Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.110551
Revised: July 10, 2025
Accepted: October 21, 2025
Published online: November 27, 2025
Processing time: 169 Days and 20 Hours
This study presents a comprehensive overview of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP), detailing its epidemiology, pathophysiology, prevention, and treatment. PEP is the most common compli
Core Tip: Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis is the most common complication of ERCP. Its pathogenesis involves mechanical injury that leads to increased pressure within the pancreatic duct, thereby causing oxidative stress and an inflammatory cascade triggered by dysbiosis. Multiple risk factors are associated with the patient, procedural aspects, and the operator. Prevention strategies primarily focus on patient risk assessment and stratified management, along with optimization of pharmacological treatment and surgical approaches. Advances in treatment emphasize precise interventions and a multidisciplinary collaborative model that integrates medical resources. Future research needs to explore genetic susceptibility, novel targeted therapies, and artificial intelligence-assisted risk assessments.
- Citation: Zhao WY, Zhao JW, Yu L, Yu ZY. Post-endoscopic retrograde cholangiopancreatography pancreatitis: Mechanistic pathways, diagnostic benchmarks, and emerging and mitochondria-targeted therapies. World J Gastrointest Surg 2025; 17(11): 110551
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/110551.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.110551
With the advent of minimally invasive imaging modalities, including computed tomography, magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS), and endoscopic retrograde cholangiopancreatography (ERCP), the field has progressed from a purely diagnostic technique to one with both diagnostic and therapeutic applications. ERCP is now the primary intervention for many pancreaticobiliary disorders, facilitating stone extraction and relieving benign or malignant biliary obstruction[1,2]. Among commonly performed endoscopic procedures, ERCP is the most technically demanding and carries the highest rates of complications and mortality. The principal ERCP-related adverse events (AEs) are cholangitis, perforation, bleeding, hypoxemia, cholecystitis, and pancreatitis, with post-ERCP pancreatitis (PEP) being the most frequent, followed by sedation-related events[3].
A multicenter prospective cohort study conducted in Japan (April 2017 to March 2018) across 36 hospitals evaluated ERCP-associated AEs in patients with biliary disease and an intact papilla. The overall AE rate was 10.1%, and ERCP-related mortality was 0.08%. PEP occurred in 258 cases (6.9%)—the highest incidence among all reported events[4].
A recent meta-analysis of 145 randomized controlled trials (RCTs) encompassing 19038 patients randomized to placebo or no-stent groups found an overall cumulative PEP incidence of 10.2% (95%CI: 9.3-11.3). Across 91 RCTs involving 14441 patients, the cumulative incidence of severe PEP was 0.5% (95%CI: 0.3-0.7), with a mortality rate of 0.2% (95%CI: 0.08-0.3). In 35 RCTs that included 3733 high-risk patients (as defined by study authors), the cumulative PEP incidence was 14.1% (95%CI: 11.5-17.2); severe PEP occurred in 0.8% (95%CI: 0.4-1.6) and mortality in 0.2% (95%CI: 0-0.3). From 1977 to 2022, the incidence of PEP among patients randomized to placebo or no-stent groups showed no significant temporal change (P = 0.48).
The study rigorously defined inclusion and exclusion criteria to minimize bias. Two independent authors performed a systematic review in line with Cochrane guidance and the Agency for Healthcare Research and Quality methods manual. Titles judged unrelated to ERCP by both reviewers were excluded. During full-text screening, only RCTs that reported PEP were retained for data extraction. One reviewer extracted the data, which a second reviewer independently verified. The meta-analysis, however, did not report use of the Jadad scale or any other formal tool to assess study quality[5].
PEP incidence varies by region and study period. In RCTs from North America, Europe, and Asia, the incidence was 13%, 8.4%, and 9.9%, respectively. Trials conducted before 2000 reported a 7.7% incidence, rising to 10% in studies conducted after 2000[6].
A retrospective cohort study utilizing inpatient sample data (2007-2016) that included all patients with ERCP ICD9-10-CM procedure codes demonstrated a continuous rise in inpatient ERCP utilization over the past decade. Although diagnostic ERCP has appropriately declined[7], therapeutic ERCP has expanded—particularly biliary and pancreatic stenting and biliary dilation—contributing to an increased PEP incidence. Resource-use trends show a shorter average hospital stay over the same period, but total inpatient costs have risen; PEP alone is estimated to cost the United States healthcare system roughly USD 200 million annually[1].
PEP remains an unavoidable and clinically significant complication[1]. This review examines the pathophysiology of PEP, strategies for its early recognition and diagnosis, and evidence-based approaches to prevention and treatment. It highlights recent advances—particularly precision interventions such as mitochondrial-targeted nanomedicines (mTWNDs) and the use of multidisciplinary team (MDT) models in PEP management—that have been under-re
Although the pathophysiology of PEP is not yet fully understood, current evidence implicates a combination of mechanical and chemical insults: (1) Mechanical trauma: Catheter insertion can injure the papilla, provoking sphincter of Oddi spasm and/or papillary edema; (2) Thermal injury: Electrocautery may damage tissue during sphincterotomy; (3) Chemical irritation: Certain contrast agents can be directly toxic to pancreatic tissue; (4) Hydrostatic stress: Excessive saline or water infusion elevates intraductal pressure; and (5) Reflux and bacterial translocation: Instrument manipulation may permit retrograde flow of intestinal enzymes or microbes, initiating inflammatory cascades[8,9].
The key cellular mechanisms underlying acute pancreatitis (AP) include mitochondrial dysfunction, accumulation of reactive oxygen species (ROS), and impaired autophagy[10]. Among these processes, mitochondrial oxidative stress is central to disease pathogenesis. Recent evidence indicates that mitochondrial ROS (mtROS) function as signaling molecules that drive the production of pro-inflammatory cytokines through both inflammasome-dependent and -independent pathways[11,12]. Specifically, the NLRP3 inflammasome interacts with mtROS, inducing the release of mitochondrial DNA (mtDNA) and thereby amplifying inflammatory activation[12]. In AP, circulating concentrations of interleukin (IL)-1β, IL-6, IL-18, and tumor necrosis factor-α (TNF-α) are consistently elevated and act as key mediators of tissue injury and necrosis[13,14]. Maturation of IL-1β and IL-18 is contingent upon NLRP3 inflammasome activation[15]. Moreover, mtDNA is recognized as a damage-associated molecular pattern that initiates immune responses—particularly via the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway—thereby propagating inflammation[16,17].
According to the Ca2+-overload hypothesis, membrane damage during AP permits extracellular Ca2+ influx, leading to intracellular Ca2+ accumulation. Excess Ca2+ impairs mitochondrial function and energy-dependent Ca2+ pumps and prematurely activates digestive enzymes, resulting in pronounced inflammatory responses. ROS can exacerbate Ca overload through multiple mechanisms, further compromising barrier integrity[18]. In pancreatic acinar cells (PACs), exposure to bile acids generates mtROS, which in turn elevates mitochondrial Ca2+ levels in both human and murine models[19].
The 2020 European Society of Gastrointestinal Endoscopy (ESGE) guidelines on ERCP-related AEs define PEP as new or worsening abdominal pain accompanied by pancreatic-enzyme elevation [amylase or lipase ≥ three-fold above the upper limit of normal (ULN)] that necessitates or prolongs hospitalization[3]. Since the earliest descriptions of ERCP complications, the definition of “clinical pancreatitis” has been refined to delineate PEP more precisely.
First, the presence of new or exacerbated abdominal pain has been formally incorporated as a diagnostic criterion[20]. However, within the first 24 hours after ERCP, it remains difficult to distinguish transient abdominal discomfort caused by postoperative intestinal distension from hyperamylasemia due to true PEP[9].
Second, controversy persists regarding the optimal timing of pancreatic-enzyme testing and the threshold that defines clinical significance. The initial definition specified a ≥ three-fold rise in serum amylase measured more than 24 hours after ERCP and required hospitalization or extension of the hospital stay by 2-3 days[21]. Tryliskyy and Bryce[22], citing Testoni et al[23], noted that in over two-thirds of patients with PEP, serum amylase exceeded fivefold the ULN 4 hours after sphincterotomy; they therefore proposed the 4 hours amylase measurement as a reliable early marker. A subsequent study by Testoni et al[23] in 1999 showed that persistent pain for 24 hours combined with an amylase level > five-fold the ULN constitutes the most dependable indicator of AP. Several studies have explored temporal changes in pancreatic enzymes to exclude PEP. In 2007, Ito et al[24] reported that serum amylase levels exceeding twice the ULN 3 hours after ERCP—but declining by 6 hours—effectively ruled out PEP. Gottlieb et al[26] likewise suggested that 2 hours serum amylase and lipase measurements can exclude the diagnosis.
More recently, Goyal et al[25] performed a meta-analysis of 18 studies involving 11790 ERCP procedures, 764 of which (6.48%) resulted in PEP. When enzyme levels were assessed 2-4 hours after ERCP, a lipase concentration < 3 × ULN reliably excluded PEP, particularly when interpreted alongside clinical findings. The authors therefore recommend multicenter RCTs to validate both lipase and amylase as early predictors of PEP in high-risk patients, as defined by the 2020 ESGE guidelines[25,26].
At present, the 2014 revised ESGE guidelines and the 2020 ESGE guidelines on AEs related to ERCP have been widely adopted and accepted.
PEP severity is stratified according to hospital-stay duration and the presence of local or systemic complications. The 1991 consensus criteria define mild PEP as a 2-3-day hospital stay, moderate PEP as 4-10 days, and severe PEP as > 10 days or the occurrence of hemorrhagic pancreatitis or pancreatic pseudocysts requiring intervention (percutaneous drainage or surgery)[21].
The revised Atlanta classification incorporates local complications, systemic complications, and organ failure—together with their persistence (> 48 hours) or resolution (≤ 48 hours)—to grade AP severity[27]. Severe disease is characterized by persistent organ failure, moderate disease by transient organ failure or local/systemic complications, and mild disease by the absence of complications[27]. In a multicenter study, the Atlanta system outperformed the 1991 consensus criteria in predicting mortality (Table 1)[28].
| Type | Severity | Advantages and disadvantages | ||
| Mild | Moderate | Severe | ||
| Consensus classification[22] | Length of hospital stay as 2-3 days | Length of hospital stay as 4-10 days | A hospital stay of more than 10 days, or the occurrence of hemorrhagic pancreatitis or pancreatic pseudocysts requiring intervention (percutaneous drainage or surgery) | Advantages: (1) Operational simplicity; (2) Strong clinical relevance; and (3) Facilitates statistical analysis and comparison. Disadvantages: (1) Lack of specificity; (2) Lack of sensitivity; and (3) Not applicable to all patient groups |
| Revised Atlanta classification[27] | The absence of complications | Transient (≤ 48 hours) organ failure or local or systemic complications | Persistent (> 48 hours) organ failure | Advantages: (1) Clear severity grading; (2) Based on clinical symptoms and signs; and (3) Promote clinical research and comparison. Disadvantages: (1) Inadequate identification of mild PEP cases; (2) Lack of reflection on the dynamic changes of the condition; and (3) Incomplete consideration of prognostic factors |
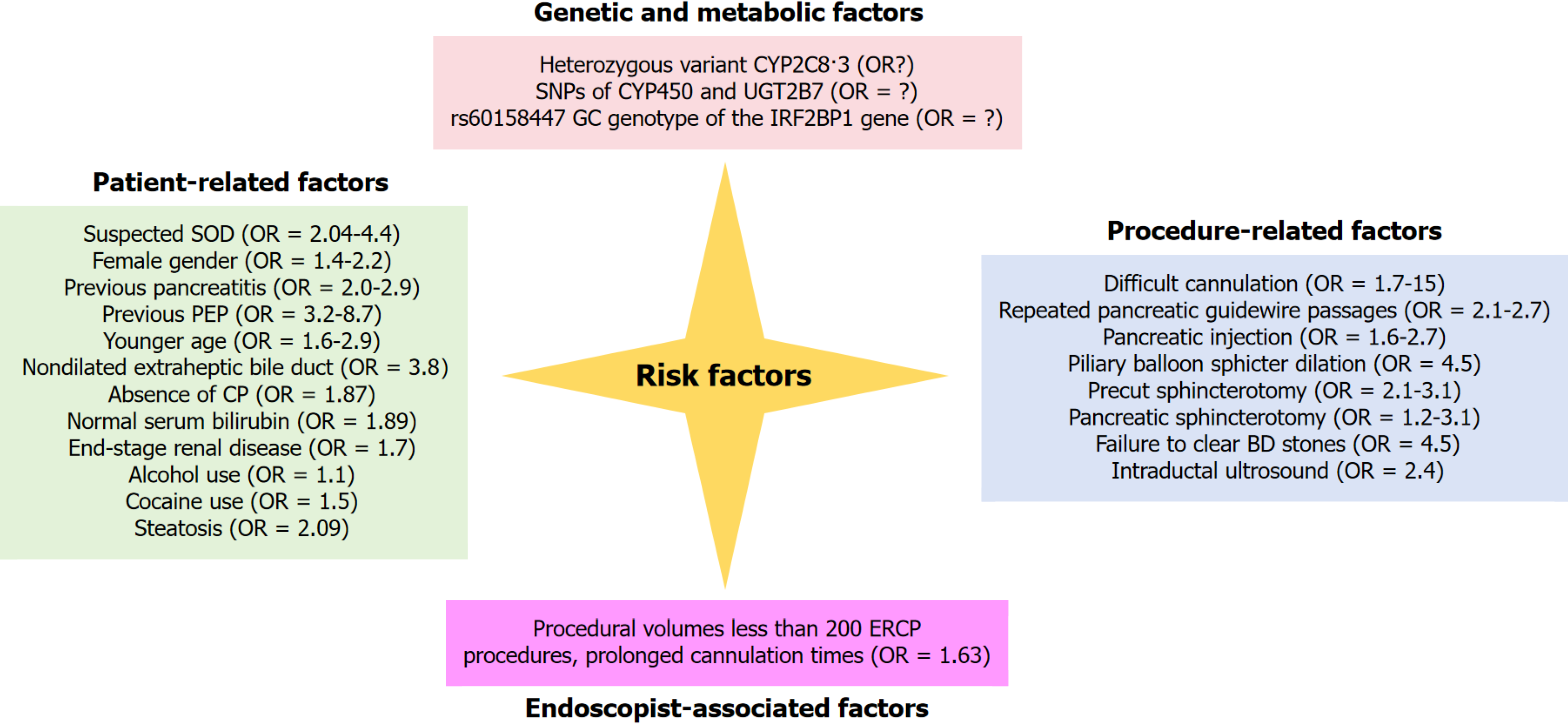
Although observational studies differ in how they define risk factors for PEP, several determinants are consistently recognized. Current evidence groups these factors into four broad categories: Patient-related, procedure-related, endoscopist-related, and genetic or metabolic (Figure 1). The 2020 ESGE guidelines further classify both patient- and procedure-related variables as either definite or possible risk factors.
Definite risk factors include suspected sphincter of Oddi dysfunction (SOD), female sex, a history of pancreatitis, and a prior episode of PEP[3,29].
Likely (possible) risk factors comprise age < 35 years, a non-dilated extrahepatic bile duct, absence of chronic pancreatitis, normal serum bilirubin, and end-stage renal disease[3]. Although one systematic review did not confirm younger age as a determinant[28], a subsequent prospective study identified age < 35 years as an independent predictor of PEP[29].
Additional contributors include modifiable factors such as alcohol or cocaine use and non-modifiable factors such as race, obesity, and congestive heart failure[30]. More recently, pancreatic steatosis has been reported as a strong risk factor for PEP[30].
Definite procedure-related risk factors for PEP include difficult cannulation, repeated pancreatic guide-wire (PGW) passages, and contrast injection into the pancreatic duct[3]. Likely procedure-related factors comprise precut sphincterotomy[3,30,31], pancreatic sphincterotomy, endoscopic papillary balloon dilation, failure to extract bile-duct stones, and the use of intraductal ultrasonography[3]. An ERCP is considered high risk for PEP when one definite factor or two likely factors are present[3].
The frequency of PEP and other ERCP-related AEs is closely linked to the endoscopist’s procedural volume and experience. A meta-analysis of 13 studies showed that high-volume endoscopists achieve a 60% greater ERCP success rate and a 30% lower overall adverse-event rate than their low-volume counterparts[32]. In a multicenter cohort of 1191 patients, operator inexperience—defined as < 200 lifetime ERCPs—emerged as an independent risk factor for PEP[33]. Less-experienced endoscopists required longer cannulation times because of greater difficulty in biliary access, and prolonged cannulation has been linked to a higher incidence of pancreatitis[29,33].
Rectal diclofenac prophylaxis reduces—but does not eliminate—the risk of PEP, partly owing to inter-individual differences in drug metabolism. Diclofenac is primarily biotransformed by cytochrome (CYP) P450 enzymes and UGT2B7[34]. Single-nucleotide polymorphisms (SNPs) in these enzymes alter diclofenac pharmacokinetics and pharmacodynamics[35]. The heterozygous CYP2C83 variant, associated with diminished diclofenac metabolic activity[36], has been linked to a lower incidence of PEP: Its prevalence was 15% in patients without PEP vs 3% in those who developed the complication. Thus, the CYP2C83 heterozygous genotype may confer protection against PEP, presumably by slowing diclofenac metabolism and prolonging its protective effect[37]. Genetic factors are increasingly recognized as contributors to the pathogenesis of PEP. Furukawa et al[38] identified an association between the risk allele of the GGT1 SNP rs5751901 and PEP. This allele up-regulates GGT1 expression within pancreatic tissue, activates the IL-6 amplifier, and thereby promotes PEP development. Their findings provide both mechanistic insight into genetic susceptibility and potential targets for personalized prevention and therapy.
The SPINK1 gene encodes a serine-protease inhibitor that protects the pancreas from autodigestion; its N34S variant has been reported as a PEP risk factor[39]. Carriers of this variant experience more frequent recurrences and more severe clinical courses, presumably because reduced trypsin inhibition allows premature activation of pancreatic enzymes[39]. Variants of CFTR, which encodes the cystic-fibrosis transmembrane conductance regulator responsible for chloride and bicarbonate transport across pancreatic ducts, have also been linked to PEP[40]. CFTR dysfunction increases juice viscosity and predisposes to inflammation[38]. Although definitive evidence is still lacking, the established association of SPINK1 N34S and CFTR variants with pancreatitis justifies further study of their role in PEP.
Current translational projects are exploring genetic and metabolic determinants of PEP to discover biomarkers and therapeutic targets. For example, next-generation sequencing is being used to identify rare variants associated with the complication. One study found that the IRF2BP1 SNP rs60158447 (GC genotype) correlates strongly with PEP incidence, suggesting a promising candidate marker for pre-procedure risk stratification[41]. Continued investigation of such variants will be essential for refining patient selection and tailoring prophylactic strategies.
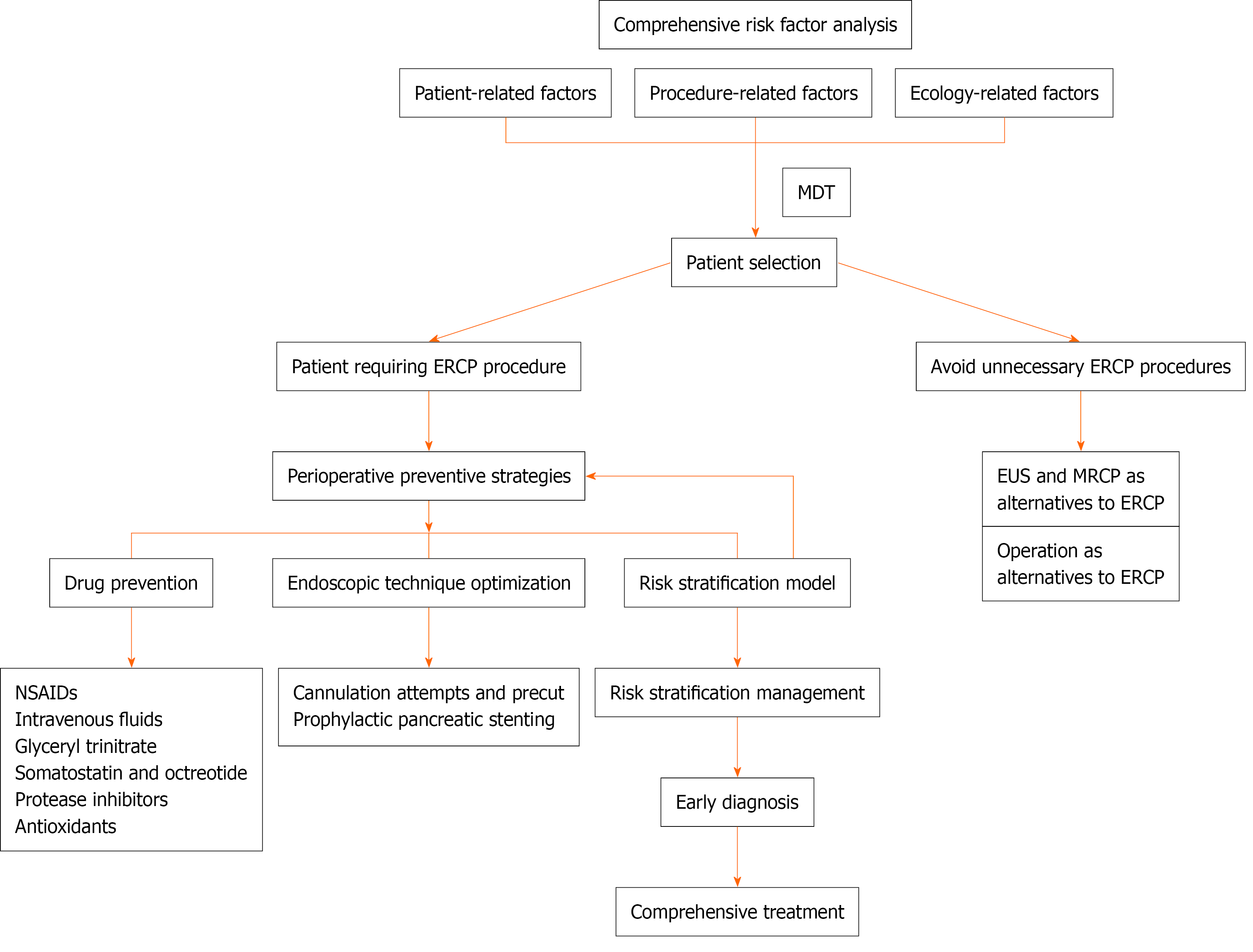
Recent evidence indicates that appropriate patient selection should consider patient-, procedure-, and operator-related risk factors to avoid unnecessary ERCP. For candidates who do undergo ERCP, prophylactic measures—pharmacological intervention, sphincterotomy, and pancreatic-duct (PD) stenting—are applied. A structured risk-stratification assessment enables comprehensive prevention, close monitoring, and early, integrated management of PEP (Figure 2). The relevant RCTs involved in the article are summarized in Table 2.
| Ref. | Study design | Participants | Intervention | Control | Results |
| Luo et al[54], 2016 | Multicentre, single-blinded, randomised controlled trial | Patients with native papilla undergoing ERCP at six centres in China | All patients received 100 mg rectal indometacin within 30 min before ERCP | High-risk patients received rectal indometacin immediately after ERCP | 4% pancreatitis in universal indometacin group vs 8% in risk-stratified group (relative risk = 0.47; 95%CI: 0.34-0.66; P < 0.0001) |
| Fogel et al[57], 2020 | Randomised, double-blind, comparative effectiveness trial | High-risk patients from six tertiary medical centres in the United States | Standard-dose group received two 50 mg indometacin suppositories and a placebo suppository; high-dose group received three 50 mg indometacin suppositories and an additional 50 mg suppository 4 hours after procedure | - | 15% pancreatitis in standard-dose group vs 12% in high-dose group (risk ratio = 1.19, 95%CI: 0.87-1.61; P = 0.32) |
| Mok et al[72], 2017 | Randomised, double-blinded, placebo-controlled trial | High-risk patients | Four groups: Normal saline + placebo, normal saline + indometacin, lactated Ringer's + placebo, lactated Ringer's + indometacin | - | 6% pancreatitis in lactated Ringer's + indometacin group vs 21% in normal saline + placebo group (P = 0.04) |
| Patel et al[67], 2022 | Randomised controlled trial | High-risk patients | Aggressive infusion of lactated Ringer's or normal saline | - | 4% pancreatitis in lactated Ringer's group vs 11% in normal saline group (relative risk = 0.38, 95%CI: 0.10-1.42; P = 0.19) |
| Concepción-Martín et al[76], 2014 | Randomised, double-blind trial | Patients undergoing ERCP at a single centre | Intravenous bolus of somatostatin followed by a 4-hour continuous infusion | Similar placebo regimen | 7.5% pancreatitis in somatostatin group vs 6.7% in placebo group (relative risk = 1.12, 95%CI: 0.59-2.1; P = 0.73) |
| Wang et al[77], 2013 | Randomised, placebo-controlled pilot trial | Patients scheduled for ERCP | Pre-ERCP somatostatin (0.5 mg/hour for 24 hours, starting 1 hour before ERCP), post-ERCP somatostatin (0.5 mg/hour for 24 hours, starting 1 hour after ERCP), or placebo (saline for 24 hours, starting 1 hour before ERCP) | - | 16.7% pancreatitis in pre-ERCP somatostatin group, 10.6% in post-ERCP somatostatin group, 14.6% in placebo group (P = 0.715) |
| Norouzi et al[101], 2023 | Double-blind randomised placebo-controlled clinical trial | High-risk patients | 100 mg rectal indometacin + 250 g somatostatin bolus followed by 500 g infusion for 2 hours | 100 mg rectal indometacin + placebo | 11.4% pancreatitis in intervention group vs 15.2% in control group (P = 0.666) |
| Yoo et al[102], 2011 | Prospective, randomised, double-blind, controlled trial | Patients undergoing ERCP | Intravenous nafamostat mesilate 60 min before and for 6 hours after ERCP | Placebo | 2.8% pancreatitis in nafamostat group vs 9.1% in placebo group (P = 0.03) |
Non-steroidal anti-inflammatory drugs: The primary driver of pancreatitis is an exaggerated inflammatory response. COX-2 modulates disease severity[39], while phospholipase A2 initiates the inflammatory cascade by generating arachidonic-acid metabolites and platelet-activating factor[40]. Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit prostaglandin synthesis, phospholipase A2 activity, and neutrophil-endothelial interactions, thereby interrupting the inflammatory cascade and potentially preventing PEP. Owing to their low cost, favorable safety profile, and ease of administration, NSAIDs have been among the most extensively studied agents for prophylaxis over the past decade[41-44].
NSAID prophylaxis is now incorporated into routine ERCP practice. The latest ESGE guidelines cite 27 meta-analyses demonstrating that NSAID administration reduces the incidence of PEP. The ESGE recommends rectal administration of 100 mg diclofenac or indomethacin immediately before ERCP in all patients who have no contraindication, irrespective of baseline risk[2,3,45].
NSAIDs can be administered intravenously, intramuscularly, orally, or rectally. Because rectal delivery causes less gastrointestinal irritation and ensures faster systemic absorption, it has become the preferred route[42]. Consistent evidence indicates that rectal NSAIDs provide superior prophylaxis against PEP compared with other routes. Yu et al[43] cited a meta-analysis by Kochar et al[6] that pooled four RCTs (n = 912) and showed a significant risk reduction with rectal NSAIDs[40]. Ding et al[7] reached a similar conclusion in a meta-analysis of 10 RCTs involving 2269 patients, and more recent syntheses have reinforced these findings[46]. Pre-procedural rectal indomethacin likewise lowers PEP risk in unselected cohorts[40,47,48].
Controversy persists, however, regarding NSAID efficacy against moderate-to-severe PEP. Yang et al[49] analyzed 12 RCTs (n = 3989) and found that NSAIDs significantly reduced this outcome, whereas Li et al[50], evaluating nine RCTs (n = 1883), reported no benefit. In high-risk patients who routinely received prophylactic pancreatic stents, Bai et al[51] (n = 607) observed no reduction in PEP incidence or severity with indomethacin. These discrepancies highlight the need for further evaluation.
Beyond the administration route, the dose, formulation, and timing require clarification. Although 100 mg is commonly used, the optimal dose remains uncertain. Okuno et al[52] reported that 25 mg of rectal diclofenac given 20 minutes before ERCP reduced PEP in a cohort of 147 patients; however, Sakai et al[53] found that this low dose did not affect either incidence or severity. A Japanese retrospective analysis suggested that 50 mg was more effective than 25 mg[54]. Conversely, recent trials indicate that 200 mg of rectal indomethacin offers no additional benefit over the standard 100 mg[55]. Taken together, these data imply a dose-dependent effect, but the ceiling of therapeutic benefit—and the dose-response relationship—remain to be defined.
Prophylactic pancreatic stenting: PD stenting promotes effective drainage, mitigates papillary oedema, and thereby lowers both the incidence and severity of PEP. It is the primary preventive measure for patients classified as high risk under the 2020 ESGE criteria, and its use in these patients is strongly endorsed by both the ASGE and ESGE[3,56]. Numerous RCTs and meta-analyses confirm its efficacy[57]. A recent meta-analysis of 17 RCTs (n = 1595) reported a 65% reduction in overall pancreatitis risk among high-risk patients who received PD stents, with no cases of severe pancreatitis in the stented group vs 13 in 1303 controls[58]. The study concluded that the benefits of prophylactic stenting outweigh the associated risks and costs[58].
Network meta-analyses favor short 5-F or 7-F stents over longer 3-F devices. When a guide-wire cannot be advanced through the stent’s proximal flange, expert panels recommend short stents to minimize trauma, but note that premature migration increases pancreatitis risk and warrants post-procedural imaging to confirm stent position[57,59,60].
Regarding duration, the ESGE advises abdominal radiography 5-10 days after ERCP to assess spontaneous stent migration. If the stent remains in situ, it should be removed promptly because retention beyond two weeks may induce ductal fibrosis and increase PEP risk 5.2-fold[3,20].
To avoid unnecessary esophagogastroduodenoscopy, radiological imaging should always precede retrieval to verify stent persistence; most centers perform fluoroscopy within the ERCP suite[3].
Consequently, routine fluoroscopic confirmation of stent migration consumes considerable endoscopy-unit resources, and cumulative radiation exposes both patients and staff[3] to avoidable risk[58]. A pilot study of 41 patients assessed the feasibility of ultrasound for detecting prophylactic pancreatic-stent position; ultrasound correctly identified retained stents in > 90% of cases, using fluoroscopy as the reference standard[58]. In a subsequent prospective trial of 88 patients, an ultrasound-based algorithm obviated X-ray imaging in 65 individuals—a 74% reduction in radiographic examinations. Among the 67 patients (76%) in whom the stent remained in situ, ultrasound visualized 54 devices, yielding a sensitivity of 81%, a positive predictive value of 83%, and a specificity of 48%; 10 of 21 migrated stents were correctly recognized (negative predictive value, 43%)[61]. These findings highlight ultrasound’s potential to curb radiation exposure for both patients and healthcare personnel.
With respect to stent composition, Yang et al[62] carried out a meta-analysis of six RCTs from 2008 to 2021 (including a total of 444 patients, with 221 using covered self-expandable metal stents (CSEMSs) and 223 using multiple plastic stents (MPSs). Compared with the MPS group, the risk of pancreatitis was significantly higher in the CSEMS group (OR = 3.34, P = 0.005). Fewer ERCP procedures were required for CSEMSs (MD = -1.56, P = 0.006). There was no statistically significant difference between the two types of stents in terms of the stenosis resolution rate or recurrence rate (OR = 0.87, P = 0.64; OR = 2.3, P = 0.18). The incidence of AEs was similar between the CSEMS and MPS groups (OR = 1.49, P = 0.07). Compared with the MPS group, the CSEMS group presented a significantly greater incidence of pancreatitis. However, it required fewer ERCP procedures, with similar rates of stenosis resolution, recurrence, and overall AE incidence.
Although pancreatic duct stenting has proven effective in reducing the incidence of PEP, its cost-effectiveness remains a critical consideration in clinical practice. Evidence indicates that the overall expense includes the price of the stent itself, procedural costs, and the management of potential complications. Although stenting markedly lowers the risk of PEP, its high cost may limit widespread adoption in resource-constrained settings. For example, the cost-effectiveness of pancreatic duct stenting appears more pronounced in high-risk patients than in the general population, suggesting that the intervention should be deployed selectively according to patient risk stratification[63]. In addition, a risk-score-based cost-effectiveness model has been proposed to evaluate the economic value of pancreatic duct stenting for individual patients. This model enables clinicians to make more personalized treatment decisions by jointly considering patient risk and economic factors[64].
Intravenous fluids: Peri-procedural fluid replacement with isotonic crystalloids reduces the incidence of PEP[65,66]. The ESGE recommends intravenous hydration for patients who have contraindications to NSAIDs and cannot undergo prophylactic PD stent placement[3]. Specifically, it advises active resuscitation with lactated Ringer’s solution (LR) at 3 mL/kg per hour during ERCP, preceded by a 20 mL/kg bolus immediately after the procedure and followed by 3 mL/kg per hour for the next 8 hours[3]. This strategy should be used cautiously in patients with heart failure, cirrhosis, or renal dysfunction[3].
In a 2014 RCT, none of the patients who received aggressive fluid therapy developed PEP, whereas 17% of those in the standard-hydration group did (P < 0.05)[2]. By contrast, a 2021 multicenter study of adults aged 18-85 years at moderate to high risk of PEP in 22 Dutch hospitals found no significant difference in AEs between aggressive and standard fluid resuscitation. These divergent findings warrant further investigation.
Regarding fluid type, Patel et al[67] compared aggressive infusions of normal saline (NS) with LR in high-risk patients (as defined by the 2020 ESGE guidelines). PEP occurred in 4% of the LR group vs 11% of the NS group, possibly because LR more closely resembles plasma and mitigates acidosis, which may otherwise trigger premature enzyme activation.
Glyceryl trinitrate: A meta-analysis of 11 RCTs involving 2095 patients showed that glyceryl trinitrate (GTN) sig
Zhang et al[68] recently conducted a meta-analysis of 10 RCTs (n = 3240) and likewise found that GTN significantly reduces the overall incidence of PEP. The risk was further decreased when GTN was combined with an NSAID compared with GTN alone. However, neither GTN alone nor GTN plus NSAIDs reduced the severity of PEP; specifically, the incidence of moderate-to-severe cases remained unchanged.
Shi et al[69] subsequently performed a network meta-analysis of 24 RCTs (n = 9416) comparing NSAIDs with GTN. The combination of rectal indomethacin and sublingual GTN emerged as the most effective strategy for preventing moderate-to-severe PEP. Consistent with these findings, Wang et al[70] reported in an RCT (n = 526) that the indomethacin + nitroglycerin regimen reduced the risk of PEP by 7% compared with prophylactic pancreatic stent placement (PSP). In difficult cannulation scenarios, this pharmacological combination was superior to PSP both in preventing PEP and in lessening its severity. Thus, indomethacin plus nitroglycerin may serve as an effective alternative to PSP for prophylaxis of PEP.
Somatostatin and its synthetic analog octreotide: Somatostatin and its synthetic analog, octreotide, have been investigated for the prophylaxis of PEP through inhibition of pancreatic secretion. Early studies, however, produced conflicting results. Bai et al[71] reported that somatostatin significantly reduced serum amylase levels and prevented PEP, whereas two other trials found no such benefit[68,72]. Subsequent evidence indicates that dose is critical. In a meta-analysis of 17 RCTs involving 2784 patients, Zhang et al[73] showed that octreotide (≥ 0.5 mg) was more effective than lower doses in preventing PEP. Likewise, Omata et al[74] pooled 17 RCTs (3818 patients) and demonstrated that high-dose somatostatin or octreotide significantly reduced the incidence of this complication.
More recent analyses have refined these findings. A meta-analysis assessing somatostatin prophylaxis found an overall risk reduction, which reached statistical significance only in patients classified as high risk according to the 2020 ESGE guidelines[9]. Consistent with this, a prospective study by Wu et al[75] showed that somatostatin decreased postoperative hyperamylasemia, improved quality of life, and lowered the incidence of PEP in high-risk patients (n = 729). In the same cohort, administering indomethacin with somatostatin within 6 hours after ERCP further reduced hyperamylasemia and improved patient-reported outcomes[76], particularly in technically demanding procedures with longer operative times and greater postoperative pain[77]. By contrast, a prospective trial by Bhat et al[78] (n = 530) reported non-significant reductions in hyperamylasemia and PEP when somatostatin was added to indomethacin.
In summary, current clinical evidence supports a protective effect of somatostatin and octreotide against PEP, especially at higher doses and in high-risk populations. Nevertheless, heterogeneity among studies persists, and further well-designed prospective trials are necessary to confirm these findings.
Protease inhibitors: Protease inhibitors are potential therapeutic agents for AP because they inhibit pancreatic proteolytic enzymes and downstream inflammatory cascades[79]. Among these agents, nafamostat has been evaluated for the prevention of PEP[79]. Yu et al[80] conducted a meta-analysis of seven RCTs involving 2956 patients and found that nafamostat significantly reduced the incidence of PEP. This protective effect is supported by another meta-analysis of eight RCTs (3210 patients), which showed that pre-procedural nafamostat prophylaxis effectively prevented PEP across different risk strata[81]. In that analysis, nafamostat reduced mild and moderate PEP but did not significantly lower the incidence of severe PEP or post-ERCP hyperamylasemia[80].
Horváth et al[79] pooled seven RCTs (2962 patients) and reported that a 20 mg dose of nafamostat lowered the overall PEP rate; a significant reduction in mild PEP was observed only in the subgroup receiving 20 mg. Overall, nafamostat decreased moderate PEP in high-risk patients (OR = 0.18) and mild PEP in low-risk patients (OR = 0.32). By contrast, a systematic review by Kim et al[81] concluded that administering nafamostat mesilate after ERCP did not prevent PEP in high-risk patients defined by the 2020 ESGE criteria, although it might lessen disease severity. Similarly, a study by Matsumoto et al[82] did not demonstrate a preventive effect. Although nafamostat shows promise for PEP prophylaxis, the evidence remains inconsistent; wider clinical adoption awaits confirmation from further rigorous prospective trials.
Cannulation attempts and precuts: To minimize papillary trauma, the number of cannulation attempts should be kept as low as possible[3,71]. The ESGE therefore recommends guidewire-assisted biliary cannulation, which both increases the success rate and avoids inadvertent pancreatic contrast injection[3,71]. Secure endoscope positioning before cannulation is essential. The papillary morphology must be inspected, the orifice precisely identified, and an optimal cannulation trajectory planned to reduce tissue injury. A thorough knowledge of the relevant anatomy and patient-specific risk factors further informs the choice of technique; for instance, the compact-disc method can facilitate targeted biliary access[3]. Some evidence suggests that endoscopist-controlled short-guidewire techniques may lower complication rates more effectively than assistant-controlled approaches[83]. Early recognition of difficult cannulation allows timely strategy modification. When standard cannulation fails, alternatives such as the double-guidewire technique, needle-knife precut sphincterotomy, pancreatic ductotomy, or fistulotomy can be employed[84]. In a recent single-center retrospective study, Tanikawa et al[85] reported that early needle-knife precut papillotomy (NKPP)—performed within 10 minutes of unsuccessful standard attempts—significantly reduced the incidence of PEP compared with delayed NKPP. When early precut (EP) is not feasible, placement of a pancreatic stent after delayed precut similarly prevents PEP, offering protection comparable to EP. These findings suggest that implementing either EP or prophylactic pancreatic stenting during protracted procedures may improve patient outcomes.
A systematic review by Mohseni Salehi Monfared et al[86] found that antioxidant interventions—such as valproate and N-acetylcysteine—did not prevent PEP, and no evidence supports the use of antioxidant therapy alone or in combination with standard prophylactic measures. A subsequent review reached the same conclusion for other antioxidants, including allopurinol, β-carotene, selenite, pantoprazole, and N-acetylcysteine, none of which reduced the incidence of PEP[87]. By contrast, recent work on nanotechnology-based, mitochondria-targeted drug-delivery systems has yielded promising in vitro and in vivo results, suggesting that natural monomeric compounds delivered in this manner may offer a novel antioxidant strategy for the prevention and treatment of PEP[44].
Best way to prevent PEP is to avoid unnecessary ERCP: Safe alternatives to ERCP should be considered where appropriate. In a single-center RCT, Jagtap et al[88] showed that, in patients with intermediate-risk common bile duct (CBD) stones, both EUS and MRCP exhibited high sensitivity (92%-98%), with no significant difference between them. The negative predictive value and positive likelihood ratio were significantly higher with EUS (P < 0.05). Using ERCP or clinical follow-up as the reference standard, false-negative examinations were rare (EUS: 2 vs MRCP: 5), as were false-positive examinations (EUS: 1 vs MRCP: 2), highlighting the diagnostic accuracy of both modalities. These findings suggest that the non-invasive techniques EUS and MRCP could replace ERCP for diagnosing CBD stones.
For CBD stones and malignant biliary strictures, surgery may also serve as an alternative to ERCP. The ESGE recommends laparoscopic CBD exploration combined with cholecystectomy for stone removal[3]. Among patients with resectable malignant strictures, those with lower bilirubin levels may benefit from early surgery, whereas ERCP should be reserved for individuals facing prolonged surgical delays or presenting with complications such as cholangitis, severe pruritus, or marked hyperbilirubinemia that predisposes to cholangitis or acute renal failure[89]. SOD has long been viewed as a risk factor for PEP. However, recent evidence—including the Evaluating Predictors and Interventions in SOD (EPISOD) study—has questioned the very existence of SOD. EPISOD demonstrated that endoscopic sphincterotomy was no more effective than a sham intervention and, by some measures, yielded worse outcomes. Consequently, ERCP for presumed SOD can no longer be recommended[90].
Risk stratification models for PEP: Risk stratification has become an integral component of clinical decision-making. This process systematically classifies patients using data that reflect their health status, lifestyle, and medical history[91]. Table 3 summarizes the principal risk-stratification models developed to date.
| Risk factors included | Predictive value | Advantages and disadvantages | Ref. |
| Pain; pancreatic duct cannulation; previous PEP; and the number of cannulation attempts | Low-risk: 1.9%; medium-risk: 6.9% and high-risk: 28% | Applicable to patients suspected of having SOD and those at high risk for PEP; not validated; a pioneer in the risk stratification model for PEP | Friedland et al[92], 2002 |
| History of gastrectomy; high DBIL; high ALB; CBD stones; papillary opening with villous type; papillary opening with nodular type; pancreatic guidewire passage; pre-cut sphincterotomy; and high procedural experience | Low-risk: 6.1%; medium-risk, 17%; high-risk: 37.5%. AUC was 0.718–0.793; sensitivity was 0.705–0.727; and specificity was 0.676–0.797 | Applicable to patients suspected of having SOD and those at high risk for PEP; not validated; a pioneer in the risk stratification model for PEP | Zheng et al[95], 2020 |
| Complete papilla; PGW-assisted biliary cannulation; difficult cannulation; pancreatic injection; absence of a pancreatic stent | Extremely high-risk: 28.79%; the predicted incidence of severe pancreatitis was 9.09%. AUC was 0.86, while the AUC for internal validation was 0.81 | Applicable to patients suspected of having SOD and those at high risk for PEP; not validated; a pioneer in the risk stratification model for PEP | Chiba et al[97], 2021 |
| A history of PEP; complete papilla; difficult cannulation; pancreatic guidewire-assisted biliary cannulation; pancreatic injection; IDUS/sampling of the pancreas; biliary IDUS/sampling | The AUC in both the training and validation sets were 0.799 and 0.791, respectively; In the high-risk, the incidence of PEP was 13.4% | Applicable to patients suspected of having SOD and those at high risk for PEP; not validated; a pioneer in the risk stratification model for PEP | Fujita et al[4], 2022 |
| Age ≤ 65 years; female sex; history of acute pancreatitis; malignant biliary obstruction | Low risk: 2.2%; medium risk: 3.8%; high risk: 6.9% | Pre-ERCP risk prediction model | Kim et al[81], 2022 |
| Age ≤ 65 years; female sex; history of acute pancreatitis; malignant biliary obstruction; pancreatic sphincterotomy | Low risk: 2.0%; medium risk: 3.4%; high risk: 18.4% | Post-ERCP risk prediction model; the model based on retrospective data, its reliability in practical application remains uncertain | Kim et al[81], 2022 |
| Extended total operation time; unexpected pancreatic duct cannulation; pancreatic imaging total operation time; unexpected pancreatic duct cannulation; and pancreatic imaging | Low-risk, medium-risk, and high-risk were 2.6%, 7.1%, and 12.6%, respectively | Using prospective cohort data; indicated that most patients without PEP had an operation time of less than 14 minutes | Kim et al[81], 2022 |
| Female gender; pancreatic duct cannulation; papillary condition; pre-cut sphincterotomy; prolonged cannulation time; bile duct stenosis; patient age; pancreatic duct stent placement | An ROC curve statistic of 0.79; a predicted-to-observed risk ratio of 1.003 | Has good discriminative ability and good calibration | Meng et al[98], 2024 |
In 2002, Friedland et al[92] devised a scoring system to predict PEP. Multivariable analysis identified four independent risk factors—procedural pain, pancreatic duct cannulation, number of cannulation attempts, and prior PEP—which were weighted to yield a composite score. Scores delineated three categories: Low risk (≤ 4 points), intermediate risk (5-8 points), and high risk (≥ 9 points), corresponding to post-procedural pancreatitis rates of 1.9%, 6.9%, and 28%, respectively[92]. The model was derived in patients with suspected SOD and in high-risk cases undergoing minor papillary cannulation[92,93]. Although subsequent studies failed to validate the tool, it pioneered structured risk assessment for PEP. Building on Haraldsson’s morphological classification of duodenal papillae (conventional, small, and prominent/pendulous)[94], Zheng et al[95] incorporated native papillary morphology into a new predictive model in 2020. Additional independent predictors included previous gastrectomy, high direct bilirubin, high albumin, CBD stones, papillary opening of the villous type, papillary opening of the nodular type, PGW passage, precut sphincterotomy, and extensive procedural experience. A new risk prediction model was developed, dividing patients into low-risk, medium-risk, and high-risk groups based on scores. The probability of PEP occurrence was significantly associated with the risk level in the three risk stratification groups. The probability of PEP was 6.1%, 17.0%, and 37.5% in the low-, medium-, and high-risk score groups, respectively (P < 0.05). The area under the curve (AUC) of the model ranged from 0.718 to 0.793, the sensitivity was 0.705-0.727, and the specificity was 0.676-0.797. A larger, multicenter study is needed to validate the predictive ability of this model for PEP[96]. In 2021, Chiba et al[97] developed a propensity-score-based model incorporating five risk factors—complete papilla, PGW-assisted biliary cannulation, difficult cannulation, pancreatic injection, and absence of a pancreatic stent. With a four-level score, the predicted pancreatitis rate in the “extremely high-risk” category was 28.79%, and the severe pancreatitis rate was 9.09%. The model showed good discrimination (AUC = 0.86; internal validation AUC = 0.81) and can be applied immediately after ERCP using a simplified scoring system[97].
Fujita et al[4] proposed a similar model based on seven variables: Prior PEP, complete papilla, difficult cannulation, PGW-assisted biliary cannulation, pancreatic injection, intraductal ultrasound (IDUS) or pancreatic sampling, and biliary IDUS/sampling. The AUCs were 0.799 (training set) and 0.791 (validation set). In patients scoring ≥ 3, the PEP rate reached 13.4%, and all severe or fatal cases occurred in this subgroup. The authors advocate aggressive prophylaxis and close post-procedural monitoring for patients in this high-risk category. They also noted that, unlike the propensity-score model of Chiba et al[97], their integer-based system does not require a lookup table, making it more practical for real-time use in the endoscopy suite.
In 2022, Park et al[96] developed a pre-ERCP risk-prediction model using multicenter retrospective data. Pre-procedural variables were assigned the following points: Age ≤ 65 years (1 point), female sex (1 point), previous AP (2 points), and malignant biliary obstruction (2 points). Patients were stratified into low- (0 point), intermediate- (1-2 points), and high-risk (≥ 3 points) categories; PEP rates did not differ significantly among these groups (2.2%, 3.8%, and 6.9%, respectively). The investigators therefore constructed a post-ERCP model that incorporated both pre- and intra-procedural factors: Age ≤ 65 years (1 point), female sex (1 point), previous AP (2 points), malignant biliary obstruction (1 point), and pancreatic sphincterotomy (2 points). In the validation cohort, the high-risk group (≥ 3 points) exhibited a markedly higher PEP incidence than the low- and intermediate-risk groups (18.4% vs 2.0% and 3.4%, respectively).
Because the original model was based on retrospective data, its applicability in routine practice remained uncertain. Park et al[96], therefore, prospectively re-evaluated and refined the score in 2025 using logistic regression. The revised model weighted extended total procedure time, unintended pancreatic duct cannulation, and prolonged pancreatic imaging as follows: Total procedure time (1 point), unintended pancreatic duct cannulation (8 points), and pancreatic imaging (8 points). PEP rates rose with increasing score: 2.6% in the low-risk group (0-2 points), 7.1% in the intermediate-risk group (3-10 points), and 12.6% in the high-risk group (≥ 11 points). Procedure duration emerged as a key de
Meng et al[98] analyzed 3021 ERCPs in a prospective multicenter study and created an eight-variable model comprising female sex, pancreatic duct cannulation, papillary morphology, precut sphincterotomy, prolonged can
The diagnosis of PEP hinges on clinical findings corroborated by laboratory data. Current guidelines recommend measuring serum amylase and lipase 2-6 hours after ERCP; levels exceeding three times the ULN strongly suggest PEP[3]. Dynamic monitoring enables the early identification of pathological changes, thereby preserving valuable time for therapeutic intervention[93].
Management strategies for PEP: Once PEP is confirmed, immediate conservative therapy—namely, bowel rest, vigorous intravenous fluid resuscitation, and symptomatic supportive care—is mandatory[93]. In severe cases, admission to an intensive care unit is indicated for close hemodynamic surveillance and early consideration of nutritional support[94]. The use of antibiotics should be judicious and limited to situations in which infection is suspected or documented, such as radiological or clinical evidence of infected pancreatic necrosis[93].
Nanotechnology-based drug delivery systems for targeting mitochondria in AP treatment: In recent years, nanotechnology-enabled drug-delivery systems have created new opportunities for disease therapy by targeting subcellular organelles. Because mitochondria are central to cellular bioenergetics and homeostasis, mitochondrial dysfunction contributes to a range of pathologies. Consequently, the design of nanodrugs that modulate mitochondrial function has become an active area of investigation[97]. Severe AP, for example, is exacerbated by mitochondrial impairment and excessive generation of ROS. Selective removal of mtROS within PACs is therefore an attractive therapeutic strategy for AP. Mitochondria-targeted nanocarriers have demonstrated substantial efficacy in vitro and in vivo[99,100].
Wang et al[100] reported a tungsten-based polyacid nanoprotectant (mTWND) that is co-modified with tannic acid and melanin; this construct efficiently scavenges mtROS in PACs, traverses the blood-pancreas barrier, and confers significant protection in experimental AP.
By contrast, consensus has not been reached on the clinical value of antioxidant therapies derived from natural products used in traditional Chinese medicine (TCM), largely because most compounds exhibit low bioavailability and require high doses that can cause adverse effects[101]. To address these limitations, Wen et al[99] developed DSPE-TK-PEG2000-KA (DTM@KA), a liposomal nanoparticle that combines kaempferol with a redox-responsive linker. DTM@KA attenuates glutathione depletion, activates the Nrf2 pathway, and restores mitochondrial dynamics and mitophagy, thereby reducing inflammation and cell death. Enhanced mitochondrial function also augments ATP production, promoting the repair and stabilization of PACs.
The studies discussed above offer promising antioxidant-based strategies for pancreatitis therapy; however, these approaches remain unsupported by clinical-trial evidence.
Compared with previous literature and guidelines—most notably the 2018 ESGE recommendations—this review not only synthesizes current knowledge on PEP but also integrates recent advances such as mitochondria-targeted nanodrug-delivery systems into prevention frameworks. We detail the encouraging in-vitro and in-vivo data demonstrating that mitochondrial nanotechnology can harness natural monomeric compounds to exert antioxidant effects against PEP. In addition, we emphasize the need for personalized prophylactic strategies, a dimension under-represented in earlier work. Accordingly, this review both updates the knowledge base and delineates new directions for future research and clinical practice.
All meta-analyses cited herein draw exclusively on publicly available data from published RCTs. Because these data contain no personally identifiable information, Institutional Review Board approval was not required under institutional policy and applicable regulations. Nevertheless, all relevant ethical guidelines were observed to ensure transparency and compliance.
Although multiple strategies are available to prevent and treat PEP, further optimization is warranted. Future research could prioritize the following avenue.
A multidisciplinary model facilitates comprehensive pre-procedural assessment, identification of patient-specific risk factors, and development of personalized prophylactic plans. During ERCP, coordinated input from endoscopists, anesthetists, radiologists, and specialized nursing staff can streamline technique, minimize PD trauma, and reduce pancreatic fluid leakage. Adequate intra-procedural crystalloid resuscitation with NS or LR helps maintain hemodynamic stability and may decrease postoperative complications. Post-procedure, collaborative management by intensive-care specialists, gastroenterologists, nutritionists, and pain-control teams enables early detection and prompt treatment of complications. Collectively, this integrated approach lowers the incidence and severity of PEP, shortens hospital stay, and reduces healthcare expenditure.
Recent studies suggest that integrating TCM with Western medicine offers meaningful advantages in the prevention and management of post-endoscopic pancreatitis[99,100]. By combining the targeted, technology-driven interventions of Western medicine with TCM’s holistic, syndrome-differentiated approach, clinicians can deliver more comprehensive and individualized care.
Western techniques—such as pharmacological prophylaxis and PD stent placement—effectively lower the incidence and severity of PEP. TCM complements these measures by modulating the patient’s overall physiological state, alleviating postoperative discomfort, and promoting convalescence. This synergistic model can enhance therapeutic efficacy while minimizing the adverse effects associated with single-modality treatments.
Ongoing research and wider adoption of TCM-Western integration are expected to yield further optimization of treatment protocols, improve clinical outcomes, and enhance quality of life for patients with PEP.
Accurately identifying high-risk populations: High-risk cohorts for PEP can be identified more precisely by integrating genetic testing with biomarker profiling. As noted earlier, heterozygosity for the CYP2C8*3 allele is linked to a reduced incidence of PEP[36]; this protective effect is thought to arise from diminished diclofenac metabolism[37].
Development of targeted pharmacotherapies: Sustained cytosolic calcium (Ca2+) overload and intracellular ATP depletion are central drivers of AP. Pharmacological inhibition of the calcium-release-activated calcium (CRAC) channel, therefore, represents a promising therapeutic strategy. The selective CRAC-channel inhibitor CM4620 (Auxora, CalciMedica), is currently in clinical development and may offer a novel treatment option for PEP[18].
Applications of AI in medicine have expanded rapidly, particularly in the management of ERCP-related complications. AI-assisted decision-making systems can integrate large clinical datasets to deliver precise risk stratification and personalized treatment recommendations, thereby enhancing the accuracy and effectiveness of clinical decision-making for PEP.
AI systems that employ machine-learning and deep-learning algorithms can analyze large clinical datasets, identify risk factors for PEP, and generate accurate risk estimates. In a recent multicenter study, investigators built and validated an AI-based model that integrated 49 clinical variables and one imaging feature derived from electronic health records; the model markedly outperformed traditional logistic-regression approaches in predicting PEP risk[98].
Another group developed a gradient-boosting-machine tool incorporating 20 established risk factors and five prophylactic measures (such as rectal NSAIDs, aggressive hydration, and PD stenting). In a prospective pilot study, this system achieved a 95% negative predictive value, allowing reliable identification of low-risk patients and reducing unnecessary postoperative surveillance[102].
Despite such promising findings, several obstacles remain—most notably data heterogeneity, limited external validation of existing models, and the need for clinician training in AI interpretation. Future research should prioritize multimodal data fusion by incorporating imaging, genomic, and molecular-biological information to further strengthen predictive accuracy and develop real-time decision-support platforms capable of guiding intra- and post-procedural management.
PEP is a serious complication of ERCP. Effective management requires preventive and therapeutic measures before, during, and after the procedure, as well as comprehensive patient education and long-term follow-up.
Patient education: Patient education is pivotal to preventing and managing PEP. Providing detailed pre-procedural information and clear postoperative instructions can markedly reduce the incidence and severity of complications. Education enables patients to understand the procedure, promotes compliance, and facilitates early intervention. Key components include: (1) Surgical-risk information: Patients should receive a clear explanation of the purpose, steps, and potential complications of ERCP, with particular emphasis on the risk of PEP; (2) Preventive measures: The rationale for prophylactic strategies (e.g., NSAIDs or PD stent placement) should be discussed; and (3) Psychological support: Addressing preoperative anxiety can improve patient confidence and cooperation.
Postoperative education: (1) Symptom monitoring: Patients should be taught to recognize early signs of PEP, such as severe abdominal pain, fever, or vomiting, and to seek immediate medical attention if these occur; (2) Dietary and activity guidance: A light, low-fat diet is recommended, and alcohol intake should be limited; and (3) Medication instructions: Patients should understand the indications and precautions for postoperative medications (e.g., analgesics and anti-inflammatory agents).
Long-term management: Long-term management of PEP aims to limit recurrent complications and improve patient quality of life. It includes structured postoperative follow-up, scheduled evaluations, lifestyle modification, and prompt management of sequelae. Ongoing patient education and coordinated care can substantially reduce both the incidence and recurrence of pancreatitis.
The principal benefits of comprehensive long-term management are: (1) Prevention of chronic pancreatitis: Although most patients recover after the acute episode, persistent inflammation and tissue injury can lead to chronic pancreatitis in some individuals; and (2) Reduction of recurrent acute attacks: Patients who experience PEP remain at elevated risk for future episodes, particularly when additional risk factors such as gallstones or alcohol use are present.
Because prognosis is influenced by individual risk profiles and therapeutic response, these patients require frequent follow-up and vigilant monitoring to detect and manage relapse promptly.
The study provides an up-to-date mechanistic cascade in which catheter-induced trauma, ductal hypertension, and electrocautery converge on mitochondrial dysfunction, mtROS generation, NLRP3 inflammasome activation, and cGAS-STING signalling, enriched by citations on Ca2+ overload and premature zymogen activation. The text links experimental ROS data to cytokine surges (IL-1β, IL-18, TNF-α) and integrates bile-acid-induced mitochondrial Ca2+ uptake, highlighting translational relevance. Yet, human biopsy or biomarker corroboration is not discussed; adding brief commentary on clinical evidence (e.g., circulating mtDNA, serum IL-6 levels) would strengthen the bedside connection. The narrative could be clarified by inserting a schematic figure illustrating mechanical vs biochemical triggers and their convergence on mitochondria. Some acronyms (e.g., “pancreatic acinar cells” for PACs) first appear later; introducing them here would improve continuity. Removing duplicate citations of ROS-Ca2+ crosstalk would tighten flow.
This section tracks the evolution from the 1991 consensus through the 2020 ESGE guideline, emphasizing pain, enzyme thresholds, and timing nuances (≥ 3 × ULN amylase/Lipase at 2-6 hours vs 24 hours). The juxtaposition of Testoni’s 4-hour ≥ 5 × ULN rule with Hemant’s meta-analysis favoring early lipase adds critical appraisal of sensitivity/specificity. However, the prose intermingles “clinical pancreatitis”, “post-ERCP pancreatitis”, and “hyperamylasemia” without explicit definitions; a short clarifying sentence would deter misinterpretation. Incorporating a concise comparison table of threshold options (2 hours, 4 hours, 24 hours) and their positive/negative predictive values would facilitate reader assimilation. The text briefly notes the difficulty of differentiating transient abdominal discomfort from PEP but could suggest routine analgesic scores or imaging algorithms as remedies. A short comment on emerging biomarkers (e.g., trypsinogen-2) would future-proof the discussion.
The authors accurately contrast the hospital-stay-based 1991 consensus (mild = 2-3 day, moderate = 4-10 day, severe > 10 day or local complications) with the revised Atlanta system stratifying by persistent organ failure and local/systemic sequelae. Citing a multicenter validation study that favored Atlanta for mortality prediction justifies its primacy. Nevertheless, the manuscript would benefit from explicitly relating severity strata to management pathways (ward vs intensive care unit, nutritional strategies, intervention thresholds). A brief acknowledgement of alternative clinical scoring (Bedside Index of Severity in Acute Pancreatitis, the Acute Physiology and Chronic Health Evaluation II) and why they were not adopted would round out the perspective. Because the authors focus heavily on emerging nanotherapies later, linking severity stages to candidate targeted interventions (e.g., mTWNDs for predicted severe cases) would improve translational continuity. Finally, the section’s heading duplicates “classification”; consolidating headings or cross-referencing would streamline structure.
This comprehensive review examines the epidemiology, pathophysiology, preventive strategies, and therapeutic advances in PEP. A critical appraisal of the most recent studies and clinical guidelines yields several key conclusions. First, prophylactic administration of rectal NSAIDs and pancreatic duct stenting is highly effective in preventing PEP, particularly in high-risk patients; these measures should therefore be adopted as standard practice. Second, emerging targeted therapies—such as mTWNDs—show considerable promise for treating established PEP by interrupting inflammatory cascades and expediting patient recovery. Third, the implementation of an MDT model has proved beneficial in the management of severe PEP. By integrating specialized expertise and optimizing the control of infection and other complications, the MDT approach markedly improves therapeutic outcomes.
| 1. | Kröner PT, Bilal M, Samuel R, Umar S, Abougergi MS, Lukens FJ, Raimondo M, Carr-Locke DL. Use of ERCP in the United States over the past decade. Endosc Int Open. 2020;8:E761-E769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Buxbaum J, Yan A, Yeh K, Lane C, Nguyen N, Laine L. Aggressive hydration with lactated Ringer's solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2014;12:303-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 581] [Article Influence: 96.8] [Reference Citation Analysis (1)] |
| 4. | Fujita K, Yazumi S, Matsumoto H, Asada M, Nebiki H, Matsumoto K, Maruo T, Takenaka M, Tomoda T, Onoyama T, Kurita A, Ueki T, Katayama T, Kawamura T, Kawamoto H; Bilio-pancreatic Study Group of West Japan. Multicenter prospective cohort study of adverse events associated with biliary endoscopic retrograde cholangiopancreatography: Incidence of adverse events and preventive measures for post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Endosc. 2022;34:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Akshintala VS, Kanthasamy K, Bhullar FA, Sperna Weiland CJ, Kamal A, Kochar B, Gurakar M, Ngamruengphong S, Kumbhari V, Brewer-Gutierrez OI, Kalloo AN, Khashab MA, van Geenen EM, Singh VK. Incidence, severity, and mortality of post-ERCP pancreatitis: an updated systematic review and meta-analysis of 145 randomized controlled trials. Gastrointest Endosc. 2023;98:1-6.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, Khashab MA, Kalloo AN, Singh VK. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143-149.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 353] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 7. | Ding X, Chen M, Huang S, Zhang S, Zou X. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: a meta-analysis. Gastrointest Endosc. 2012;76:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Pekgöz M. Post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review for prevention and treatment. World J Gastroenterol. 2019;25:4019-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Cahyadi O, Tehami N, de-Madaria E, Siau K. Post-ERCP Pancreatitis: Prevention, Diagnosis and Management. Medicina (Kaunas). 2022;58:1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 10. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 578] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 11. | Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 617] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 12. | Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3253] [Cited by in RCA: 4456] [Article Influence: 278.5] [Reference Citation Analysis (0)] |
| 13. | Rafaqat S, Patoulias D, Behnoush AH, Sharif S, Klisic A. Interleukins: pathophysiological role in acute pancreatitis. Arch Med Sci. 2024;20:138-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Papantoniou K, Aggeletopoulou I, Michailides C, Pastras P, Triantos C. Understanding the Role of NLRP3 Inflammasome in Acute Pancreatitis. Biology (Basel). 2024;13:945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Willemsen J, Neuhoff MT, Hoyler T, Noir E, Tessier C, Sarret S, Thorsen TN, Littlewood-Evans A, Zhang J, Hasan M, Rush JS, Guerini D, Siegel RM. TNF leads to mtDNA release and cGAS/STING-dependent interferon responses that support inflammatory arthritis. Cell Rep. 2021;37:109977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 17. | Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, Ying W, Hoffman HM, Shadel GS, Karin M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55:1370-1385.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 508] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 18. | Pallagi P, Madácsy T, Varga Á, Maléth J. Intracellular Ca(2+) Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives. Int J Mol Sci. 2020;21:4005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Booth DM, Murphy JA, Mukherjee R, Awais M, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Petersen OH, Sutton R, Criddle DN. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Cha SW, Leung WD, Lehman GA, Watkins JL, McHenry L, Fogel EL, Sherman S. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest Endosc. 2013;77:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2082] [Article Influence: 59.5] [Reference Citation Analysis (1)] |
| 22. | Tryliskyy Y, Bryce GJ. Post-ERCP pancreatitis: Pathophysiology, early identification and risk stratification. Adv Clin Exp Med. 2018;27:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Testoni PA, Bagnolo F, Caporuscio S, Lella F. Serum amylase measured four hours after endoscopic sphincterotomy is a reliable predictor of postprocedure pancreatitis. Am J Gastroenterol. 1999;94:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Relationship between post-ERCP pancreatitis and the change of serum amylase level after the procedure. World J Gastroenterol. 2007;13:3855-3860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Goyal H, Sachdeva S, Sherazi SAA, Gupta S, Perisetti A, Ali A, Chandan S, Tharian B, Sharma N, Thosani N. Early prediction of post-ERCP pancreatitis by post-procedure amylase and lipase levels: A systematic review and meta-analysis. Endosc Int Open. 2022;10:E952-E970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Gottlieb K, Sherman S, Pezzi J, Esber E, Lehman GA. Early recognition of post-ERCP pancreatitis by clinical assessment and serum pancreatic enzymes. Am J Gastroenterol. 1996;91:1553-1557. [PubMed] |
| 27. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4673] [Article Influence: 359.5] [Reference Citation Analysis (48)] |
| 28. | Smeets X, Bouhouch N, Buxbaum J, Zhang H, Cho J, Verdonk RC, Römkens T, Venneman NG, Kats I, Vrolijk JM, Hemmink G, Otten A, Tan A, Elmunzer BJ, Cotton PB, Drenth J, van Geenen E. The revised Atlanta criteria more accurately reflect severity of post-ERCP pancreatitis compared to the consensus criteria. United European Gastroenterol J. 2019;7:557-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Lee YS, Cho CM, Cho KB, Heo J, Jung MK, Kim SB, Kim KH, Kim TN, Lee DW, Han J, Kim HG, Kim D, Kim H. Difficult Biliary Cannulation from the Perspective of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: Identifying the Optimal Timing for the Rescue Cannulation Technique. Gut Liver. 2021;15:459-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Prouvot C, Boumaiza M, Maoui K, Peaucelle AS, Mohamed S, Boutallaka H, Boutet C, Roblin X, Phelip JM, Grange R, Williet N. Pancreatic steatosis is a strong risk factor for post-ERCP pancreatitis: An emerging concept. Dig Liver Dis. 2025;57:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Ding X, Zhang F, Wang Y. Risk factors for post-ERCP pancreatitis: A systematic review and meta-analysis. Surgeon. 2015;13:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Keswani RN, Qumseya BJ, O'Dwyer LC, Wani S. Association Between Endoscopist and Center Endoscopic Retrograde Cholangiopancreatography Volume With Procedure Success and Adverse Outcomes: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1866-1875.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Lee HJ, Cho CM, Heo J, Jung MK, Kim TN, Kim KH, Kim H, Cho KB, Kim HG, Han J, Lee DW, Lee YS. Impact of Hospital Volume and the Experience of Endoscopist on Adverse Events Related to Endoscopic Retrograde Cholangiopancreatography: A Prospective Observational Study. Gut Liver. 2020;14:257-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Tang W. The metabolism of diclofenac--enzymology and toxicology perspectives. Curr Drug Metab. 2003;4:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Kirchheiner J, Tsahuridu M, Jabrane W, Roots I, Brockmöller J. The CYP2C9 polymorphism: from enzyme kinetics to clinical dose recommendations. Per Med. 2004;1:63-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Dorado P, Cavaco I, Cáceres MC, Piedade R, Ribeiro V, Llerena A. Relationship between CYP2C8 genotypes and diclofenac 5-hydroxylation in healthy Spanish volunteers. Eur J Clin Pharmacol. 2008;64:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | de Jong MJP, Kuipers RN, Drenth JPH, Te Morsche RHM, van Delft F, Siersema PD; Dutch Pancreatitis Study Group, Sperna Weiland CJ, Verdonk RC, Venneman NG, Hadithi M, Bisseling TM, Bruno MJ, van Geenen EM. Heterozygous Variant of CYP2C8∗3 is a Protective Factor for Developing Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Clin Gastroenterol Hepatol. 2025;23:1068-1070.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Furukawa R, Kuwatani M, Jiang JJ, Tanaka Y, Hasebe R, Murakami K, Tanaka K, Hirata N, Ohki I, Takahashi I, Yamasaki T, Shinohara Y, Nozawa S, Hojyo S, Kubota SI, Hashimoto S, Hirano S, Sakamoto N, Murakami M. GGT1 is a SNP eQTL gene involved in STAT3 activation and associated with the development of Post-ERCP pancreatitis. Sci Rep. 2024;14:12224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Jena SS, Pati GK, Uthansingh K, Venkatesh GV, Mallick P, Behera M, Narayan J, Mishra D, Agarwal S, Sahu MK. SPINK1 Mutation in Idiopathic Chronic Pancreatitis: How Pertinent Is It in Coastal Eastern India? Cureus. 2021;13:e14427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Suzuki M, Minowa K, Nakano S, Isayama H, Shimizu T. Genetic Abnormalities in Pancreatitis: An Update on Diagnosis, Clinical Features, and Treatment. Diagnostics (Basel). 2020;11:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Choi YH, Lim Y, Jang DK, Ahn DW, Ryu JK, Paik WH, Kim YT, Kim JH, Lee SH. Genetic susceptibility to post-endoscopic retrograde cholangiopancreatography pancreatitis identified in propensity score-matched analysis. Korean J Intern Med. 2023;38:854-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Yu LM, Zhao KJ, Lu B. Use of NSAIDs via the Rectal Route for the Prevention of Pancreatitis after ERCP in All-Risk Patients: An Updated Meta-Analysis. Gastroenterol Res Pract. 2018;2018:1027530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Mäkelä A, Kuusi T, Schröder T. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scand J Clin Lab Invest. 1997;57:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33:184-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 265] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 46. | Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 47. | Zhao XW, Bao JJ, Hu C, Ding H, Liu XC, Mei Q, Xu JM. Effect of diclofenac on the levels of lipoxin A4 and Resolvin D1 and E1 in the post-ERCP pancreatitis. Dig Dis Sci. 2014;59:2992-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Buxbaum JL, Freeman M, Amateau SK, Chalhoub JM, Coelho-Prabhu N, Desai M, Elhanafi SE, Forbes N, Fujii-Lau LL, Kohli DR, Kwon RS, Machicado JD, Marya NB, Pawa S, Ruan WH, Sheth SG, Thiruvengadam NR, Thosani NC, Qumseya BJ; (ASGE Standards of Practice Committee Chair). American Society for Gastrointestinal Endoscopy guideline on post-ERCP pancreatitis prevention strategies: summary and recommendations. Gastrointest Endosc. 2023;97:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 49. | Yang C, Zhao Y, Li W, Zhu S, Yang H, Zhang Y, Liu X, Peng N, Fan P, Jin X. Rectal nonsteroidal anti-inflammatory drugs administration is effective for the prevention of post-ERCP pancreatitis: An updated meta-analysis of randomized controlled trials. Pancreatology. 2017;17:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Li X, Tao LP, Wang CH. Effectiveness of nonsteroidal anti-inflammatory drugs in prevention of post-ERCP pancreatitis: a meta-analysis. World J Gastroenterol. 2014;20:12322-12329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Bai B, Wang S, Du Y, Li M, Huang Q, Liu S, Zhang C, Fang Y, Chen X, Hong J, Li Y, Xu Z, Liu X, Hong R, Bao J, Mei Q. Indomethacin Does Not Reduce Post-ERCP Pancreatitis in High-Risk Patients Receiving Pancreatic Stenting. Dig Dis Sci. 2024;69:3442-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | Okuno M, Shiroko J, Taguchi D, Yamaguchi K, Takada J, Imai S, Sato H, Thanabashi S. The Effectiveness of the Rectal Administration of Low-dose Diclofenac for the Prevention of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis. Intern Med. 2018;57:2289-2294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Sakai H, Iwai N, Sakagami J, Okuda T, Ohara T, Hattori C, Taniguchi M, Oka K, Hara T, Tsuji T, Komaki T, Kagawa K, Dohi O, Yasuda H, Konishi H, Itoh Y. Rectal administration of low-dose diclofenac does not reduce post-endoscopic retrograde cholangiopancreatography pancreatitis: a propensity score matching analysis. Surg Endosc. 2023;37:2698-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 54. | Luo H, Zhao L, Leung J, Zhang R, Liu Z, Wang X, Wang B, Nie Z, Lei T, Li X, Zhou W, Zhang L, Wang Q, Li M, Zhou Y, Liu Q, Sun H, Wang Z, Liang S, Guo X, Tao Q, Wu K, Pan Y, Guo X, Fan D. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet. 2016;387:2293-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 55. | Agarwal A, Mahapatra SJ, Sethia R, Agarwal S, Elhence A, Mohta S, Gunjan D, Garg PK. Universal prophylactic rectal nonsteroidal anti-inflammatory drugs with a policy of selective pancreatic duct stenting significantly reduce post-endoscopic retrograde cholangiopancreatography pancreatitis. Indian J Gastroenterol. 2023;42:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Yoshihara T, Horimoto M, Kitamura T, Osugi N, Ikezoe T, Kotani K, Sanada T, Higashi C, Yamaguchi D, Ota M, Mizuno T, Gotoh Y, Okuda Y, Suzuki K. 25 mg versus 50 mg dose of rectal diclofenac for prevention of post-ERCP pancreatitis in Japanese patients: a retrospective study. BMJ Open. 2015;5:e006950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Fogel EL, Lehman GA, Tarnasky P, Cote GA, Schmidt SE, Waljee AK, Higgins PDR, Watkins JL, Sherman S, Kwon RSY, Elta GH, Easler JJ, Pleskow DK, Scheiman JM, El Hajj II, Guda NM, Gromski MA, McHenry L Jr, Arol S, Korsnes S, Suarez AL, Spitzer R, Miller M, Hofbauer M, Elmunzer BJ; US Cooperative for Outcomes Research in Endoscopy (USCORE). Rectal indometacin dose escalation for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography in high-risk patients: a double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Akshintala VS, Sperna Weiland CJ, Bhullar FA, Kamal A, Kanthasamy K, Kuo A, Tomasetti C, Gurakar M, Drenth JPH, Yadav D, Elmunzer BJ, Reddy DN, Goenka MK, Kochhar R, Kalloo AN, Khashab MA, van Geenen EJM, Singh VK. Non-steroidal anti-inflammatory drugs, intravenous fluids, pancreatic stents, or their combinations for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 59. | ASGE Standards of Practice Committee; Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 587] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 60. | Beran A, Aboursheid T, Ali AH, Nayfeh T, Albunni H, Vargas A, Mohamed MF, Elfert K, Shaear M, Obaitan I, Saleem N, Ahmed A, Gromski MA, DeWitt JM, Al-Haddad M, Watkins JL, Fogel E, Easler JJ. Predictors of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis: A Comprehensive Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2025;23:1905-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 61. | Afghani E, Akshintala VS, Khashab MA, Law JK, Hutfless SM, Kim KJ, Lennon AM, Kalloo AN, Singh VK. 5-Fr vs. 3-Fr pancreatic stents for the prevention of post-ERCP pancreatitis in high-risk patients: a systematic review and network meta-analysis. Endoscopy. 2014;46:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Yang H, Yang Z, Hong J. Post-ERCP pancreatitis occurs more frequently in self-expandable metallic stents than multiple plastic stents on benign biliary strictures: a meta-analysis. Ann Med. 2022;54:2439-2449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 63. | Kerdsirichairat T, Attam R, Arain M, Bakman Y, Radosevich D, Freeman M. Urgent ERCP with pancreatic stent placement or replacement for salvage of post-ERCP pancreatitis. Endoscopy. 2014;46:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Thiruvengadam NR, Saumoy M, Schneider Y, Attala S, Triggs J, Lee P, Kochman ML. A Cost-Effectiveness Analysis for Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis Prophylaxis in the United States. Clin Gastroenterol Hepatol. 2022;20:216-226.e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Menon S, Abbasi A. S121 Cost-Effectiveness of Biodegradable Pancreatic Stents in Post-ERCP Pancreatitis Prophylaxis. Am J Gastroenterol. 2024;119:S88. [DOI] [Full Text] |
| 66. | Michael FA, Gerber L, Weiler N, Hunyady PM, Abedin N, de la Vera AL, Stoffers P, Filmann N, Zeuzem S, Bojunga J, Friedrich-Rust M, Dultz G. Transabdominal ultrasonography to reduce the burden of X-ray imaging in prophylactic pancreatic stent localization after ERCP-A prospective trial. United European Gastroenterol J. 2021;9:469-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 67. | Patel R, Bertran-Rodriguez C, Kumar A, Brady P, Gomez-Esquivel R, Changela K, Niknam N, Taunk P. Efficacy of aggressive hydration with normal saline versus lactated Ringer's solution for the prevention of post-ERCP pancreatitis in high-risk patients: a randomized controlled trial. Endosc Int Open. 2022;10:E933-E939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 68. | Zhang X, Zhang JM, Wei W, Lin H. Nitroglycerin combined with NSAIDs for prevention of post-ERCP pancreatitis: A meta-analysis of prospective, randomized, controlled trials. Medicine (Baltimore). 2024;103:e38764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Shi QQ, Huang GX, Li W, Yang JR, Ning XY. Rectal nonsteroidal anti-inflammatory drugs, glyceryl trinitrate, or combinations for prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: A network meta-analysis. World J Clin Cases. 2022;10:7859-7871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 70. | Wang Y, Xu B, Zhang W, Lin J, Li G, Qiu W, Wang Y, Sun D, Wang Y. Prophylactic effect of rectal indomethacin plus nitroglycerin administration for preventing pancreatitis after endoscopic retrograde cholangiopancreatography in female patients. Ann Palliat Med. 2020;9:4029-4037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Bai Y, Ren X, Zhang XF, Lv NH, Guo XG, Wan XJ, Nie ZG, Han ST, Bie P, Tian DA, Ji M, Li ZS. Prophylactic somatostatin can reduce incidence of post-ERCP pancreatitis: multicenter randomized controlled trial. Endoscopy. 2015;47:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Mok SRS, Ho HC, Shah P, Patel M, Gaughan JP, Elfant AB. Lactated Ringer's solution in combination with rectal indomethacin for prevention of post-ERCP pancreatitis and readmission: a prospective randomized, double-blinded, placebo-controlled trial. Gastrointest Endosc. 2017;85:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Zhang Y, Chen QB, Gao ZY, Xie WF. Meta-analysis: octreotide prevents post-ERCP pancreatitis, but only at sufficient doses. Aliment Pharmacol Ther. 2009;29:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Omata F, Deshpande G, Tokuda Y, Takahashi O, Ohde S, Carr-Locke DL, Jacobs JL, Mine T, Fukui T. Meta-analysis: somatostatin or its long-acting analogue, octreotide, for prophylaxis against post-ERCP pancreatitis. J Gastroenterol. 2010;45:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Wu Z, Xiao G, Wang G, Xiong L, Qiu P, Tan S. Effects of Somatostatin and Indomethacin Mono or Combination Therapy on High-risk Hyperamylasemia and Post-pancreatitis Endoscopic Retrograde Cholangiopancreatography Patients: A Randomized Study. Surg Laparosc Endosc Percutan Tech. 2023;33:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 76. | Concepción-Martín M, Gómez-Oliva C, Juanes A, Díez X, Prieto-Alhambra D, Torras X, Sainz S, Villanueva C, Farre A, Guarner-Argente C, Guarner C. Somatostatin for prevention of post-ERCP pancreatitis: a randomized, double-blind trial. Endoscopy. 2014;46:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Wang ZK, Yang YS, Cai FC, Wang YH, Shi XL, Ding C, Li W. Is prophylactic somatostatin effective to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis or hyperamylasemia? A randomized, placebo-controlled pilot trial. Chin Med J (Engl). 2013;126:2403-2408. [PubMed] |
| 78. | Bhat SZ, Salvatori R. Current role of pasireotide in the treatment of acromegaly. Best Pract Res Clin Endocrinol Metab. 2024;38:101875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 79. | Horváth IL, Kleiner D, Nagy R, Fehérvári P, Hankó B, Hegyi P, Csupor D. Nafamostat Reduces the Incidence of post-ERCP Pancreatitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin Pharmacol Ther. 2024;115:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Yu G, Li S, Wan R, Wang X, Hu G. Nafamostat mesilate for prevention of post-ERCP pancreatitis: a meta-analysis of prospective, randomized, controlled trials. Pancreas. 2015;44:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Kim JS, Lee SH, Park N, Huh G, Chun JW, Choi JH, Cho IR, Paik WH, Ryu JK, Kim YT. The effect of nafamostat mesilate infusion after ERCP for post-ERCP pancreatitis. BMC Gastroenterol. 2022;22:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Matsumoto T, Okuwaki K, Imaizumi H, Kida M, Iwai T, Yamauchi H, Kaneko T, Hasegawa R, Masutani H, Tadehara M, Adachi K, Watanabe M, Kurosu T, Tamaki A, Kikuchi H, Ohno T, Koizumi W. Nafamostat Mesylate is Not Effective in Preventing Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Dig Dis Sci. 2021;66:4475-4484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Buxbaum J, Leonor P, Tung J, Lane C, Sahakian A, Laine L. Randomized Trial of Endoscopist-Controlled vs. Assistant-Controlled Wire-Guided Cannulation of the Bile Duct. Am J Gastroenterol. 2016;111:1841-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Leerhøy B, Elmunzer BJ. How to Avoid Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Gastrointest Endosc Clin N Am. 2018;28:439-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Tanikawa T, Miyake K, Kawada M, Ishii K, Fushimi T, Urata N, Wada N, Nishino K, Suehiro M, Kawanaka M, Shiraha H, Haruma K, Kawamoto H. Can early precut reduce post-endoscopic retrograde cholangiopancreatography pancreatitis in patients with difficult bile duct cannulation? World J Gastrointest Endosc. 2024;16:519-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Mohseni Salehi Monfared SS, Vahidi H, Abdolghaffari AH, Nikfar S, Abdollahi M. Antioxidant therapy in the management of acute, chronic and post-ERCP pancreatitis: a systematic review. World J Gastroenterol. 2009;15:4481-4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Fuentes-Orozco C, Dávalos-Cobián C, García-Correa J, Ambriz-González G, Macías-Amezcua MD, García-Rentería J, Rendón-Félix J, Chávez-Tostado M, Cuesta-Márquez LA, Alvarez-Villaseñor AS, Cortés-Flores AO, González-Ojeda A. Antioxidant drugs to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis: What does evidence suggest? World J Gastroenterol. 2015;21:6745-6753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Jagtap N, Kumar JK, Chavan R, Basha J, Tandan M, Lakhtakia S, Kalapala R, Nabi Z, Gupta R, Ramchandani M, Talukdar R, Reddy M, Yarlagadda R, Singh J, Memon SF, Venkat Rao G, Reddy DN. EUS versus MRCP to perform ERCP in patients with intermediate likelihood of choledocholithiasis: a randomised controlled trial. Gut. 2022;gutjnl-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Impellizzeri G, Grassini MV, Donato G, De Angelis CG, Pagano N. An Approach to and Treatment of Indeterminate Biliary Strictures: A Comprehensive Review of the Literature. J Clin Med. 2024;14:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 90. | Cotton PB, Pauls Q, Keith J, Thornhill A, Drossman D, Williams A, Durkalski-Mauldin V. The EPISOD study: long-term outcomes. Gastrointest Endosc. 2018;87:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Dom Dera J. Risk Stratification: A Two-Step Process for Identifying Your Sickest Patients. Fam Pract Manag. 2019;26:21-26. [PubMed] |
| 92. | Friedland S, Soetikno RM, Vandervoort J, Montes H, Tham T, Carr-Locke DL. Bedside scoring system to predict the risk of developing pancreatitis following ERCP. Endoscopy. 2002;34:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, Shaw MJ, Snady HW, Erickson RV, Moore JP, Roel JP. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 852] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 94. | Haraldsson E, Kylänpää L, Grönroos J, Saarela A, Toth E, Qvigstad G, Hult M, Lindström O, Laine S, Karjula H, Hauge T, Sadik R, Arnelo U. Macroscopic appearance of the major duodenal papilla influences bile duct cannulation: a prospective multicenter study by the Scandinavian Association for Digestive Endoscopy Study Group for ERCP. Gastrointest Endosc. 2019;90:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | Zheng R, Chen M, Wang X, Li B, He T, Wang L, Xu G, Yao Y, Cao J, Shen Y, Wang Y, Zhu H, Zhang B, Wu H, Zou X, He G. Development and validation of a risk prediction model and scoring system for post-endoscopic retrograde cholangiopancreatography pancreatitis. Ann Transl Med. 2020;8:1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Park CH, Park SW, Yang MJ, Moon SH, Park DH. Pre- and post-procedure risk prediction models for post-endoscopic retrograde cholangiopancreatography pancreatitis. Surg Endosc. 2022;36:2052-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 97. | Chiba M, Kato M, Kinoshita Y, Shimamoto N, Tomita Y, Abe T, Kanazawa K, Tsukinaga S, Nakano M, Torisu Y, Toyoizumi H, Sumiyama K. The milestone for preventing post-ERCP pancreatitis using novel simplified predictive scoring system: a propensity score analysis. Surg Endosc. 2021;35:6696-6707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Meng ZW, Ruan Y, Fisher S, Bishay K, Chau M, Howarth M, Cartwright S, Chen YI, Dixon E, Heitman SJ, Brenner DR, Forbes N. Development and validation of a practical clinical risk prediction model for post-endoscopic retrograde cholangiopancreatography pancreatitis. DEN Open. 2024;4:e355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 99. | Wen E, Cao Y, He S, Zhang Y, You L, Wang T, Wang Z, He J, Feng Y. The mitochondria-targeted Kaempferol nanoparticle ameliorates severe acute pancreatitis. J Nanobiotechnology. 2024;22:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 100. | Wang D, Wang S, Liu J, Shi X, Xiong T, Li R, Wei W, Ji L, Huang Q, Gong X, Ai K. Nanomedicine Penetrating Blood-Pancreas Barrier for Effective Treatment of Acute Pancreatitis. Adv Sci (Weinh). 2025;12:e2413925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Norouzi A, Ghasem Poori E, Kaabe S, Norouzi Z, Sohrabi A, Amlashi FI, Tavasoli S, Besharat S, Ezabadi Z, Amiriani T. Effect of Adding Intravenous Somatostatin to Rectal Indomethacin on Post-Endoscopic Retrograde Cholangiopancreatography (ERCP) Pancreatitis in High-risk Patients: A Double-blind Randomized Placebo-controlled Clinical Trial. J Clin Gastroenterol. 2023;57:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 102. | Yoo KS, Huh KR, Kim YJ, Kim KO, Park CH, Hahn T, Park SH, Kim JH, Park CK, Kwon YJ, Lehman GA. Nafamostat mesilate for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a prospective, randomized, double-blind, controlled trial. Pancreas. 2011;40:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |