Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.111004
Revised: July 15, 2025
Accepted: August 20, 2025
Published online: October 27, 2025
Processing time: 126 Days and 4.3 Hours
Small intestinal fistulas following drainage tube removal are rare but can cause significant morbidity. These complications are most commonly seen in the context of postoperative abdominal surgery. A small intestinal fistula secondary to drai
We report a case of an 80-year-old man who developed a small intestinal fistula following the removal of an abdominal drainage tube after radical surgery for sigmoid colon cancer. On postoperative Day 8, the patient experienced abdominal pain and nausea immediately after the drainage tube was removed. Imaging studies and contrast injection confirmed the presence of a small intestinal fistula. The patient underwent a second surgery for partial small intestine resection and end-to-end anastomosis, followed by supportive care.
This case highlights the importance of recognizing the potential for small intes
Core Tip: Small intestinal fistulas after drainage tube removal are rare, especially after sigmoid colon cancer radical surgery. Nonspecific symptoms often delay diagnosis. This case reports an 80-year-old man who developed such a fistula, successfully treated with surgical repair and supportive care, highlighting the need for awareness of this rare complication.
- Citation: Pan XF, Cai Y, Cao YG. Small intestinal fistula caused by drainage tube removal: A case report. World J Gastrointest Surg 2025; 17(10): 111004
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/111004.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.111004
In clinical practice, drainage tubes are an important auxiliary treatment tool after abdominal surgery[1], mainly used to drain fluid from the abdominal cavity, help wound healing, and prevent infection. However, the process of removing the drainage tube may also lead to complications[2], such as difficulty in removing the tube, intestinal adhesions, broken drainage tube, incomplete formation of drainage tube sinus tract, resulting in leakage and bleeding after tube removal. However, small intestinal fistulas caused by the removal of drainage tubes after surgery are not common. We present a case of a small intestinal fistula resulting from the removal of an abdominal drainage tube after radical surgery for sigmoid colon cancer, and analyze the causes to deepen our understanding of this complication as primary surgeons.
An 80-year-old Han Chinese male patient from mainland China was admitted to the hospital due to abdominal pain with hematochezia for 3 days, with no significant past medical history. Physical examination upon admission revealed no significant abnormalities. Enhanced abdominal computed tomography (CT) on admission showed mild thickening of the walls of the descending colon and sigmoid colon, with peripheral exudative changes. Endoscopic colonoscopy suggested sigmoid colon cancer and colonic polyps.
The patient was admitted to the hospital due to abdominal pain and melena for 3 days, with no significant past medical history. Physical examination upon admission revealed no significant abnormalities. Enhanced abdominal CT showed mild thickening of the walls of the descending colon and sigmoid colon, with peripheral exudative changes. Colonoscopy suggested sigmoid colon cancer and colonic polyps.
No significant past medical history.
The patient did not provide family history information.
Physical examination upon admission revealed no significant abnormalities.
Laboratory tests showed a white blood cell count of 12.8 × 109/L, C-reactive protein (CRP) of 53.2 mg/L, and procalcitonin (PCT) of 0.517 µg/L.
Consequently, the patient underwent a laparoscopy-assisted radical resection of colon cancer. During surgery, a tumor measuring approximately 5.0 cm × 5.0 cm was found in the middle segment of the sigmoid colon, with a hard texture and unclear margins. The rectum was resected using a stapling device, with the proximal cut made approximately 7 cm above the tumor and the distal cut approximately 5 cm below it. A drainage tube was placed in the left pelvic cavity near the anastomosis, with a depth of 20 cm. Postoperative pathology revealed moderately to poorly differentiated adenocarcinoma with metastasis to 1 out of 15 regional lymph nodes, but no distant metastasis.
Postoperative diagnosis: Malignant tumor of the sigmoid colon (T4N1M0, Stage IIIb).
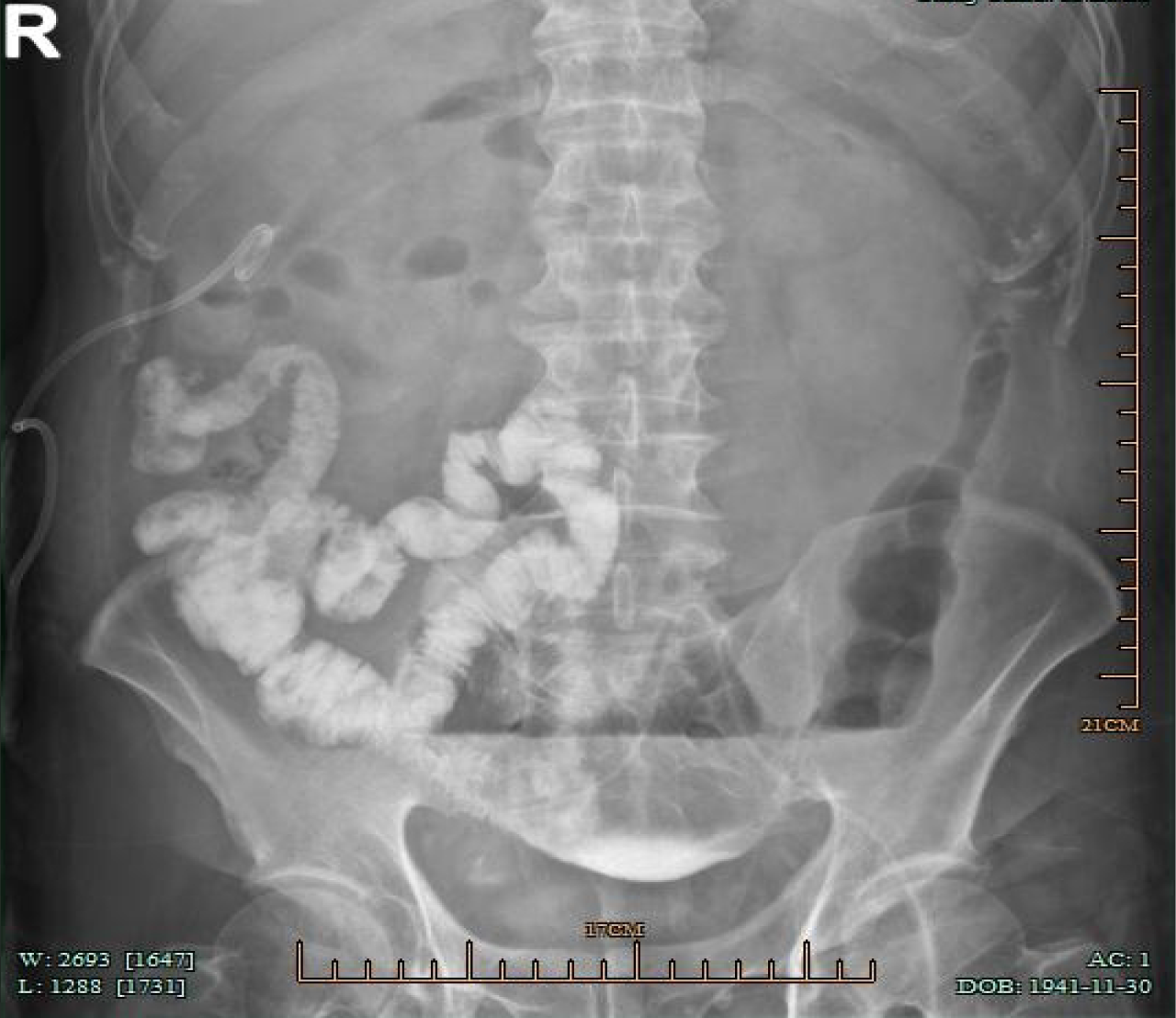
On postoperative day 8, the drainage from the left abdominal drain significantly decreased to 5 mL per day. Therefore, it was decided to remove the abdominal drainage tube. After confirming that the drainage tube could be moved smoothly, it was slowly withdrawn. During the removal process, the patient did not experience any significant discomfort. However, 12 hours after the removal of the abdominal drainage tube, the patient developed abdominal colic, which worsened intermittently, accompanied by nausea. Laboratory tests showed a white blood cell count of 12.8 × 109/L, CRP of 53.2 mg/L, and PCT of 0.517 µg/L. It is considered that there may be anastomosis leakage. The abdominal enhanced CT scan showed postoperative changes of the sigmoid colon tumor with a small amount of pelvic fluid accumulation. Conservative treatment was given, including fasting, anti-infection measures, nutritional support, and octreotide to inhibit digestive fluid secretion for five days. However, the patient's abdominal pain and bloating did not improve. Fecal-like fluid was also observed draining from the original tube, with a daily volume ranging from 100-200 mL. On the 14th day after surgery, a follow-up abdominal CT scan was performed, revealing multiple effusions in the abdominal and pelvic cavities. Injecting contrast agent through the original abdominal drainage tube in the lower left abdomen, the small intestines in the middle and lower right abdomen were filled with contrast agent, indicating a small intestinal fistula (Figure 1).
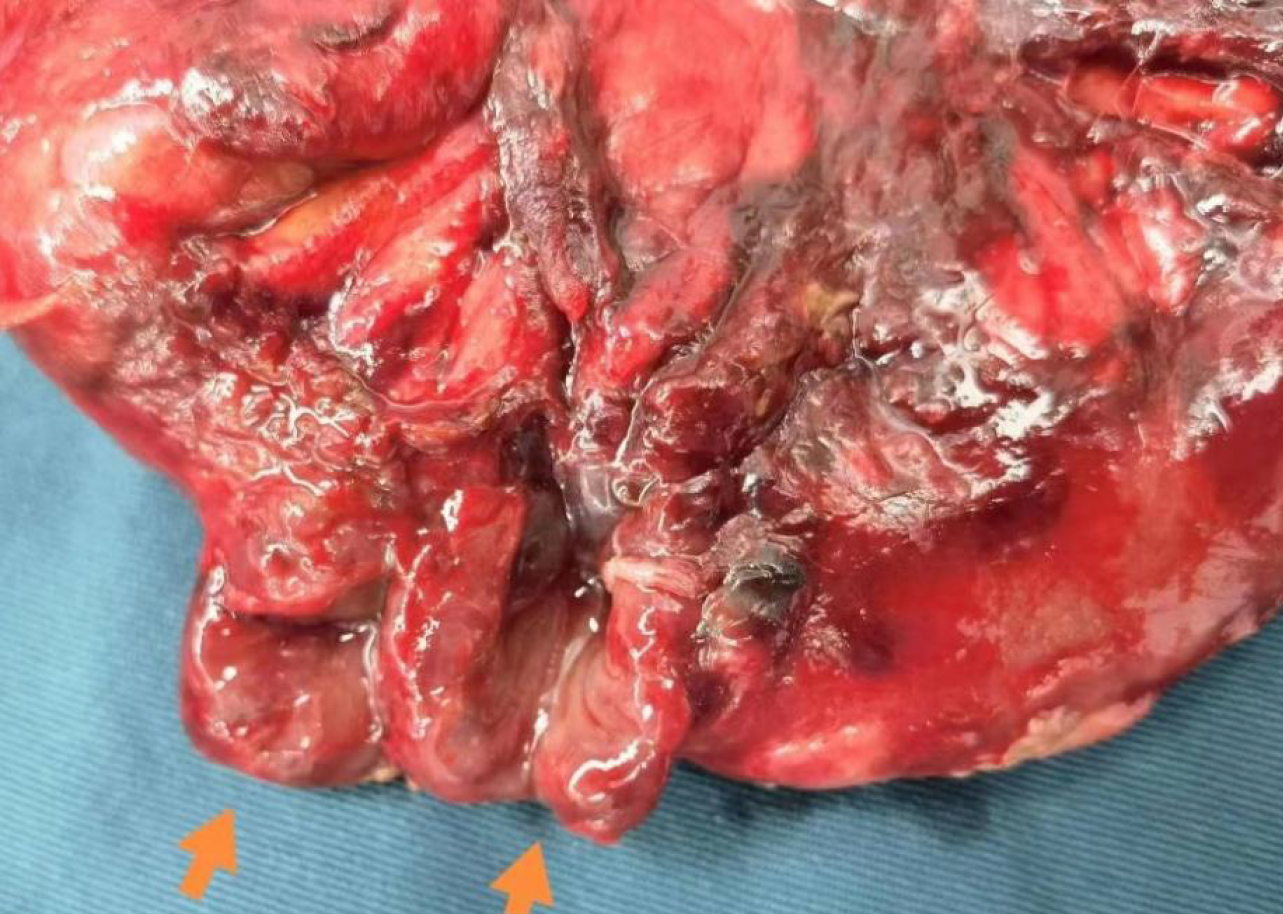
After the surgical contraindications were eliminated, the patient underwent exploratory laparotomy. During the surgery, it was found that the anastomosis of the rectum had healed well. However, two adjacent ruptures, each approximately 1.5 cm in diameter, were discovered about 1 meter from the ileocecal valve of the ileum (Figure 2). Intestinal fluid was overflowing from the ruptures, and the surrounding tissues had formed an encasement. A straight forceps could be inserted along the sinus tract into the intestinal lumen. Exploration revealed significant congestion and edema in the small intestine, large intestine, and parietal peritoneum. There was about 500 mL of pale yellow fluid accumulation in the upper abdominal cavity, with no other abnormalities observed. Consequently, partial resection of the small intestine involving the ruptured segment was performed. The postoperative pathology report indicated chronic inflammation of the intestinal wall mucosa accompanied by congestion, edema, bleeding, and local necrosis, with inflammatory exudate attached to the serosal surface. The postoperative diagnosis was small intestinal fistula and postoperative sigmoid colon malignant tumor.
After the surgery, the patient was treated with anti-inflammatory therapy and enteral nutrition support and was discharged smoothly. During the 3-month follow-up after discharge, the patient had smooth bowel movements and no abdominal discomfort.
Intestinal fistula refers to an abnormal passage between the intestine and other organs, or between the intestine and the abdominal cavity or outside the abdominal wall. In patients who have undergone gastrointestinal surgery, it is a relatively common postoperative complication[3]. When an intestinal fistula develops, intestinal contents may leak into other parts of the body or adjacent organs, triggering a range of pathological and physiological issues, including infection, fluid loss, malnutrition, and organ dysfunction[4]. The condition is often caused by surgical procedures, trauma, tumors, infections, or radiation damage, and it frequently results in severe infections and malnutrition. Treatment is difficult, and patient prognosis is poor, with a mortality rate of 15% to 20%[5]. The patient recovered well after radical surgery for colon cancer but developed abdominal pain and peritoneal irritation signs following the removal of the abdominal drainage tube. Retrograde contrast imaging at the drainage tube site revealed small bowel opacification. A subsequent surgery confirmed the presence of a small bowel fistula caused by the removal of the drainage tube. Pathological findings further supported that the fistula resulted from bowel perforation due to tube removal.
Small intestinal fistula should be differentiated from other postoperative complications such as anastomotic leakage, intra-abdominal abscess, postoperative ileus, and early small bowel obstruction. While symptoms like abdominal pain and distension are common to all, small intestinal fistula is characterized by the presence of enteric contents in drainage or imaging-confirmed communication with the intestine. Contrast-enhanced CT and fistulography are essential tools for distinguishing small intestinal fistula from these conditions and guiding appropriate treatment[6].
The formation of a small intestinal fistula in this case is likely multifactorial. Although a temporal association was observed between drainage tube removal and fistula onset, a definitive causal relationship cannot be established. Other contributing factors, such as subclinical anastomotic leakage, intestinal ischemia, or postoperative adhesions, may also have played a role in the development of the fistula. First, the side holes of the drainage tube, when excessively enlarged during trimming, may have caused localized mechanical pressure on the small intestinal wall. This mechanical pressure can lead to impaired local blood flow, resulting in ischemia and subsequent necrosis of the intestinal wall[7]. Secondly, the patient's postoperative intestinal motility may facilitate the movement of the drainage tube, causing it to come into closer contact with the small intestine. The continuous movement and peristalsis of the intestines may lead to friction between the tube and the intestinal wall, resulting in erosion and ultimately perforation[8]. In this case, the drainage tube was placed from the left abdominal wall into the posterior pelvic cavity on the left side during surgery. Postoperatively, with the patient's movements and intestinal motility, the drainage tube may have migrated to the mid-abdominal region, creating an opportunity for the small intestine to be drawn into the side holes of the drainage tube; Additionally, the negative pressure generated during routine maintenance or removal of the drainage tube may cause the intestinal wall to adhere to the side holes of the drainage tube, thereby exacerbating this issue[9]. Moreover, the patient's advanced age and underlying conditions may have also played a role in the development of the small intestinal fistula[10]. Advanced age is associated with decreased intestinal perfusion and slower intestinal wound healing, both of which could have con
To reduce the incidence of small bowel fistula complications caused by the removal of abdominal drainage tubes, prevention is paramount. Several studies have highlighted unnecessary placement of drainage tubes should be mi
For patients requiring drainage tube placement, meticulous attention to tube placement details, judicious timing of tube removal, and gentle manipulation can effectively prevent the occurrence of small bowel fistula. During surgery, avoid excessive trimming of the side holes and prevent the tube’s tip from direct contact with the omentum or mesentery. Ensure the abdominal wall puncture site is adequately dilated, position the drainage tube holes close to the target area while avoiding sharp angles and abdominal wall vessels, and secure the tube properly. Remove the drainage tube promptly once its purpose is fulfilled[16]. Previous studies have reported iatrogenic duodenal fistula caused by drainage-tube penetration after hepatectomy, which may be due to the long-term placement of the drainage tube near the duodenum[17]. If the removal of the tube proves to be challenging, flexible approaches should be employed to address the issue[9]. In cases where patients experience abdominal pain and bloating after drainage tube removal, or show signs of peritoneal irritation on physical examination, intestinal fistula should be suspected. Immediate completion of abdominal CT, complete blood count, and biochemical blood tests is essential to aid diagnosis and assess the patient’s condition. Concurrent measures should include fasting, anti-inflammatory treatment, nutritional support, and administration of somatostatin, to create conditions for early reoperation if needed. In summary, this case highlights the importance of judicious drainage tube use for primary care surgeons, aiming to reduce associated complications, alleviate patient suffering and burden, and improve prognosis. At present, the patient is in stable condition during follow-up, with satisfactory recovery. However, long-term complications following small intestinal fistula repair must be considered. These include nutritional and metabolic disturbances due to chronic loss of intestinal fluid, potentially leading to severe malnutrition, protein-energy deficiency, and electrolyte (Na+, K+, Mg2+) or trace element (zinc, selenium) depletion. Vitamin B12 deficiency, particularly in cases involving ileal involvement, may cause anemia and neurological symptoms. Mechanical complications such as intestinal obstruction may arise from intra-abdominal adhesions or stenosis at or distal to the fistula site, possibly requiring repeated surgical intervention. Careful long-term monitoring and nutritional support are essential to reduce the risk of these sequelae[18-22].
This study is a single case report, which inherently limits the generalizability of the findings. The conclusions drawn from this case may not be applicable to all patients undergoing similar procedures. Additionally, the follow-up duration was relatively short, and long-term outcomes require further observation. Comprehensive biochemical monitoring and nutritional assessments were also limited, which may affect the completeness of the evaluation of postoperative complications. Future studies with larger sample sizes and longer follow-up periods are needed to validate and expand upon these observations. As this is a single case report, the findings and clinical interpretations should be viewed with caution. The observations may not be directly generalizable to a broader patient population or incorporated into clinical guide
This case report describes a rare case of an 80-year-old male patient who developed a small intestinal fistula following the removal of a drainage tube after radical surgery for sigmoid colon cancer. The patient underwent a second surgery, which included partial small intestine resection and end-to-end anastomosis, and received supportive care. Follow-up at three months postoperatively showed that the patient had recovered well, with no abdominal discomfort. This case highlights the importance of recognizing the potential for small intestinal fistulas following drainage tube removal in postoperative patients. Timely diagnosis and surgical intervention can lead to successful management and recovery. Additionally, this case emphasizes the need for careful manipulation during the placement and removal of drainage tubes to reduce the incidence of related complications, alleviate patient suffering and burden, and improve prognosis.
| 1. | Ozer MT, Uzar AI, Eryilmaz M, Altinel O, Demirbas S, Arslan I, Tufan CT. A novel laparoscopic suction device for applying precise aspiration during laparoscopic surgery: sponge-tip suction tube. J Laparoendosc Adv Surg Tech A. 2008;18:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Guo Y, Guo X, Wang J, Li K, Xu G, Yan W, Zhang J, Lian D, Fan Q, Han Z, Liu S, Wang W, Amin B, Gong K, Zhang N, Peng J, Song M, Zhang B, Zhu B. Abdominal infectious complications associated with the dislocation of intraperitoneal part of drainage tube and poor drainage after major surgeries. Int Wound J. 2020;17:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Hu KY, Peterson CY. Enteric fistula: An overlooked but significant healthcare burden. Am J Surg. 2021;221:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Lee SH. Surgical management of enterocutaneous fistula. Korean J Radiol. 2012;13 Suppl 1:S17-S20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Stevens P, Foulkes RE, Hartford-Beynon JS, Delicata RJ. Systematic review and meta-analysis of the role of somatostatin and its analogues in the treatment of enterocutaneous fistula. Eur J Gastroenterol Hepatol. 2011;23:912-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Tonolini M, Magistrelli P. Enterocutaneous fistulas: a primer for radiologists with emphasis on CT and MRI. Insights Imaging. 2017;8:537-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Pecoraro F, Rancic Z, Lachat M, Mayer D, Amann-Vesti B, Pfammatter T, Bajardi G, Veith FJ. Chronic mesenteric ischemia: critical review and guidelines for management. Ann Vasc Surg. 2013;27:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | EDMUNDS LH Jr, WILLIAMS GM, WELCH CE. External fistulas arising from the gastro-intestinal tract. Ann Surg. 1960;152:445-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Shi W, Huang Y, Xu Z, Huang X, Tong G, Lin J, Zhou Y, Yang Q, Chen C. Application effect of standardized nursing process of abdominal drainage tube in cholelithiasis: a single-center retrospective cohort study. BMC Gastroenterol. 2025;25:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Denicu MM, Cartu D, Ciorbagiu M, Nemes RN, Surlin V, Ramboiu S, Chiuțu LC. Therapeutic Options in Postoperative Enterocutaneous Fistula-A Retrospective Case Series. Medicina (Kaunas). 2022;58:880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Liu Y, Ji Y, Jiang R, Fang C, Shi G, Cheng L, Zuo Y, Ye Y, Su X, Li J, Wang H, Wang Y, Lin Y, Dai L, Zhang S, Deng H. Reduced smooth muscle-fibroblasts transformation potentially decreases intestinal wound healing and colitis-associated cancer in ageing mice. Signal Transduct Target Ther. 2023;8:294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 12. | Mariani P, Slim K. Enhanced recovery after gastro-intestinal surgery: The scientific background. J Visc Surg. 2016;153:S19-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Samaiya A. To Drain or Not to Drain after Colorectal Cancer Surgery. Indian J Surg. 2015;77:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Menahem B, Vallois A, Alves A, Lubrano J. Prophylactic pelvic drainage after rectal resection with extraperitoneal anastomosis: is it worthwhile? A meta-analysis of randomized controlled trials. Int J Colorectal Dis. 2017;32:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Nguyen MT, Pham AV. Early small bowel obstruction as a complication of abdominal drain in colon cancer surgery: a case report and literature review. Ann Med Surg (Lond). 2023;85:5804-5808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, Miyazawa M, Uchiyama K, Yamaue H. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 375] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 17. | Chen F, Wang J, Li F. A rare case of iatrogenic duodenal fistula secondary to drainage-tube penetration. Asian J Surg. 2024;47:4182-4183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Lloyd DA, Gabe SM, Windsor AC. Nutrition and management of enterocutaneous fistula. Br J Surg. 2006;93:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Martinez JL, Luque-de-León E, Ballinas-Oseguera G, Mendez JD, Juárez-Oropeza MA, Román-Ramos R. Factors predictive of recurrence and mortality after surgical repair of enterocutaneous fistula. J Gastrointest Surg. 2012;16:156-63; discussion 163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Schecter WP, Hirshberg A, Chang DS, Harris HW, Napolitano LM, Wexner SD, Dudrick SJ. Enteric fistulas: principles of management. J Am Coll Surg. 2009;209:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Ghimire P. Management of Enterocutaneous Fistula: A Review. JNMA J Nepal Med Assoc. 2022;60:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Visschers RG, Olde Damink SW, Winkens B, Soeters PB, van Gemert WG. Treatment strategies in 135 consecutive patients with enterocutaneous fistulas. World J Surg. 2008;32:445-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/