Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.113401
Revised: September 14, 2025
Accepted: October 10, 2025
Published online: November 27, 2025
Processing time: 93 Days and 4.5 Hours
The largest multi-institutional cohort analysis of bile spillage in incidental gallbladder cancer was presented by van Dooren et al The study offers important insights, though certain methodological limitations and interpretative challenges temper the strength of its conclusions. We address these, clarify how statistical findings intersect with clinical relevance for bile spillage, propose a refined classification system, and provide global epidemiological context.
Core Tip: Bile spillage remains clinically relevant in incidental gallbladder cancer despite loss of statistical independence in multivariate overall survival analysis. This letter contextualizes gallbladder cancer incidence globally, identifies methodological flaws and a key limitation in van Dooren et al, and proposes a bile spillage classification framework.
- Citation: Liau MYQ, Shelat VG. Rethinking the prognostic significance of bile spillage in gallbladder cancer. World J Gastrointest Surg 2025; 17(11): 113401
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/113401.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.113401
We read with interest the article by van Dooren et al[1] on bile spillage and survival in incidental gallbladder cancer (iGBC). For clarity, our letter uses the term ‘bile spillage’ to denote intraoperative rupture or contamination, in line with the original study, and avoids conflation with postoperative ‘bile leak’, which is a distinct complication. We commend van Dooren et al[1] for compiling the largest multi-institutional dataset to date on iGBC, which enhances the statistical power and external validity of their findings. The systematic collection of operative details, survival outcomes, and re-resection data across diverse centers represents a significant effort that strengthens the field’s understanding of bile spillage in this uncommon but clinically consequential disease. While the study adds valuable data, methodological weaknesses should be considered in tandem with its conclusions.
Gallbladder cancer (GBC) is considered relatively rare at the global level, with an age-standardized incidence rate of around 2.5 per 100000 population in 2021[2]. However, substantial regional heterogeneity exists, with rates exceeding 10-20 per 100000 in high-incidence areas such as northern India, Chile, and Japan[3]. In such regions, GBC meets the threshold of a ‘common’ cancer despite its rarity on a worldwide scale. Thus, labeling GBC as a ‘rare cancer’ does not account for regional variation as incidence patterns are population- and geography-dependent. Emerging evidence suggests that factors such as biliary Helicobacter pylori colonization may contribute to carcinogenesis and could partly underlie geographic heterogeneity; however, detection rates and causal pathways vary by setting and remain incompletely defined[4].
In the current study, univariate analysis demonstrated that bile spillage nearly doubled mortality risk [hazard ratio (HR) = 1.97, P < 0.001] and was associated with a markedly reduced median overall survival (OS)[1]. However, in multivariate analysis, the association lost statistical significance (HR = 1.21, P = 0.313), likely due to confounding by factors such as advanced tumor stage, comorbidity burden, or surgical complexity. Importantly, this statistical neutrality does not diminish its clinical relevance, as bile spillage remains a potentially modifiable intraoperative event with implications for recurrence patterns and postoperative management, because clinicians act on biological plausibility and patient outcomes, not P-values alone. Importantly, although independence for OS may attenuate in some multivariable models, multiple series demonstrate consistent, independent associations with disease-free survival (DFS), aligning with biological plausibility and recurrence patterns. In a single-center series, Lee et al[5] reported that patients with bile spillage had dramatically shorter DFS (20.9 months vs 71.4 months; P = 0.028) and OS (25.8 months vs 72.6 months; P = 0.014) compared with those without spillage, despite similar tumor stages. In a population-based cohort from Alberta, Horkoff et al[6] found bile spillage to be an independent predictor of shorter DFS [HR = 1.99; 95% confidence interval (CI): 1.07-3.67] on multivariable analysis, underscoring its prognostic importance even when adjusted for re-resection rates and nodal status.
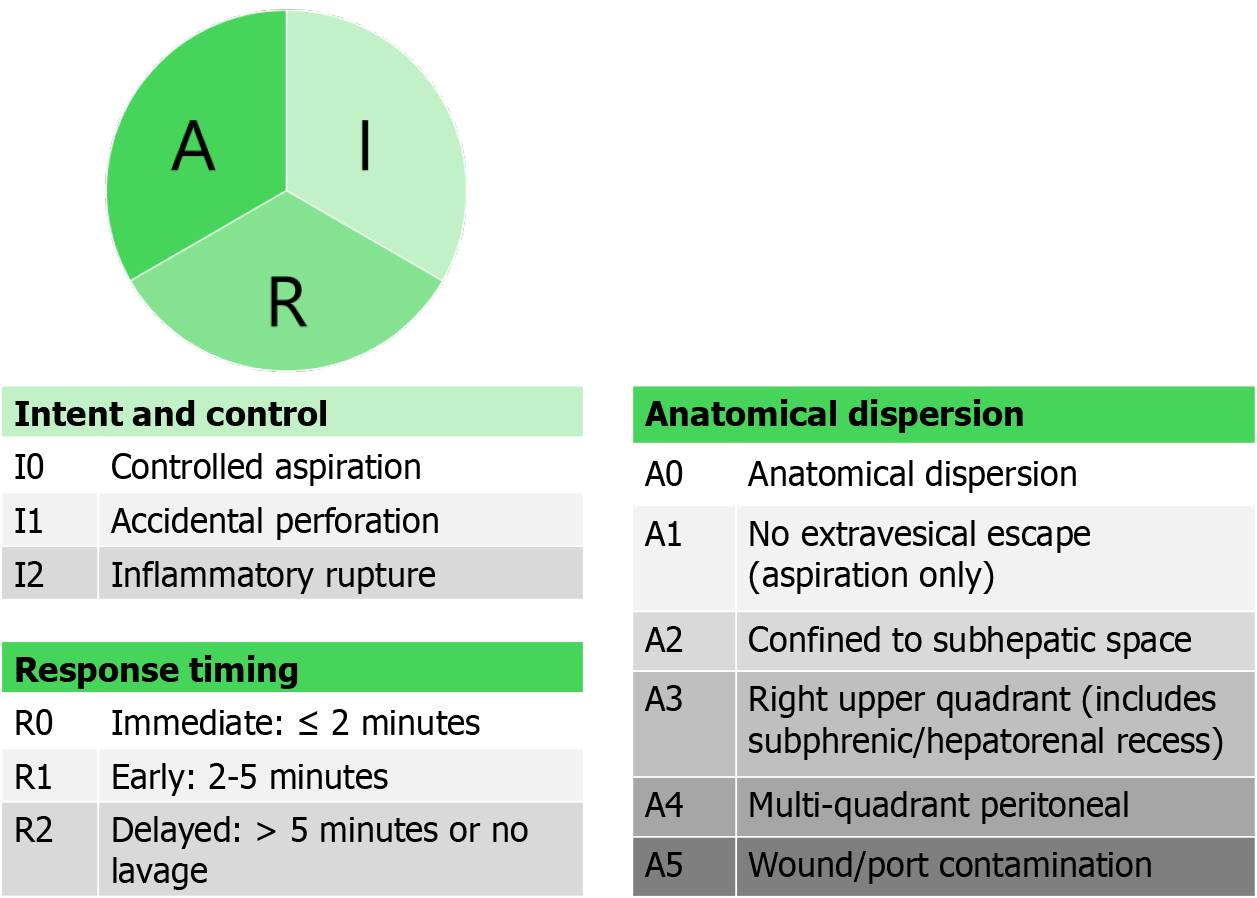
We propose a three-dimensional descriptive framework to standardize reporting and enable risk stratification: (1) Intent and control; (2) Anatomical dispersion; and (3) Response timing (Figure 1). Our proposed operational definitions are as follows: A0 - anatomical dispersion; A1 - no extra-vesical escape (aspiration only); A2 - confined to subhepatic space; A3 - right upper quadrant (includes subphrenic/hepatorenal recess); A4 - multi-quadrant peritoneal; A5 - wound/port contamination.
We are of the view that adequate response timing can be graded as: (a) Immediate: ≤ 2 minutes; (b) early: 2-5 minutes; and (c) delayed: > 5 minutes or no lavage. This framework may help standardize reporting, enable more precise risk assessment, and inform targeted perioperative strategies in iGBC. Intent and control are recorded to support root-cause analysis and quality improvement; when dimensions 2-3 are captured, its independent prognostic value may be limited. By differentiating between an intentional aspiration vs an inadvertent rupture with diffuse contamination, clinicians can better anticipate recurrence patterns and tailor surveillance intensity. For example, a patient with accidental perforation and widespread peritoneal contamination who did not receive prompt lavage may be counseled differently regarding re-resection or systemic therapy than one with contained spillage that was immediately managed.
The study has methodological weaknesses which may impact the reliability of its conclusions, and we will discuss these. First, misclassification bias is particularly likely because ‘no documentation’ appears to have been treated as ‘no bile spillage,’ despite well-described gaps in operative recording for laparoscopic cholecystectomy. Narrative operative notes frequently omit key intraoperative steps and do not consistently reflect reality when benchmarked against video, and completion of dictated reports is inferior to synoptic templates (e.g., 76% vs 99.7%)[7]. Video (and audio-augmented video) captures intraoperative events more completely than written notes (e.g., 92.3% vs 78% depiction), and adverse intraoperative events are sometimes not recorded at all in narrative reports[8]. Taken together, equating absence of documentation with absence of spillage likely underestimates true exposure and biases associations, particularly in centers without standardized, synoptic reporting that explicitly prompts for perforation, spillage, and lavage details. Our proposed three-dimensional classification directly addresses this gap by offering a structured template that can be embedded in operative reporting. By prompting surgeons to document whether spillage was intentional or accidental, whether contamination was localized or diffuse, and how promptly it was managed, this framework minimizes misclassification bias and enhances dataset reliability. Second, misclassification bias is compounded by a discordance in data handling: While the methods section states that non-documentation was treated as absence, the results section reports exclusion of 41 patients due to unavailable spillage information, raising questions about uniformity. Third, nodal staging fidelity is problematic. The study’s definition of regional lymph nodes is inconsistent with American Joint Committee on Cancer 8th edition, which stages nodal status by number (N0: 0; N1: 1-3; N2: ≥ 4) rather than location. When this deviation is coupled with a minimal harvest (nearly all patients had only 1-2 nodes retrieved), the risks of understating, prognostic misclassification, and biased survival comparisons are substantial. Supporting this concern, a Surveillance, Epidemiology, and End Results analysis of 1754 GBC resections showed that examining ≥ 2 negative lymph nodes (NLNs) was associated with markedly better 5-year cause-specific survival than < 2 NLNs (40.9% vs 23.1%; P < 0.001) and that NLN count remained an independent prognostic factor (HR = 0.761; 95%CI: 0.627-0.925, P = 0.006)[9]. Notably, patients with < 2 NLNs had fewer total regional nodes assessed (median 1) than those with ≥ 2 NLNs (median 5), and NLN and total node counts were strongly correlated (r = 0.929). This underscores how low yields impair staging fidelity. The survival separation with ≥ 2 NLNs persisted across stages (e.g., stage I/II 73.3% vs 54.2%; stage III/IV 20.0% vs 13.7%; all P < 0.001), making it likely that a dataset dominated by 1-2 nodes examined will misclassify biologically node-positive disease as N0 and dilute treatment effects. Reports of robotic radical cholecystectomy demonstrate that higher lymph node yields (> 6) are achievable, but this is ancillary to the central question of spillage. Moreover, Blakely et al[10] showed that intraoperative bile spillage independently predicted significantly worse progression-free survival (HR = 6.5; 95%CI: 2.46-17.4; P = 0.0002), highlighting its biological impact beyond nodal and margin status. Fourth, selection bias further limits validity. The exclusion of patients with perioperative suspicion of GBC from certain subgroup analyses is inconsistent with the study’s own definition of iGBC, reducing generalizability. Finally, although the dataset is described as “high quality”, the authors acknowledge substantial missing and heterogeneous data, possible under-reporting of bile spillage, frequent omission of re-resection, and limited sample size, all of which undermine the robustness of their conclusions.
The reported finding that 20% of patients undergoing surgery for presumed cholecystitis had T3/T4 tumors raises concern, as such advanced lesions would typically be detectable on preoperative imaging or arouse intraoperative suspicion, suggesting potential gaps in diagnostic evaluation. The Gallbladder Reporting and Data System, a consensus-driven ultrasound lexicon, has shown to standardize reporting, enhance detection of malignancy, and guide multiphasic scan[11]. The author’s statement that no study has shown bile spillage to be an independent predictor of OS is technically correct for multivariate OS analyses. However, multiple studies demonstrate strong univariate associations with OS and independent associations with DFS. A recent meta-analysis by Sugumar et al[12], pooling 11 studies (n = 1116), demonstrated that biliary spillage conferred worse OS (pooled HR = 1.68; 95%CI: 1.32-2.14) and DFS (pooled HR = 2.19; 95%CI: 1.30-3.68), as well as a nine-fold increase in peritoneal recurrence (odds ratio = 9.37; 95%CI: 3.49-25.2). Furthermore, the study lacks important details on adjuvant chemotherapy and radiotherapy, including regimen differences between palliative and curative intent, the clinical indications for treatment, and the anatomical sites targeted, limiting the ability to interpret the potential influence of adjuvant therapy on outcomes.
The findings of this study, even with their limitations, carry practical implications for clinical practice. Intraoperative documentation of spillage (extent and response) and multidisciplinary review can help tailor surveillance and adjuvant therapy; however, current GBC staging systems do not incorporate bile spillage, and there is no evidence-based justification for using spillage as a formal prognostic parameter or for recommending radical re-resection solely for uncontrolled spillage in the absence of confirmed malignancy. Intraperitoneal strategies (e.g., hyperthermic intraperitoneal chemotherapy) remain experimental in incidental GBC with no prospective data supporting routine use. That said, uncontrolled, widespread, or inadequately managed spillage may adversely influence recurrence and survival, warranting careful documentation and rigorous evaluation in future studies. Where spillage is uncontrolled and multi-quadrant, or when intraoperative findings suggest advanced disease, selective referral to hepato-pancreato-biliary specialists is reasonable.
The largest multi-institutional dataset to date on bile spillage in iGBC has been provided by van Dooren et al[1], adding valuable information to a scarce evidence base. Nonetheless, methodological flaws, definitional inconsistencies, and incomplete or heterogeneous data limit the strength of the conclusions that can be drawn. Given the established univariate association with survival and the potential for bile spillage to influence recurrence patterns, it should remain an important intraoperative consideration. Future studies incorporating standardized spillage classification and robust nodal staging will help refine prognostication and improve patient outcomes.
| 1. | van Dooren M, de Savornin Lohman EA, van der Post RS, Hoogwater FJ, van den Boezem PB, Groot Koerkamp B, Erdmann JI, de Reuver PR. Bile spillage in incidental gallbladder cancer is not an independent predictor for survival: A multi-institute retrospective cohort study. World J Gastrointest Surg. 2025;17:106919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Xie D, Liu F, Zhou D, Zhu Q, Xiao F, Zhang K. Global burden and cross-country inequalities in gallbladder and biliary tract cancer (1990-2021) with projections to 2050: insights from the global burden of disease study 2021. Front Med (Lausanne). 2025;12:1520714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Vuthaluru S, Sharma P, Chowdhury S, Are C. Global epidemiological trends and variations in the burden of gallbladder cancer. J Surg Oncol. 2023;128:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (1)] |
| 4. | Lim KPK, Lee AJL, Jiang X, Teng TZJ, Shelat VG. The link between Helicobacter pylori infection and gallbladder and biliary tract diseases: A review. Ann Hepatobiliary Pancreat Surg. 2023;27:241-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Lee JM, Kim BW, Kim WH, Wang HJ, Kim MW. Clinical implication of bile spillage in patients undergoing laparoscopic cholecystectomy for gallbladder cancer. Am Surg. 2011;77:697-701. [PubMed] |
| 6. | Horkoff MJ, Ahmed Z, Xu Y, Sutherland FR, Dixon E, Ball CG, Bathe OF. Adverse Outcomes After Bile Spillage in Incidental Gallbladder Cancers: A Population-based Study. Ann Surg. 2021;273:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Deal SB, D'Angelica MI, Hawkins WG, Pucci M, Ujiki M, Brunt LM, Wexner S, Alseidi AA. Synoptic operative reporting for laparoscopic cholecystectomy and pancreaticoduodenectomy: A multi institutional pilot study evaluating completeness and surgeon perceptions. Am J Surg. 2018;216:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | O'Connor N, Sugrue M, Melly C, McGeehan G, Bucholc M, Crawford A, O'Connor P, Abu-Zidan F, Wani I, Balogh ZJ, Shelat VG, Tebala GD, De Simone B, Eid HO, Chirica M, Fraga GP, Di Saverio S, Picetti E, Bonavina L, Ceresoli M, Fette A, Sakakushe B, Pikoulis E, Coimbra R, Ten Broek R, Hecker A, Leppäniemi A, Litvin A, Stahel P, Tan E, Koike K, Catena F, Pisano M, Coccolini F, Johnston A. It's time for a minimum synoptic operation template in patients undergoing laparoscopic cholecystectomy: a systematic review. World J Emerg Surg. 2022;17:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Lin JY, Bai DS, Zhou BH, Chen P, Qian JJ, Jin SJ, Jiang GQ. Positive relationship between number of negative lymph nodes and duration of gallbladder cancer cause-specific survival after surgery. Cancer Manag Res. 2018;10:6961-6969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Blakely AM, Wong P, Chu P, Warner SG, Raoof M, Singh G, Fong Y, Melstrom LG. Intraoperative bile spillage is associated with worse survival in gallbladder adenocarcinoma. J Surg Oncol. 2019;120:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Gupta P, Dutta U, Rana P, Singhal M, Gulati A, Kalra N, Soundararajan R, Kalage D, Chhabra M, Sharma V, Gupta V, Yadav TD, Kaman L, Irrinki S, Singh H, Sakaray Y, Das CK, Saikia U, Nada R, Srinivasan R, Sandhu MS, Sharma R, Shetty N, Eapen A, Kaur H, Kambadakone A, de Haas R, Kapoor VK, Barreto SG, Sharma AK, Patel A, Garg P, Pal SK, Goel M, Patkar S, Behari A, Agarwal AK, Sirohi B, Javle M, Garcea G, Nervi F, Adsay V, Roa JC, Han HS. Gallbladder reporting and data system (GB-RADS) for risk stratification of gallbladder wall thickening on ultrasonography: an international expert consensus. Abdom Radiol (NY). 2022;47:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Sugumar K, De Mond J, Vijay A, Paramesh AS, Jeon H, Pointer DT, Corsetti RL. Bile Spillage as a Prognostic Factor for Gall Bladder Cancer: A Systematic Review and Meta-Analysis. J Surg Res. 2024;299:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/