Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.3048
Revised: July 2, 2024
Accepted: July 10, 2024
Published online: September 27, 2024
Processing time: 174 Days and 20.9 Hours
Clostridium difficile (C. difficile) infection (CDI) is a rare clinical disease caused by changes in the intestinal microenvironment, which has a variety of causes and a poor prognosis, and for which there is no standardized clinical treatment.
A patient experienced recurrent difficulty in bowel movements over the past decade. Recently, symptoms worsened within the last ten days, leading to a clinic visit due to constipation. The patient was subsequently referred to our depart
CDI is the leading cause of nosocomial post-operative care, with limited clinical cases and poor patient prognosis, and comprehensive clinical treatment guidelines are still lacking. This infection can be triggered by a variety of factors, including intestinal hypoxia, inappropriate antibiotic use, and bile acid circulation disorders. In patients with chronic bowel disease and related etiologies, prompt preoperative attention to possible CDI and preoperative bowel preparation is critical. Adequate and prolonged medication should be maintained in the treatment of CDI to prevent recurrence of the disease.
Core Tip: Clostridium difficile infection, a rare and serious condition, often lacks standardized treatment. A case involved obstructed colon and subsequent infection post-surgery, successfully treated with vancomycin and linezolid. However, non-adherence to medication led to patient fatality. Timely preoperative assessment and prolonged treatment are crucial for chronic bowel disease patients. More comprehensive clinical guidelines are needed.
- Citation: Ke F, Dong ZH, Bu F, Li CN, He QT, Liu ZC, Lu J, Yu K, Wang DG, Xu HN, Ye CT. Clostridium difficile infection following colon subtotal resection in a patient with gallstones: A case report and review of literature. World J Gastrointest Surg 2024; 16(9): 3048-3056
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/3048.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.3048
Clostridium difficile (C. difficile), a bacterium predominantly found in soil and the intestinal environment, was initially isolated from the feces of newborns and termed "C. difficile" by Hall and O'Toole[1]. This bacterium constitutes a part of the normal gut microbiota, which accounts for 70% of the total microbial population in the human body. Alterations in the gut microbiota, triggered by factors such as the use of antibiotics, disruptions in the bile acid cycle, and chronic intestinal hypoxia, can lead to the development of C. difficile infection (CDI)[2]. In clinical settings, CDI often manifest with multidrug resistance. Currently, the primary diagnostic approach involves confirming the presence of toxigenic strains or toxins in fecal samples based on clinical symptoms and laboratory test results[3]. While relatively rare in clinical practice, we now present a case of a cholecystolithiasis patient from the First Hospital of Jilin University, who developed a CDI following a colon subtotal resection. In this report, the patient has given written consent, all identifiable information has been removed to protect privacy, and the use of clinical data and images has been done with the patient's consent and in compliance with the data use policy of the relevant healthcare institution, we collected various data since his onset, as described below.
A 58-year-old male patient presented with a history of constipation that commenced a decade ago. He experienced infrequent bowel movements occurring every 3 to 4 days, without accompanying abdominal pain or distention.
Over the years, he resorted to self-administered laxatives, which provided temporary relief. However, in the past ten days, the patient's condition deteriorated, and he ceased to pass stools, coupled with progressive abdominal distension.
Seeking further medical intervention, the patient sought outpatient care at our institution and was admitted to our department on October 8, 2022, with a primary diagnosis of "constipation".
The patient denied any family history of malignant tumours.
The physical examination found no positive signs.
No significant abnormal results were found in laboratory tests.
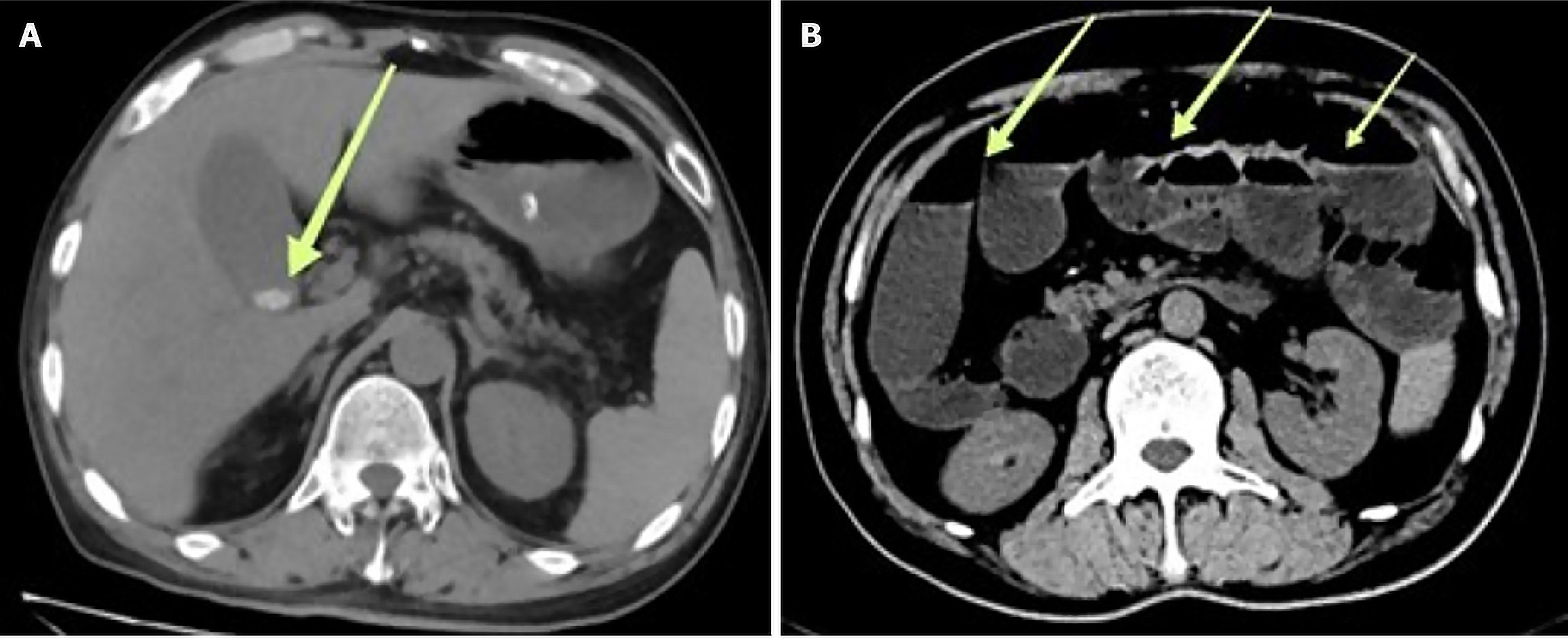
An abdominal contrast-enhanced computed tomography (CT) scan revealed the presence of gallstones and a substantial amount of colonic content. Colonic transit studies demonstrated a pronounced reduction in colonic transit speed (refer to Figure 1). Physical examination revealed a flat abdominal contour without significant tenderness or rebound tenderness.
Gallstones can be treated conservatively.
Combined with the patient’s medical history, the final diagnosis was CDI.
Prior to surgery, the patient received a cefazolin (2 g) prophylactic treatment on October 9th and orally administered a compound polyethylene glycol electrolyte solution. Subsequently, on October 10th, the patient underwent laparoscopic subtotal colectomy, appendectomy, ascending colon-rectal end-to-end anastomosis, and adhesiolysis. During the procedure, slight dilation of the proximal rectal segment, as well as the absence of cecal pouches in the ascending colon, transverse colon, descending colon, and sigmoid colon, was observed. Combining the patient's medical history and preoperative examination, intraoperative diagnosis was adhesive small bowel obstruction. Postoperatively, the patient received empiric antimicrobial therapy with cefepime.
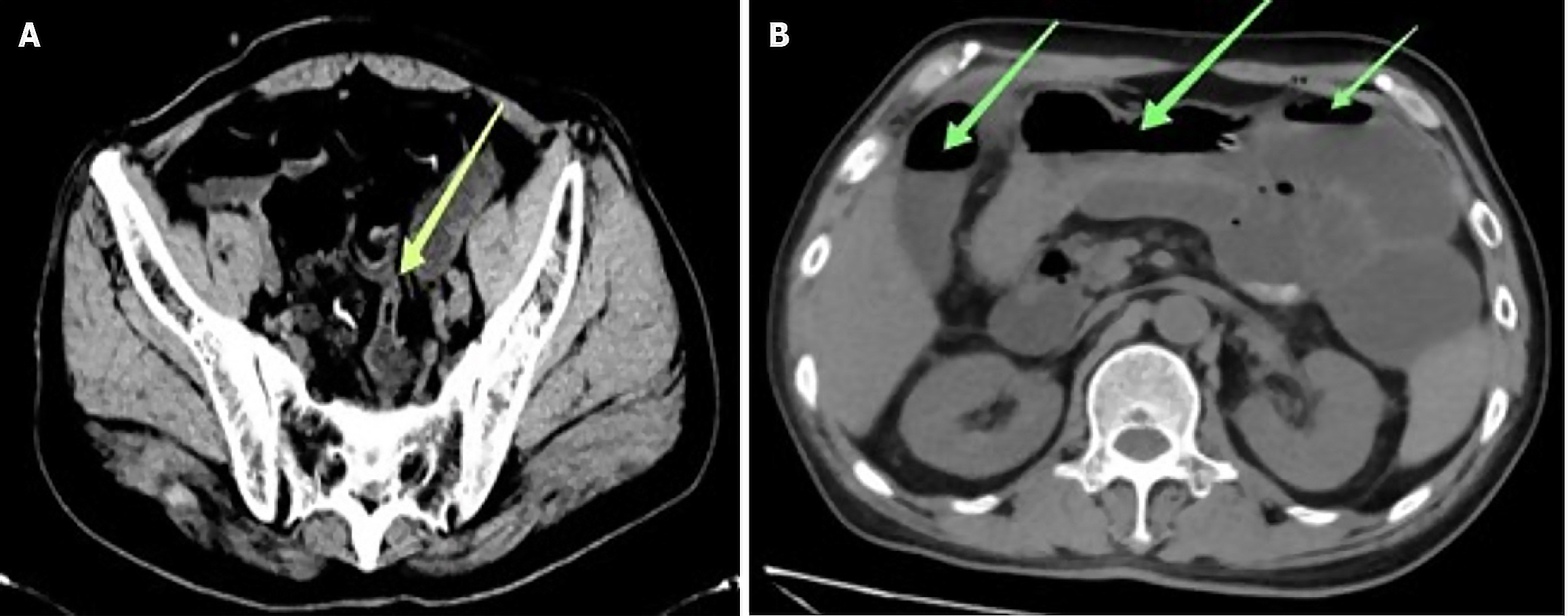
During the first and second days of postoperative rehabilitation and care, the results of a complete blood count showed a gradual increase in white blood cell count. Although the patient did not exhibit obvious discomfort, considering the physiologic stress response induced by the surgery, we conducted dynamic monitoring. However, on the fourth postoperative day, the patient suddenly developed high fever accompanied by watery diarrhea, with more than 10 bowel movements on that day, presenting with pronounced foul odor. The nursing team informed the doctor in charge of the situation in time, and the doctor in charge adjusted the medication plan in time after checking the patient's situation. Meanwhile, the nursing team carried out the nursing task more strictly and recorded the patient's subsequent defecation frequency and fecal characteristics in detail. Concurrently, the white blood cell count continued to decrease. Two abdominal CT scans reported fluid and gas accumulation within the small intestine, though no significant intestinal constriction was evident, raising the possibility of intestinal infection (Figure 2). The patient received oral montmorillonite and symptomatic supportive therapy to stabilize the internal environment. Furthermore, stool and blood samples were collected for culture, including both aerobic and anaerobic bacteria.
Figure 2 illustrates two significant observations in the patient's medical records. In Figure 2A, dated October 15, 2022, we observe evidence of pelvic small bowel constriction. Subsequently, in Figure 2B, recorded on October 17, 2022, there is a notable presence of intestinal fluid accumulation and gas retention within the small intestine.
Postoperative day 5-6: The patient experienced intermittent fever, with temperatures reaching up to 38.5 degrees celsius, and worsening diarrhea, leading to frequent bowel movements. Symptomatic treatment was administered, involving ibuprofen and fluid replacement therapy.
Postoperative Day 7: Results of the bowel culture indicated the presence of C. difficile DNA, specifically the non-toxigenic 027 strain, while blood cultures remained negative. These findings confirmed the diagnosis of "postoperative CDI." Following a consultation with the pharmacy department, the patient received vancomycin (0.5 g vancomycin + 50 mL warm water via nasogastric tube, every 6 hours) and intravenous metronidazole (600 mL, every 12 hours) as part of the antimicrobial treatment. Additionally, a probiotic supplement containing bifidobacterium quadruple live bacteria tablets (0.5 g, three times a day) was incorporated into the treatment regimen.
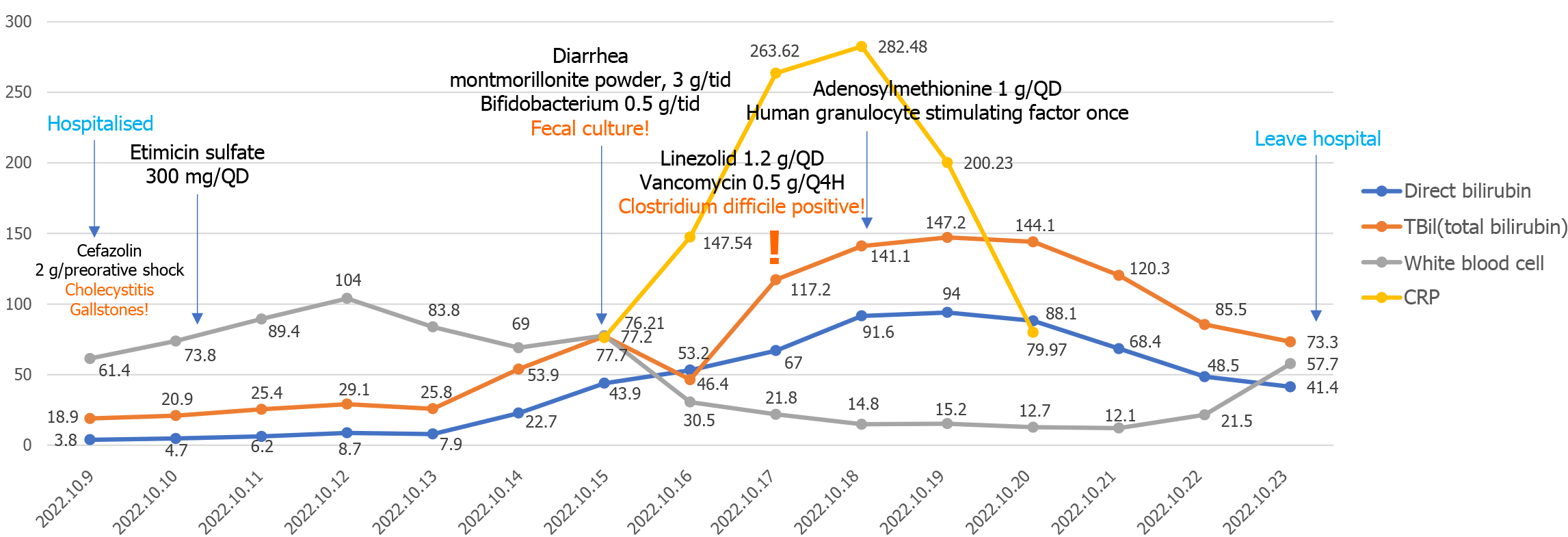
Subsequently: The patient's symptoms, clinical signs, and laboratory results gradually improved during the treatment period. Key indicators such as high-sensitivity C-reactive protein (CRP), white blood cell count, and total bile acid exhibited changes, as depicted in Figure 3. CRP reached its peak on the 8th postoperative day, gradually decreasing and approaching normal levels. White blood cell count increased on the 2nd postoperative day, subsequently decreasing, with a brief increase on the 5th day, and continued elevation from the 11th day, stabilizing around the 13th day. Total bile acid levels began to rise on the 2nd day, peaked on the 8th day, and then gradually decreased.
On October 23, 2022, the patient was discharged with instructions to continue oral vancomycin for two additional weeks. However, during a follow-up two months after discharge, it was noted that the patient, due to non-compliance with the prescribed antibiotic regimen, experienced a recurrence of symptoms, including high fever, diarrhea, and vomiting related to gastrointestinal infection. The patient sought care at a local hospital, but the symptoms progressed, ultimately leading to multi-organ failure and an unfortunate demise.
In this case, the use of cefoperazone (2 g) prior to surgery and ertapenem following it emerged as significant risk factors for the challenging CDI. Furthermore, the patient had a long history of constipation, which may have contributed to a chronically anaerobic intestinal environment and potentially altered the composition of the gut microbiota, leading to an increased proportion of anaerobic bacteria, thus creating a favorable postoperative environment for CDI to some extent.
Additionally, under normal circumstances, cholesterol in the human body undergoes metabolism to synthesize primary bile acids, which are then excreted into the intestines with bile. These primary bile acids are further transformed into secondary bile acids through the action of the gut microbiota. For patients with a history of gallstones, this could signify the presence of bile stasis and impaired excretion, resulting in reduced primary bile acids in the intestines. Moreover, antibiotic usage may alter the gut microbiota, leading to decreased synthesis of secondary bile acids. Consequently, the relative abundance of primary bile acids may increase. Primary bile acids stimulate the growth of C. difficile, while secondary bile acids inhibit C. difficile production[4]. Therefore, it is of particular importance to focus on protecting the liver and promoting bile acid circulation in patients with gallstones to maintain a normal bile acid composition ratio.
As the use of antibiotics continues to advance, the incidence of CDI has been steadily increasing. This increase is primarily associated with alterations in the gut microbiota balance resulting from antibiotic use[5]. CDI typically has an incubation period of 2-3 days, and patients with gallstones often experience disturbances in the bile acid cycle[6]. Some gallstone patients who undergo gastrointestinal surgery during their hospitalization may subsequently develop CDI, and there is even a school of thought suggesting that patients may already carry latent infections upon admission[7].
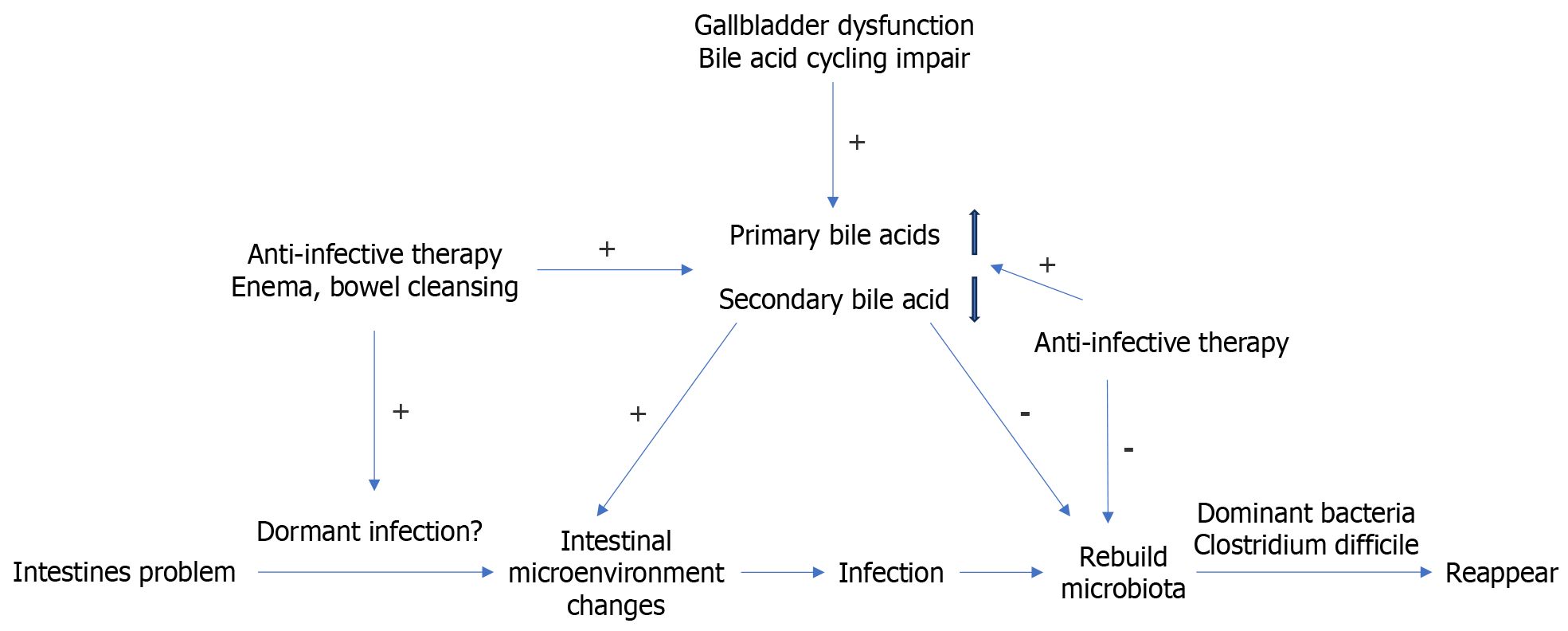
In the conventional antibiotic treatment of patients following CDI, there is a need to reestablish a healthy gut microbiota[8], which typically requires 2-3 years. If C. difficile continues to maintain dominance in the intestinal microbiome after the microbiota reconstitution, it can potentially lead to the recurrence of CDI[9]. The potential mechanisms for this phenomenon are illustrated in Figure 4.
The primary cause of post-operative care within hospitals is CDI, commonly found in intensive care units or critically ill patients. Clinical manifestations of CDI typically include mild to moderate diarrhea, which can be watery, bloody, or accompanied by mucous and pus in the stool; characteristic patches or filamentous pseudomembranes might be visible in the stool[10]. Presently, detecting C. difficile toxin B remains the most specific method for CDI diagnosis[11]. The usage of different antibiotics, especially clindamycin, second and third-generation cephalosporins, fluoroquinolones, and macrolides, can alter the proportions of various microbial communities, disrupt the intestinal microbiota balance, and increase the risk of CDI. Furthermore, research indicates that mechanical bowel preparation before surgery and the addition of oral antibiotics during this preparation do not increase the risk of post-colon surgery CDI, emphasizing the importance of proactive preoperative preparation[12]. However, studies also suggest cautious use of laxatives, first and second-generation narrow-spectrum cephalosporins, or metronidazole, as they may have protective effects against CDI[13].
Patients often experience symptoms such as fever, lower abdominal pain, tenderness, and bloating. Therefore, we recommend routine examinations for patients exhibiting these symptoms postoperatively to exclude CDI. Early detection and implementation of preventive and therapeutic measures are crucial to preventing outbreaks of nosocomial CDI. Moreover, most patients infected with C. difficile usually exhibit an elevated white blood cell count. However, in this case, the patient's white blood cell count decreased to a critical level after reaching its peak, possibly related to C. difficile-induced bone marrow suppression, although the specific mechanism remains unclear. During follow-ups after surgery, patients might face the risk of death, including recurrence of CDI due to inadequate use of vancomycin. Hence, healthcare professionals should actively explain the necessity of complete antibiotic usage to patients, aiming to improve patient compliance and consequently enhance the prognosis of patients after colon surgery.
Previous reports have documented cases of difficult CDI occurring after total colectomy[14], total hip arthroplasty[15], and ileal pouch-anal anastomosis surgery[16]. As a result, there is a need to standardize the treatment approaches for CDI. Currently, recommended treatments for CDI include oral metronidazole (for milder cases) and oral vancomycin or fidaxomicin (for more severe cases), typically administered for a 10-day course per dose. Severe CDI cases often necessitate colectomy. For the treatment of the first recurrence, the same medication used for the initial episode is typically employed. In cases of a second recurrence, medication dosages are usually tapered or prolonged courses of vancomycin are considered to facilitate gut microbiota restoration, or alternative medications like fidaxomicin may be explored. There have also been reports of successful treatment of patients with nonmotile intestinal obstruction and concurrent CDI using intracolonic vancomycin[17].
In contrast to antibiotic therapy, ileostomy or colon irrigation serves as an alternative to colectomy for the treatment of severe and complicated CDI, aiming to preserve the colon[18]. Additionally, intestinal oxygen therapy has been successfully employed in the treatment of pediatric C. difficile colitis[19]. Bacterial therapy through fecal microbiota transplantation is also considered an emerging approach for the management of recurrent cases[20].
Linazolid may potentially offer protective effects against CDI and can be considered for selective use. But the drug is generally more effective at preventing CDI, and vancomycin is still the treatment of choice after an infection has already occurred[21]. The antibacterial effect of Tidazoid in vitro has been proven[22], and it can also become a new drug for clinical treatment after clinical trials in the future. In the event of a CDI, healthcare personnel should pay special attention to surgical hygiene to prevent cross-infections. Patients should be isolated individually and provided with dedicated restroom facilities to minimize the risk of C. difficile transmission. Additionally, pulsed xenon ultraviolet disinfection can be effectively employed to address surface contamination by C. difficile.
C. difficile primarily spreads the disease through spore transmission, leading to a recurrence rate as high as 30%[23]. Its primary mechanism may be related to alterations in fecal bile acids induced by the antibiotics used in treatment, resulting in a reduction in primary bile acids and an increase in secondary bile acids[24]. Primary bile acids promote the germination of C. difficile spores, while secondary bile acids have the opposite effect[25]. Recurrent patients often experience disruptions in bile acid circulation; thus, post CDI, regulating the patient's bile acid circulation is necessary to prevent disease recurrence.
In the study conducted by Maseda et al[26] initial CDI was primarily associated with the use of two broad-spectrum antibiotics, cephalosporins, and clindamycin. Other research indicates that proton pump inhibitors, nonsteroidal anti-inflammatory drugs, and the laxative polyethylene glycol solution may also increase the incidence of C. difficile by damaging the gastrointestinal mucosa and altering intestinal colonization resistance[27-29].
As research delves deeper, preventing CDI in the intestinal tract appears to be more intricate than safeguarding against the spore-forming CDI. Owing to the diversity in clinical presentations, the severity of symptoms can vary greatly; milder cases may manifest with abdominal pain and diarrhea, while more severe cases may involve severe diarrhea, toxic megacolon, intestinal obstruction, and colonic dilation. In the case of initial CDI, where the intestinal mucosa is mildly damaged, probiotics are commonly employed to expedite the restoration of intestinal microbiota[30]. Initial cases of CDI are typically treated with a combination of vancomycin and other high-level antibiotics, which has shown effective outcomes. However, due to the broad-spectrum nature of these antibiotics, they have a widespread impact on the intestinal microbiota. Therefore, current research is gradually expanding to investigate the use of narrow-spectrum antibiotics such as fidaxomicin[31,32]. Failure of patients to adhere to the prescribed antibiotic course could lead to a fatal outcome, increasing the likelihood of recurrent CDI. Hence, healthcare professionals should actively emphasize the necessity of completing the full course of antibiotics to improve patient compliance and subsequently enhance the prognosis of CDI patients following colon surgery.
In cases of recurrent and refractory CDI, the intestinal mucosa and normal intestinal microbiota typically endure more severe damage. The natural recovery of intestinal microbiota is relatively slow in such situations. In these instances, fecal microbiota transplantation with a success rate ranging from 85% to 95%[33-35] is a viable option. Compared to natural recovery, exogenous microbiota can expedite the restoration of intestinal microbiota balance, inhibit the growth of refractory C. difficile, and thereby achieve therapeutic effectiveness[27].
Post-gastrointestinal surgery CDI invariably arises from C. difficile becoming the dominant microbial community within the intestinal tract, often presenting as pseudomembranous enteritis. If left untreated, pseudomembranous colitis can lead to severe diarrhea, hypovolemic shock, toxic colonic dilatation, cecal perforation, bleeding, and death. Consequently, in patients with concurrent intestinal obstruction, severe abdominal pain, noticeable tenderness, immunosuppression, advanced age, high white blood cell count, acute renal dysfunction, altered mental status, or cardiopulmonary impairment, an early surgical consultation should be considered, with emergency surgical intervention if necessary[36].
For patients with vancomycin-resistant clostridium difficile infection, nursing care should include the following meticulous measures: (1) Strictly implement isolation measures to ensure that patients are isolated individually and, if necessary, transferred to the intensive care unit for treatment, and that healthcare workers wear the appropriate level of protective gear; (2) Pay close attention to the patient's condition, and record and report any symptomatic changes in a timely manner. In addition, keep the environment in which the patient is living clean, emphasizing regular disinfection and cleaning of contaminated items such as those in the ward[37]; and (3) Patient care should be combined with medical measures, medication should be administered according to prescribed protocols, and doctors should be cooperated in monitoring and evaluating the condition and making timely adjustments to the treatment program[38]. These nursing measures should be implemented according to the specific conditions of the patient and under the guidance of the doctor to ensure that the patient receives scientific and effective care and treatment.
CDI post colon subtotal resection: Case review limitations of this study mainly include the small number of patients, which may have some individual variability, and the fact that patient deaths occurred out-of-hospital, which may have some confounding factors. In the future, with CDI, they should be examined for the presence of specific complications similar to methicillin-resistant staphylococcus aureus pneumonia, which may be an influential factor in the prognosis of infected patients. We may need prospective studies on the etiology of CDI to further explore the mechanism of CDI.
In conclusion, this case highlights the importance of being vigilant for CDI in patients undergoing gastrointestinal surgery, especially those with risk factors such as antibiotic use and a history of constipation. Early diagnosis and prompt treatment are crucial in managing C. difficile infections post-surgery. Healthcare providers should focus on preventive measures, routine monitoring, and patient adherence to treatment regimens to improve outcomes and minimize the risk of recurrent infections. Further research is needed to explore effective strategies for preventing and treating C. difficile infections in surgical patients.
Thank for all the patients in this research, thank for all the scholars in this article. Thank for all the teammates for supporting this research. We are also particularly grateful to our colleagues in The First Affiliated Hospital of Jilin University for their contributions.
| 1. | Burnham CA, Carroll KC. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013;26:604-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 2. | Vindigni SM, Surawicz CM. The gut microbiome: a clinically significant player in transplantation? Expert Rev Clin Immunol. 2015;11:781-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 4. | Theriot CM, Young VB. Microbial and metabolic interactions between the gastrointestinal tract and Clostridium difficile infection. Gut Microbes. 2014;5:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Dailey FE, Turse EP, Daglilar E, Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. 2019;49:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Taubes G. Collateral damage. The rise of resistant C. difficile. Science. 2008;321:360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol. 2016;14:609-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 8. | Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017;56:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (1)] |
| 9. | Crobach MJT, Vernon JJ, Loo VG, Kong LY, Péchiné S, Wilcox MH, Kuijper EJ. Understanding Clostridium difficile Colonization. Clin Microbiol Rev. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 10. | Abad CLR, Safdar N. A Review of Clostridioides difficile Infection and Antibiotic-Associated Diarrhea. Gastroenterol Clin North Am. 2021;50:323-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Gerding DN, Brazier JS. Optimal methods for identifying Clostridium difficile infections. Clin Infect Dis. 1993;16 Suppl 4:S439-S442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Krapohl GL, Phillips LR, Campbell DA Jr, Hendren S, Banerjee M, Metzger B, Morris AM. Bowel preparation for colectomy and risk of Clostridium difficile infection. Dis Colon Rectum. 2011;54:810-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Bibbò S, Lopetuso LR, Ianiro G, Di Rienzo T, Gasbarrini A, Cammarota G. Role of microbiota and innate immunity in recurrent Clostridium difficile infection. J Immunol Res. 2014;2014:462740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Freiler JF, Durning SJ, Ender PT. Clostridium difficile small bowel enteritis occurring after total colectomy. Clin Infect Dis. 2001;33:1429-31; discussion 1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Loloi J, Mrowczynski O, Claxton B, Abdulbasit M, Schade M. Clostridium difficile Infection of a Total Hip Arthroplasty: Case Report and Review of the Literature. JBJS Case Connect. 2020;10:e0266. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Shen BO, Jiang ZD, Fazio VW, Remzi FH, Rodriguez L, Bennett AE, Lopez R, Queener E, Dupont HL. Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Fawley J, Napolitano LM. Vancomycin Enema in the Treatment of Clostridium difficile Infection. Surg Infect (Larchmt). 2019;20:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011;254:423-7; discussion 427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Scherbakov A, Malisheva D, Sukhotskaya A, Rzheutskaya R, Pervunina T, Bairov V, Mazurok V. Case report of successful treatment of clostridial colitis in a child using enteral oxygen therapy. Anaesthesiol Intensive Ther. 2022;54:97-98. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Al-Jashaami LS, DuPont HL. Management of Clostridium difficile Infection. Gastroenterol Hepatol (N Y). 2016;12:609-616. [PubMed] |
| 21. | Valerio M, Pedromingo M, Muñoz P, Alcalá L, Marin M, Peláez T, Giannella M, Bouza E. Potential protective role of linezolid against Clostridium difficile infection. Int J Antimicrob Agents. 2012;39:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Binyamin D, Nitzan O, Azrad M, Hamo Z, Koren O, Peretz A. In Vitro Activity of Tedizolid, Dalbavancin, and Ceftobiprole Against Clostridium difficile. Front Microbiol. 2018;9:1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 377] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 24. | Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 502] [Article Influence: 71.7] [Reference Citation Analysis (1)] |
| 25. | Park YH, Seong JM, Cho S, Han HW, Kim JY, An SH, Gwak HS. Effects of proton pump inhibitor use on risk of Clostridium difficile infection: a hospital cohort study. J Gastroenterol. 2019;54:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Maseda D, Zackular JP, Trindade B, Kirk L, Roxas JL, Rogers LM, Washington MK, Du L, Koyama T, Viswanathan VK, Vedantam G, Schloss PD, Crofford LJ, Skaar EP, Aronoff DM. Nonsteroidal Anti-inflammatory Drugs Alter the Microbiota and Exacerbate Clostridium difficile Colitis while Dysregulating the Inflammatory Response. mBio. 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Gilboa M, Houri-Levi E, Cohen C, Tal I, Rubin C, Feld-Simon O, Brom A, Eden-Friedman Y, Segal S, Rahav G, Regev-Yochay G; ShIC research group. Environmental shedding of toxigenic Clostridioides difficile by asymptomatic carriers: A prospective observational study. Clin Microbiol Infect. 2020;26:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Lee JC, Hung YP, Tsai BY, Tsai PJ, Ko WC. Severe Clostridium difficile infections in intensive care units: Diverse clinical presentations. J Microbiol Immunol Infect. 2021;54:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Tomkovich S, Taylor A, King J, Colovas J, Bishop L, McBride K, Royzenblat S, Lesniak NA, Bergin IL, Schloss PD. An Osmotic Laxative Renders Mice Susceptible to Prolonged Clostridioides difficile Colonization and Hinders Clearance. mSphere. 2021;6:e0062921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner-Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, Elinav E. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 2018;174:1406-1423.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 799] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 31. | Poylin V, Hawkins AT, Bhama AR, Boutros M, Lightner AL, Khanna S, Paquette IM, Feingold DL; Prepared by the Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Clostridioides difficile Infection. Dis Colon Rectum. 2021;64:650-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Ajami NJ, Cope JL, Wong MC, Petrosino JF, Chesnel L. Impact of Oral Fidaxomicin Administration on the Intestinal Microbiota and Susceptibility to Clostridium difficile Colonization in Mice. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Dembrovszky F, Gede N, Szakács Z, Hegyi P, Kiss S, Farkas N, Molnár Z, Imrei M, Dohos D, Péterfi Z. Fecal Microbiota Transplantation May Be the Best Option in Treating Multiple Clostridioides difficile Infection: A Network Meta-Analysis. Infect Dis Ther. 2021;10:201-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Wei S, Jespersen ML, Baunwall SMD, Myers PN, Smith EM, Dahlerup JF, Rasmussen S, Nielsen HB, Licht TR, Bahl MI, Hvas CL. Cross-generational bacterial strain transfer to an infant after fecal microbiota transplantation to a pregnant patient: a case report. Microbiome. 2022;10:193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Allegretti JR, Allegretti AS, Phelps E, Xu H, Kassam Z, Fischer M. Asymptomatic Clostridium difficile carriage rate post-fecal microbiota transplant is low: a prospective clinical and stool assessment. Clin Microbiol Infect. 2018;24:780.e1-780.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Carchman EH, Peitzman AB, Simmons RL, Zuckerbraun BS. The role of acute care surgery in the treatment of severe, complicated Clostridium difficile-associated disease. J Trauma Acute Care Surg. 2012;73:789-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Bouza E, Aguado JM, Alcalá L, Almirante B, Alonso-Fernández P, Borges M, Cobo J, Guardiola J, Horcajada JP, Maseda E, Mensa J, Merchante N, Muñoz P, Pérez Sáenz JL, Pujol M, Reigadas E, Salavert M, Barberán J. Recommendations for the diagnosis and treatment of Clostridioides difficile infection: An official clinical practice guideline of the Spanish Society of Chemotherapy (SEQ), Spanish Society of Internal Medicine (SEMI) and the working group of Postoperative Infection of the Spanish Society of Anesthesia and Reanimation (SEDAR). Rev Esp Quimioter. 2020;33:151-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | van der Wilden GM, Subramanian MP, Chang Y, Lottenberg L, Sawyer R, Davies SW, Ferrada P, Han J, Beekley A, Velmahos GC, de Moya MA. Antibiotic Regimen after a Total Abdominal Colectomy with Ileostomy for Fulminant Clostridium difficile Colitis: A Multi-Institutional Study. Surg Infect (Larchmt). 2015;16:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/