² = 0.98). For the detection of cell NO, it was necessary to select a control cell group (blank control group: No probe added; negative control group: Probe added but no DEANO added; experimental group: Probe and different concentrations of DEANO added), deduct the endogenous background NO, and analyze according to the fluorescent-concentration curve.
Sprague-Dawley rats were used to establish esophagitis cancer models, and the animals were randomly divided into 4 groups with 10 rats per group: (1) Normal control group (n = 10); (2) Esophagitis model group (n = 10); (3) Esophagitis cancer model group (n = 10); and (4) The drug [5-fluorouracil (5-Fu), 10 mg/kg] + radiotherapy (2 Gy dose) intervention group (n = 10). The esophagitis model was established using 0.15% deoxycholic acid by gavage (10 mL/kg, once a day for 4 weeks); the esophagitis cancer model was established by continuing to administer N-methyl-N-nitrosourea (20 mg/kg) by gavage once a week for 8 weeks on the basis of the esophagitis model. The modeling success standard was achieved when a pathological section showed that the esophageal mucosa had severe dysplasia or carcinoma in situ. The DAF-FM probe was used to monitor the NO concentration changes and fluorescence intensity in the esophageal tissue of rats in each group, once a week for 4 consecutive weeks. In addition, the volume of esophageal tumors in rats was measured (volume = length × width2/2), and tumor growth was recorded. At each time point, 3 rats per group were randomly selected for tissue sampling to avoid repeated sampling bias. In addition, rat esophageal tissue samples from each group were collected, and immunohistochemistry was conducted to detect the expression of Ki-67 (proliferation index) and caspase-3 (apoptosis index), and the correlation between the probe monitoring results and tumor proliferation and apoptosis was analyzed.
Fifty patients with suspected esophagitis cancer (28 males, 22 females; age range: 45-68 years, mean age: 56.2 ± 7.8 years) were prospectively enrolled from the Department of Gastroenterology, Quanzhou First Hospital Affiliated to Fujian Medical University between January 2025 and March 2025. All patients met the following inclusion criteria: (1) Presence of clinical symptoms (e.g., dysphagia, retrosternal pain); (2) No prior esophageal surgery or radiotherapy/chemotherapy; and (3) Willingness to undergo DAF-FM probe detection, endoscopic biopsy, and histopathological examination. Exclusion criteria included: (1) Severe liver/kidney dysfunction; (2) Autoimmune diseases; and (3) Pregnancy or lactation. DAF-FM probe detection, endoscopic biopsy and histopathological examination were performed, respectively. Using the histopathological examination results as the gold standard, the sensitivity [sensitivity = number of true positive cases/(number of true positive cases + number of false negative cases) × 100%], specificity [specificity = number of true negative cases/(number of true negative cases + number of false positive cases) × 100%], accuracy [accuracy = (number of true positive cases + number of true negative cases)/total number of cases × 100%] and detection time of the three methods were calculated for statistical analysis.
To clarify the specific mechanism of NO in esophagitis carcinogenesis and the molecular logic of DAF-FM reflecting carcinogenesis by monitoring NO, the following experiments were conducted.
Western blot detection: Esophageal cancer cells were treated with different concentrations of NO donors (0 nM, 50 nM, 100 nM, 200 nM, 300 nM) for 24 hours. Three replicate wells were included for each concentration group, and the experiment was repeated 3 times independently to ensure result reproducibility. Total protein was extracted from cells, and the expression levels of nuclear factor-kappa B (NF-κB) (p65), c-Myc, Bcl-2, and p53 proteins were detected by western blot. β-actin was used as the internal reference. The gray value of protein bands was analyzed by Image J software, and the relative expression level of each protein was calculated (relative expression level = gray value of target protein band/gray value of β-actin band).
Quantitative real-time polymerase chain reaction detection: The same NO concentration-treated esophageal cancer cells as above were used, with 3 replicate wells per group. Total RNA was extracted using TRIzol reagent (Invitrogen, United States), and RNA purity was verified by measuring A260/A280 ratio (1.8-2.0) using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, United States). Total RNA was extracted, reverse-transcribed into cDNA, and quantitative real-time polymerase chain reaction (qRT-PCR) was performed to detect the mRNA expression levels of NF-κB (p65), c-Myc, Bcl-2, and p53 genes. GAPDH was used as the internal reference. The relative expression level of mRNA was calculated by the 2-ΔΔCt method.
Immunofluorescence colocalization experiment: Esophageal cancer cells were incubated with 5 μM DAF-FM probe and 1 μg/mL NF-κB (p65) primary antibody (Abcam, ab16502) at 37 °C for 2 hours, then incubated with Cy3-labeled secondary antibody (Abcam, ab6939) for 1 hour. Three replicate wells were included, and laser confocal microscopy (LSM710, Zeiss, Germany) was used for imaging. Z-stack images were acquired at 0.5 μm intervals to ensure full colocalization analysis, and ImageJ software (with Coloc 2 plugin) was used to calculate the Pearson correlation coefficient between DAF-FM fluorescence signals and NF-κB (p65) immunofluorescence signals, to analyze the association between NO (monitored by DAF-FM) and the NF-κB signaling pathway.
Statistical analysis was performed using Statistical Package for the Social Sciences 25.0 (IBM, United States). The results are expressed as mean ± SD. The difference between the two groups was determined by the unpaired student t test. In addition, one-way analysis of variance (ANOVA) with the minimal significant difference post facto test was used to compare the mean values between multiple groups, two-tailed two-factor ANOVA was used in multiple comparisons, and then Bonferroni post facto analysis was used to determine interactions. For the comparison of diagnostic methods, the χ2 test was used to compare the sensitivity, specificity, and accuracy between the groups. P values less than 0.05 were considered statistically significant.
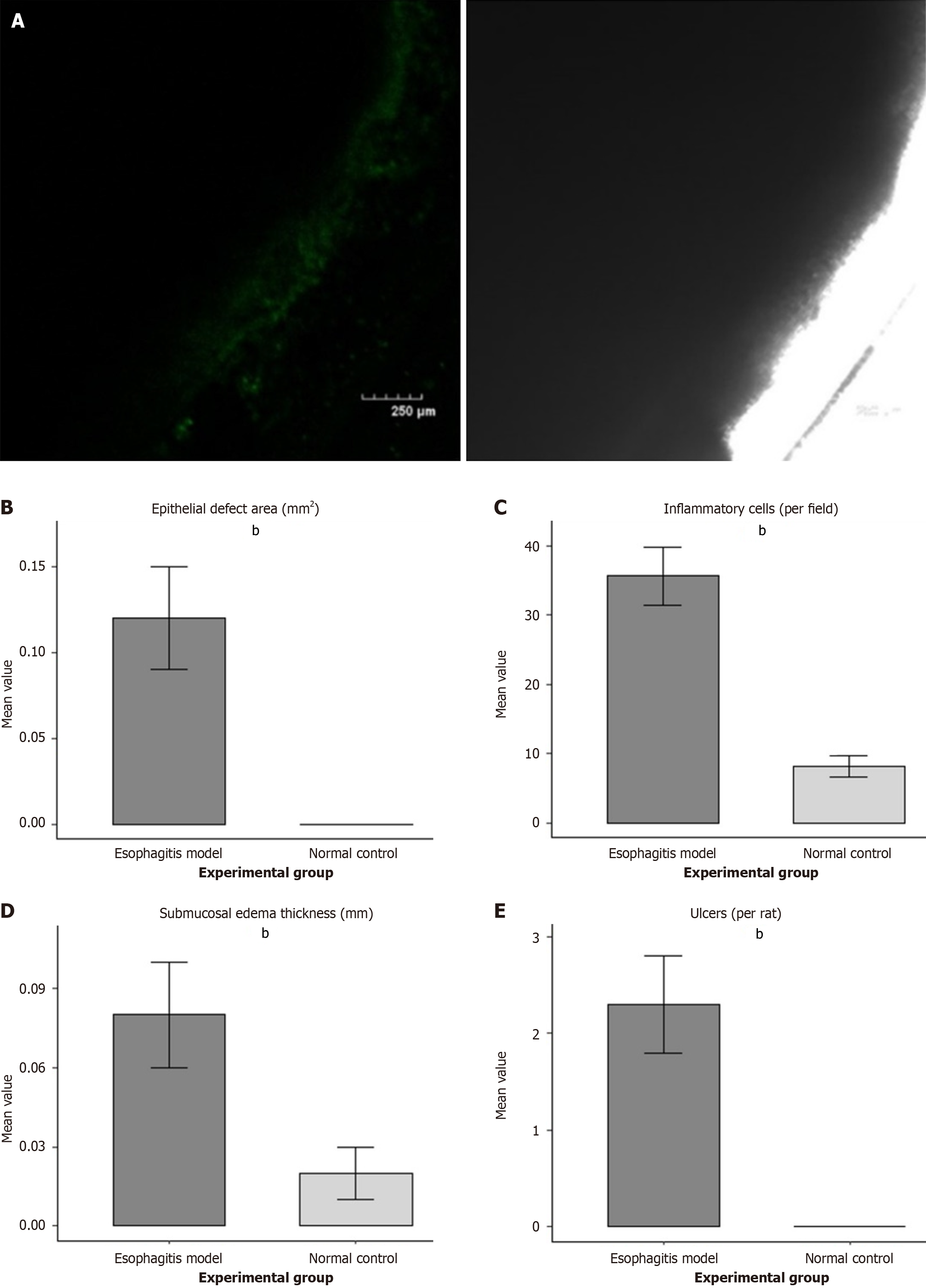
Imaging of the rat model of esophagitis showed that the esophageal mucosa was rough, infiltration of inflammatory cells was serious, inflammation of the lower esophageal mucosa had changed, that is, inflammatory cells such as lymphocytes and neutrophils had infiltrated, epithelial defects, submucosal tissue edema and capillary dilatation were present, and mucosal damage and ulcers were significantly changed. In terms of quantitative data, in the esophagitis model group (n = 10), the number of inflammatory cells per field of view was 35.6 ± 4.2, which was significantly higher than that in the normal control group (8.2 ± 1.5, P < 0.01); the epithelial defect area was 0.12 ± 0.03 mm² (normal control group: 0 mm, P < 0.01); the submucosal edema thickness was 0.08 ± 0.02 mm (normal control group: 0.02 ± 0.01 mm, P < 0.01); the number of ulcers per rat was 2.3 ± 0.5 (normal control group: 0 mm, P < 0.01) (Figure 1).
The DAF-FM probe showed a concentration-dependent relationship with NO, and the fluorescence intensity increased significantly with time. In terms of quantitative data, 0 μM NO: (1) 0 minute fluorescence intensity 52 ± 3 arbitrary units (a.u.); and (2) 60 minutes fluorescence intensity 55 ± 4 a.u.; 50 μM NO: (1) 0 minute fluorescence intensity 89 ± 5 a.u.; and (2) 60 minutes fluorescence intensity 187 ± 8 a.u.; 150 μM NO: (1) 0 minute fluorescence intensity 156 ± 7 a.u.; and (2) 60 minutes fluorescence intensity 312 ± 11 a.u.; 300 μM NO: (1) 0 minute fluorescence intensity 223 ± 9 a.u.; and (2) 60 minutes fluorescence intensity 458 ± 15 a.u. All concentration groups showed significant differences in fluorescence intensity at the different time points (P < 0.05) (Figure 2).
The results of the CCK-8 experiment showed that the cell activity of the fluorescent probe decreased significantly with increased CCK-8 concentration. In terms of quantitative data, 0 μM (control group) cell survival rate was 100% ± 2.1%; 10 μM cell survival rate was 96.5% ± 3.2%; 20 μM cell survival rate was 92.3% ± 2.8%; 30 μM cell survival rate was 88.7% ± 3.5%; 40 μM cell survival rate was 85.2% ± 4.0%; 50 μM cell survival rate was 82.3% ± 4.1%. Compared with the control group, the cell survival rate decreased significantly when the probe concentration was ≥ 30 μM (P < 0.05), but remained above 80% even at 50 μM (Figure 3).
The DAF-FM probe showed targeted colocalization with lysosomes. In terms of quantitative data, the Pearson colocalization coefficient between DAF-FM and Lyso-Tracker Green (lysosome dye) was 0.82 ± 0.03 (n = 3), while the colocalization coefficients with Mito-Tracker Green (mitochondrial dye) and BODIPY493/593 (liposome dye) were 0.21 ± 0.04 and 0.18 ± 0.03, respectively. The colocalization coefficient with lysosomes was significantly higher than that with mitochondria and liposomes (P < 0.01) (Figure 4).
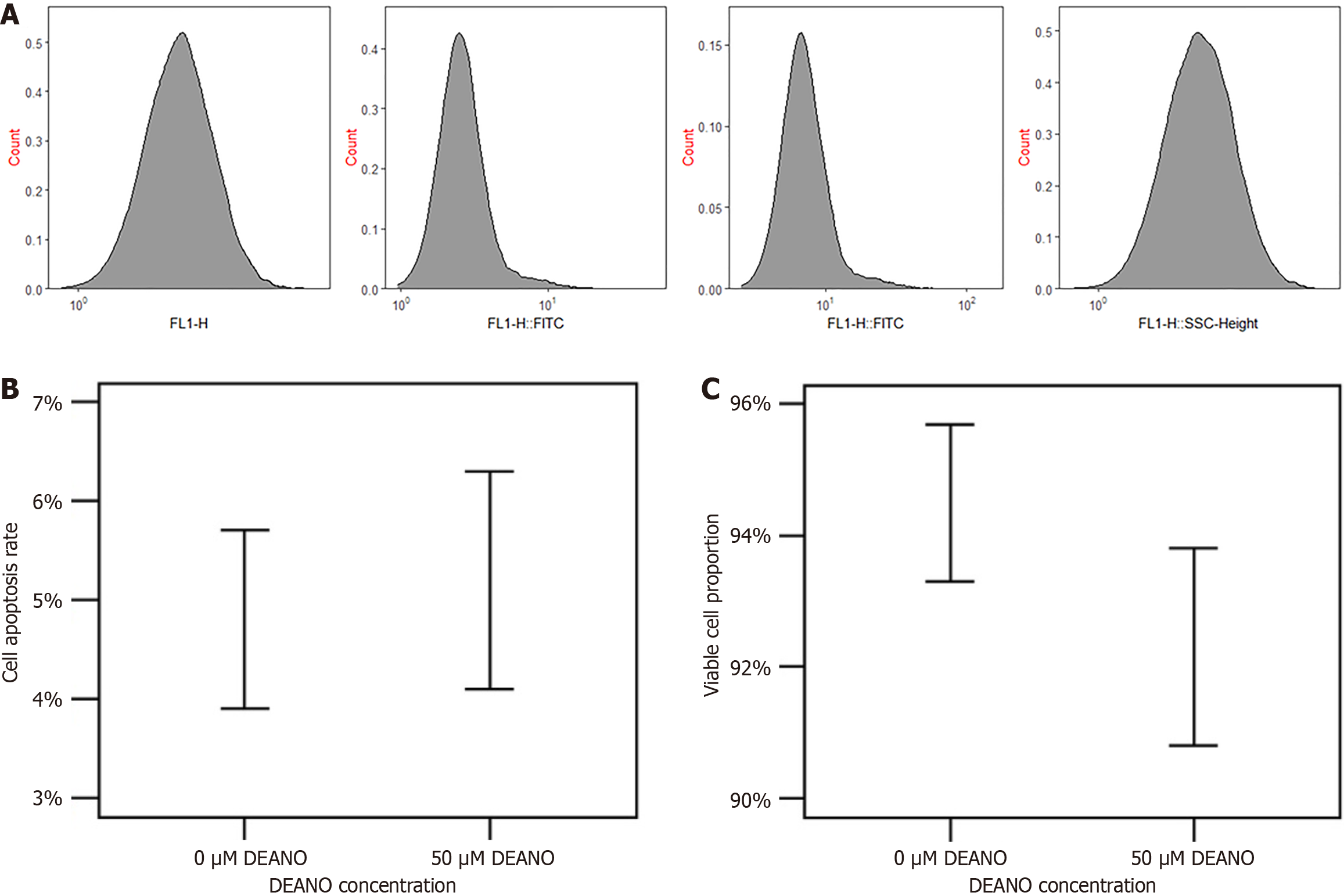
The DAF-FM probe was less cytotoxic as shown by flow cytometry. In terms of quantitative data, following treatment with 50 μM DAF-FM for 24 hours, the apoptotic rate of esophageal cancer cells was 5.2% ± 1.1% (control group: 4.8% ± 0.9%, P > 0.05); the proportion of living cells was 92.3% ± 1.5% (control group: 94.5% ± 1.2%, P > 0.05) (Figure 5).
In the esophagitis cancer model group (n = 10), the NO concentration in esophageal tissue increased gradually with time (week 1: 89.2 ± 7.3 nM; week 4: 126.5 ± 9.8 nM, P < 0.05), and the tumor volume increased from 52.3 ± 8.5 mm3 (week 1) to 189.6 ± 15.2 mm3 (week 4, P < 0.05). In the drug + radiotherapy intervention group (n = 10), the NO concentration showed a rapid decrease at 24 hours after 5-Fu administration: Pre-intervention (112.3 ± 8.7 nM) vs post-intervention (69.2 ± 7.1 nM), a reduction of 38.6% ± 5.2% (P < 0.05). At 48 hours after radiotherapy (2 Gy), the NO concentration further decreased to 59.5 ± 6.8 nM (pre-radiotherapy: 108.5 ± 9.2 nM), a reduction of 45.3% ± 6.1% (P < 0.05). The tumor volume in the intervention group was 89.2 ± 10.3 mm3 at week 4, which was significantly lower than that in the model group (P < 0.05). The linear correlation coefficient between NO concentration (monitored by DAF-FM) and tumor volume was R² = 0.87 (95%CI: 0.79-0.93, P < 0.05), indicating a strong positive correlation. In addition, the Ki-67 positive rate (a marker of cell proliferation) in the intervention group was 32.5% ± 4.2% (model group: 68.7% ± 5.3%, P < 0.05), and the caspase-3 positive rate (a marker of apoptosis) was 28.6% ± 3.5% (model group: 12.3% ± 2.1%, P < 0.05). These results confirmed that changes in NO concentration monitored by DAF-FM were consistent with tumor proliferation and apoptosis trends, verifying the probe’s value in efficacy evaluation.
Among 50 patients with suspected esophagitis cancer, 32 cases were confirmed as esophagitis cancer by histopathological examination (gold standard). The sensitivity of DAF-FM probe detection was 92.5% (29/32), which was significantly higher than that of endoscopic biopsy (78.3%, 25/32, χ² = 4.28, P = 0.039) and comparable to histopathological examination (81.7%, 26/32, χ² = 2.91, P = 0.088); the specificity was 89.3% (16/18), which was not significantly different from that of endoscopic biopsy (85.6%, 15/18, χ² = 0.18, P = 0.672) and histopathological examination (87.2%, 15/18, χ² = 0.08, P = 0.778); the accuracy was 90.0% (45/50), which was higher than that of endoscopic biopsy (80.0%, 40/50, χ² = 3.20, P = 0.074) and histopathological examination (82.0%, 41/50, χ² = 2.17, P = 0.141), but the difference was not statistically significant. The average detection time of the DAF-FM probe was 30 ± 5 minutes, which was significantly shorter than that of endoscopic biopsy (48 ± 2 hours, t = 68.32, P < 0.001) and histopathological examination (72 ± 3 hours, t = 92.56, P < 0.001).
Western blot showed that with increasing NO concentration (0-300 nM), the expression of NF-κB (p65), c-Myc, and Bcl-2 proteins first increased and then decreased: Peak expression was at 50 nM (NF-κB: 2.3 ± 0.2 times the control; c-Myc: 2.5 ± 0.3 times; Bcl-2: 2.1 ± 0.2 times, P < 0.05), and decreased to 0.8 ± 0.1 times, 0.7 ± 0.1 times, and 0.6 ± 0.1 times the control at 300 nM (P < 0.05). The expression of p53 protein (a tumor suppressor gene) increased with NO concentration (300 nM: 3.5 ± 0.3 times the control, P < 0.05). The qRT-PCR results were consistent with those of Western blot. These findings indicate that NO regulates esophagitis carcinogenesis in a concentration-dependent manner: Low NO concentrations (10-50 nM) activate the NF-κB pathway to promote oncogene expression and cell proliferation, while high NO concentrations (100-300 nM) inhibit NF-κB and upregulate p53 to induce apoptosis. Immunofluorescence colocalization showed that the Pearson correlation coefficient between DAF-FM fluorescence signals and NF-κB (p65) signals was 0.78 ± 0.05 (P < 0.05), indicating a strong association between NO and NF-κB activation. This clarifies the molecular logic of DAF-FM in reflecting carcinogenesis: Changes in NO concentration (monitored by DAF-FM) directly correspond to the activation/inhibition of the NF-κB signaling pathway, thus indicating different stages of esophagitis carcinogenesis.
As early as 1863, the German pathologist Virchow confirmed that a large number of white blood cells infiltrate tumor tissues, and believed that tumors often occurred at the site of chronic inflammation, thus proposing the hypothesis that swelling pain originated from chronic inflammation[19]. Unfortunately, this hypothesis did not attract enough attention from scholars at that time. It was not until the early 21st century that the relationship between inflammation and tumor again aroused great interest. Scholars have found that chronic inflammation is involved in the whole process of malig
NO is one of the smallest and simplest bioactive molecules in nature. It is widely found in mammals and is involved in regulating blood multi-cellular activities, including blood vessel growth, smooth muscle relaxation, immune response, cell apoptosis and transmission of synaptic information[27]. In addition to playing a role in normal physiological activities, a large number of studies have confirmed that NO is closely related to the occurrence and development of many diseases, especially tumors[28]. NO can be produced by NO synthase in vivo through oxidation reactions with L-arginine and molecular oxygen as substrates. Nitrogen-containing free radicals such as NO2, NO2-, NO3- and ONOO- generated during NO molecule and NO anabolic processes are collectively referred to as reactive nitrogen metabolites[29]. Existing data show that there is a dual relationship between NO and tumor: An appropriate concentration of NO can promote tumor growth, while high concentrations of NO are not conducive to tumor growth and have anti-tumor effects[30]. In general, the concentration of NO when it exerts anti-tumor effects is 10-100 times higher than that when it promotes tumor growth[31]. Sustained low concentration of NO can promote tumor cell growth, participate in tumor angiogenesis and inhibit tumor cell apoptosis[32].
A high concentration of NO mainly has anti-tumor effects, and its mechanism mediates the tumor-killing effect of macrophages. It also mediates the oncolytic effect of endothelial cells[33]. Binding with superoxide anions in cells to generate nitrogen/oxygen free radicals, damage DNA, and thus produce cytotoxicity; the energy metabolism of cells is affected, and tumor cells die due to energy metabolism disorder[34]. The apoptosis of tumor cells is induced by activating p53 expression. Inhibiting tumor metastasis by inhibiting platelet aggregation can increase the sensitivity of tumor cells to chemotherapy drugs[35]. On the other hand, a large number of studies have found that NO is also widely involved in the process of tumor chemotherapy and immunotherapy, interacting with chemotherapy drugs and cytokines, etc., and affecting the tumor killing effect of drugs[36].
NO plays a role in different stages of carcinogenesis and progression, and is an important factor in the process of tumor initiation, growth and metastasis[37]. Fluorescent probes bind as targeted NO donor molecules and can be used as biomarkers for inflammatory cancer transformation[38]. Our results show that the DAF-FM probe had a concentration-dependent relationship with NO, and the fluorescence intensity increased significantly with time. The DAF-FM probe showed targeted colocalization with lysosomes. The DAF-FM probe revealed less cytotoxicity as shown by flow cytometry. DAF-FM, a high-performance fluorescent probe, may provide a new research idea for the transformation of esophageal cancer. Furthermore, the mechanism exploration results confirmed that NO regulates esophagitis carcinogenesis through the NF-κB signaling pathway: Low-concentrations of NO (10-50 nM) activated NF-κB to promote oncogene expression and cell proliferation, while high-concentrations of NO (100-500 nM) inhibited NF-κB and upregulated p53 to induce apoptosis. This explains the molecular logic of DAF-FM monitoring NO to reflect carcinogenesis as changes in NO concentration directly correspond to the activation/inhibition of carcinogenic signaling pathways, and thus to different stages of esophagitis carcinogenesis.
Efficacy evaluation results further verified the practical value of DAF-FM as the probe can dynamically monitor the changes in NO concentration following drug/radiotherapy intervention. The monitoring results were also positively correlated with tumor volume, Ki-67 (proliferation), and caspase-3 (apoptosis), indicating that DAF-FM can be used as a non-invasive indicator of efficacy in esophagitis cancer treatment. In addition, compared with traditional diagnostic methods, DAF-FM had higher sensitivity and shorter detection time, which is more suitable for early screening of esophagitis carcinogenesis. Its high sensitivity can reduce the missed diagnosis rate of early small lesions, and rapid detection can improve clinical efficiency.
The limitations of the study include the following: (1) The rat esophagitis cancer model, although similar to humans in esophageal structure, still has species differences – for example, the speed of inflammatory carcinogenesis in rats is faster than that in humans, and the response to drugs/radiotherapy may differ to that in humans, which may affect the clinical transformation of DAF-FM; (2) The sample size of patients in the clinical diagnostic comparison was small (50 cases), and multi-center, large-sample studies are needed to further verify the diagnostic performance of DAF-FM; (3) The mechanism exploration focused on the NF-κB signaling pathway, but NO may also regulate esophagitis carcinogenesis through other pathways (such as mitogen-activated protein kinase, phosphatidylinositol 3-kinase/protein kinase B), and the interaction between these pathways and NO remains to be clarified; and (4) The current study only evaluated the short-term efficacy of DAF-FM in monitoring treatment response (4 weeks), and long-term follow-up data (e.g., 6 months to 1 year) are required to confirm whether the probe can predict long-term tumor recurrence or progression.
In the present study, the DAF-FM probe was used to further reveal the physiological mechanism of inflammatory cancer transformation. It was demonstrated that the DAF-FM probe has a good visual screening and efficacy evaluation function in the transformation of esophagitis cancer; thus, providing an important tool for exploring the diagnosis and treatment of esophagitis cancer transformation.
| 1. | Liao CP, Booker RC, Brosseau JP, Chen Z, Mo J, Tchegnon E, Wang Y, Clapp DW, Le LQ. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J Clin Invest. 2018;128:2848-2861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 943] [Article Influence: 157.2] [Reference Citation Analysis (0)] |
| 3. | Manjili SH, Isbell M, Ghochaghi N, Perkinson T, Manjili MH. Multifaceted functions of chronic inflammation in regulating tumor dormancy and relapse. Semin Cancer Biol. 2022;78:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Dominguez C, David JM, Palena C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol. 2017;47:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Li S, Li L, Lin X, Chen C, Luo C, Huang Y. Targeted Inhibition of Tumor Inflammation and Tumor-Platelet Crosstalk by Nanoparticle-Mediated Drug Delivery Mitigates Cancer Metastasis. ACS Nano. 2022;16:50-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Nisticò P, Ciliberto G. Biological mechanisms linked to inflammation in cancer: Discovery of tumor microenvironment-related biomarkers and their clinical application in solid tumors. Int J Biol Markers. 2020;35:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Marques P, de Vries F, Dekkers OM, Korbonits M, Biermasz NR, Pereira AM. Serum Inflammation-based Scores in Endocrine Tumors. J Clin Endocrinol Metab. 2021;106:e3796-e3819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Radomska KJ, Coulpier F, Gresset A, Schmitt A, Debbiche A, Lemoine S, Wolkenstein P, Vallat JM, Charnay P, Topilko P. Cellular Origin, Tumor Progression, and Pathogenic Mechanisms of Cutaneous Neurofibromas Revealed by Mice with Nf1 Knockout in Boundary Cap Cells. Cancer Discov. 2019;9:130-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Marelli G, Sica A, Vannucci L, Allavena P. Inflammation as target in cancer therapy. Curr Opin Pharmacol. 2017;35:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Na H, Song Y, Lee HW. Emphasis on Adipocyte Transformation: Anti-Inflammatory Agents to Prevent the Development of Cancer-Associated Adipocytes. Cancers (Basel). 2023;15:502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | Huysseune A, Larsen UG, Larionova D, Matthiesen CL, Petersen SV, Muller M, Witten PE. Bone Formation in Zebrafish: The Significance of DAF-FM DA Staining for Nitric Oxide Detection. Biomolecules. 2023;13:1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Oiki S, Nasuno R, Urayama SI, Takagi H, Hagiwara D. Intracellular production of reactive oxygen species and a DAF-FM-related compound in Aspergillus fumigatus in response to antifungal agent exposure. Sci Rep. 2022;12:13516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Yang B, Tu C, Ping Y, Chen S, Wu T, Zhao Z, Mao Y, Yang Z, Cao Z, Li J, Huang K, Ding X, Wu G, Zou P, Deng Z, Sun X. Mitochondrial impairment and downregulation of Drp1 phosphorylation underlie the antiproliferative and proapoptotic effects of alantolactone on oral squamous cell carcinoma cells. J Transl Med. 2023;21:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Kumari A, Bhatoee M, Singh P, Kaladhar VC, Yadav N, Paul D, Loake GJ, Gupta KJ. Detection of Nitric Oxide from Chickpea Using DAF Fluorescence and Chemiluminescence Methods. Curr Protoc. 2022;2:e420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Gardiner B, Dougherty JA, Ponnalagu D, Singh H, Angelos M, Chen CA, Khan M. Measurement of Oxidative Stress Markers In Vitro Using Commercially Available Kits. 2020 Aug 9. In: Measuring Oxidants and Oxidative Stress in Biological Systems [Internet]. Cham (CH): Springer; 2020–. [PubMed] |
| 16. | Datta R, Heaster TM, Sharick JT, Gillette AA, Skala MC. Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications. J Biomed Opt. 2020;25:1-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 449] [Cited by in RCA: 500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 17. | Okuno H, Ieda N, Hotta Y, Kawaguchi M, Kimura K, Nakagawa H. A yellowish-green-light-controllable nitric oxide donor based on N-nitrosoaminophenol applicable for photocontrolled vasodilation. Org Biomol Chem. 2017;15:2791-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Gu YY, Tan XH, Song WP, Song WD, Yuan YM, Xin ZC, Wang JD, Fang D, Guan RL. Icariside Ⅱ Attenuates Palmitic Acid-Induced Endothelial Dysfunction Through SRPK1-Akt-eNOS Signaling Pathway. Front Pharmacol. 2022;13:920601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I, Henneman L, Kas SM, Prekovic S, Hau CS, Vrijland K, Drenth AP, de Korte-Grimmerink R, Schut E, van der Heijden I, Zwart W, Wessels LFA, Schumacher TN, Jonkers J, de Visser KE. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572:538-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 20. | Orisaka M, Mizutani T, Miyazaki Y, Shirafuji A, Tamamura C, Fujita M, Tsuyoshi H, Yoshida Y. Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front Endocrinol (Lausanne). 2023;14:1324429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 21. | Tang F, Wang Y, Hemmings BA, Rüegg C, Xue G. PKB/Akt-dependent regulation of inflammation in cancer. Semin Cancer Biol. 2018;48:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Catalano M, Roviello G, Santi R, Villari D, Spatafora P, Galli IC, Sessa F, Conte FL, Mini E, Cai T, Nesi G. Inflammation in Urological Malignancies: The Silent Killer. Int J Mol Sci. 2023;24:866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Liu K, He X, Huang J, Yu S, Cui M, Gao M, Liu L, Qian Y, Xie Y, Hui M, Hong Y, Nie X. Short-chain fatty acid-butyric acid ameliorates granulosa cells inflammation through regulating METTL3-mediated N6-methyladenosine modification of FOSL2 in polycystic ovarian syndrome. Clin Epigenetics. 2023;15:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 24. | Ritter B, Greten FR. Modulating inflammation for cancer therapy. J Exp Med. 2019;216:1234-1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Çıtar Dazıroğlu ME, Acar Tek N. The Effect on Inflammation of Adherence to the Mediterranean Diet in Polycystic Ovary Syndrome. Curr Nutr Rep. 2023;12:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 26. | Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11:805-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 442] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 27. | López-Sánchez LM, Aranda E, Rodríguez-Ariza A. Nitric oxide and tumor metabolic reprogramming. Biochem Pharmacol. 2020;176:113769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Hu Y, Xiang J, Su L, Tang X. The regulation of nitric oxide in tumor progression and therapy. J Int Med Res. 2020;48:300060520905985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | McGinity CL, Palmieri EM, Somasundaram V, Bhattacharyya DD, Ridnour LA, Cheng RYS, Ryan AE, Glynn SA, Thomas DD, Miranda KM, Anderson SK, Lockett SJ, McVicar DW, Wink DA. Nitric Oxide Modulates Metabolic Processes in the Tumor Immune Microenvironment. Int J Mol Sci. 2021;22:7068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Girotti AW, Fahey JM. Upregulation of pro-tumor nitric oxide by anti-tumor photodynamic therapy. Biochem Pharmacol. 2020;176:113750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Girotti AW, Bazak J, Korytowski W. Pro-Tumor Activity of Endogenous Nitric Oxide in Anti-Tumor Photodynamic Therapy: Recently Recognized Bystander Effects. Int J Mol Sci. 2023;24:11559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Girotti AW, Fahey JF, Korytowski W. Role of nitric oxide in hyper-aggressiveness of tumor cells that survive various anti-cancer therapies. Crit Rev Oncol Hematol. 2022;179:103805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 33. | He Q, Qu M, Xu C, Shi W, Hussain M, Jin G, Zhu H, Zeng LH, Wu X. The emerging roles of nitric oxide in ferroptosis and pyroptosis of tumor cells. Life Sci. 2022;290:120257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Girotti AW. Upregulation of nitric oxide in tumor cells as a negative adaptation to photodynamic therapy. Lasers Surg Med. 2018;50:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Vedenko A, Panara K, Goldstein G, Ramasamy R, Arora H. Tumor Microenvironment and Nitric Oxide: Concepts and Mechanisms. Adv Exp Med Biol. 2020;1277:143-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Holotiuk VV, Kryzhanivska AY, Churpiy IK, Tataryn BB, Ivasiutyn DY. Role of nitric oxide in pathogenesis of tumor growth and its possible application in cancer treatment. Exp Oncol. 2019;41:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Qin Y, You SH, Zhang Y, Venu A, Hong Y, Min JJ. Genetic Programming by Nitric Oxide-Sensing Gene Switch System in Tumor-Targeting Bacteria. Biosensors (Basel). 2023;13:266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 38. | Li CY, Anuraga G, Chang CP, Weng TY, Hsu HP, Ta HDK, Su PF, Chiu PH, Yang SJ, Chen FW, Ye PH, Wang CY, Lai MD. Repurposing nitric oxide donating drugs in cancer therapy through immune modulation. J Exp Clin Cancer Res. 2023;42:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/