Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2853
Revised: August 16, 2024
Accepted: August 21, 2024
Published online: September 27, 2024
Processing time: 173 Days and 23.6 Hours
In laparoscopic proximal gastrectomy (LPG), the prolapse of the hepatic left lateral lobe near the lesser curvature and esophageal hiatus can obstruct the field of vision and operation. Therefore, it is necessary to retract or obstruct the hepatic left lateral lobe to ensure a clear field of vision.
To investigate the safety and clinical efficacy of the modified hepatic left lateral lobe inversion technique for LPG.
A retrospective analysis was conducted on the clinical data of 13 consecutive patients with early-stage upper gastric adenocarcinoma or adenocarcinoma of the esophagogastric junction treated with LPG from January to December 2023 at the Department of Gastrointestinal Surgery, Second Affiliated Hospital of Fujian Medical University. The modified hepatic left lateral lobe inversion technique was used to expose the surgical field in all patients, and short-term outcomes were observed.
In all 13 patients, the modified hepatic left lateral lobe inversion technique was successful during surgery without the need for re-retraction or alteration of the liver traction method. There were no instances of esophageal hiatus occlusion, eliminating the need for forceps to assist in exposure. There was no occurrence of intraoperative hepatic hemorrhage, hepatic vein injury, or hepatic congestion. No postoperative digestive complications of Clavien-Dindo grade ≥ II occurred wi
The modified hepatic left lateral lobe inversion technique has demonstrated satisfactory results, offering ad
Core Tip: This study retrospectively analyzed the clinicopathological data of patients who underwent laparoscopic proximal gastrectomy (LPG) with the modified hepatic left lateral lobe inversion technique. According to our research, the modified hepatic left lateral lobe inversion technique in LPG can facilitate surgical procedures, reduce surgical trauma, and protect the liver.
- Citation: Lin JA, Wu CY, Ye K. Modified hepatic left lateral lobe inversion in laparoscopic proximal gastrectomy: An analysis of 13 cases. World J Gastrointest Surg 2024; 16(9): 2853-2859
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2853.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2853
In laparoscopic proximal gastrectomy (LPG), prolapse of the hepatic left lateral lobe near the lesser curvature of the stomach and the esophageal hiatus obstruct the field of vision and operation. Except for a few cases in which the hepatic left lateral lobe adheres to the diaphragm, the hepatic left lateral lobe should be retracted or obstructed to obtain a good field of view[1]. This procedure is more critical in surgeries for adenocarcinomas of the esophagogastric junction (AEG). Various methods to reduce liver obstruction have been clinically applied in LPG, including Nathanson liver retractors, suture suspensions, silicone disc retraction and liver adhesion[2-5]. However, these methods have disadvantages, such as increased auxiliary incisions, intraoperative liver damage, poor surgical field exposure and the use of new consumables that increase surgical costs[6]. To overcome these limitations, Nakamura et al[7] first reported hepatic left lateral lobe inversion, which completely isolated the hepatic left lateral lobe outside the surgical field and significantly reduced liver damage. Harada et al[6] simplified this method, shortening the operative time to approximately 16 minutes. In January 2023, our center pioneered the use of hepatic left lateral lobe inversion in the LPG and subsequently made additional enhancements to the technique. The results are reported below.
The clinical data from 13 consecutive patients with early upper gastric cancer or adenocarcinoma of the AEG who un
| Number | Sex | Age | BMI (kg/m2) | Tumor location | Pathological stage | Complicating liver disease | Preoperative ALT (U/L) | Preoperative AST (U/L) | Digestive tract reconstruction method |
| 1 | Male | 79 | 22.491 | Siewert II AEG | II | None | 13.1 | 15.9 | Double tract |
| 2 | Male | 81 | 22.649 | Upper stomach | II | None | 10.7 | 20.4 | Double tract |
| 3 | Male | 79 | 30.041 | Upper stomach | I | Hepatic cyst | 13.2 | 17.9 | Kamikawa |
| 4 | Male | 68 | 23.473 | Siewert II AEG | I | None | 11.7 | 8.0 | Double tract |
| 5 | Female | 56 | 22.342 | Upper stomach | I | None | 20.4 | 19.1 | Kamikawa |
| 6 | Male | 70 | 20.415 | Upper stomach | II | Hepatic cyst | 10.6 | 15.2 | Kamikawa |
| 7 | Male | 60 | 19.030 | Upper stomach | I | None | 13.0 | 15.3 | Kamikawa |
| 8 | Male | 79 | 21.764 | Siewert II AEG | II | Hepatic cyst | 10.6 | 19.6 | Double tract |
| 9 | Male | 63 | 20.384 | Upper stomach | I | Hepatitis B and fatty liver | 17.8 | 19.8 | Kamikawa |
| 10 | Male | 34 | 20.529 | Upper stomach | I | None | 41.1 | 27.8 | Kamikawa |
| 11 | Male | 65 | 21.139 | Upper stomach | I | None | 13.8 | 22.7 | Side overlap |
| 12 | Male | 62 | 25.952 | Upper stomach | I | Hepatic cyst | 20.0 | 17.5 | Kamikawa |
| 13 | Male | 63 | 18.365 | Siewert II AEG | I | Hepatic cyst | 38.8 | 38.0 | Side overlap |
After routine preoperative preparation and successful anesthesia, the patients were placed in the split-leg supine position. Using the conventional five-hole approach, surgeons stood on the right of the patients, with a main operating hole of 12 mm located superolateral to the right observing hole and the remaining operating holes of 5 mm. D1+ lymph node dis
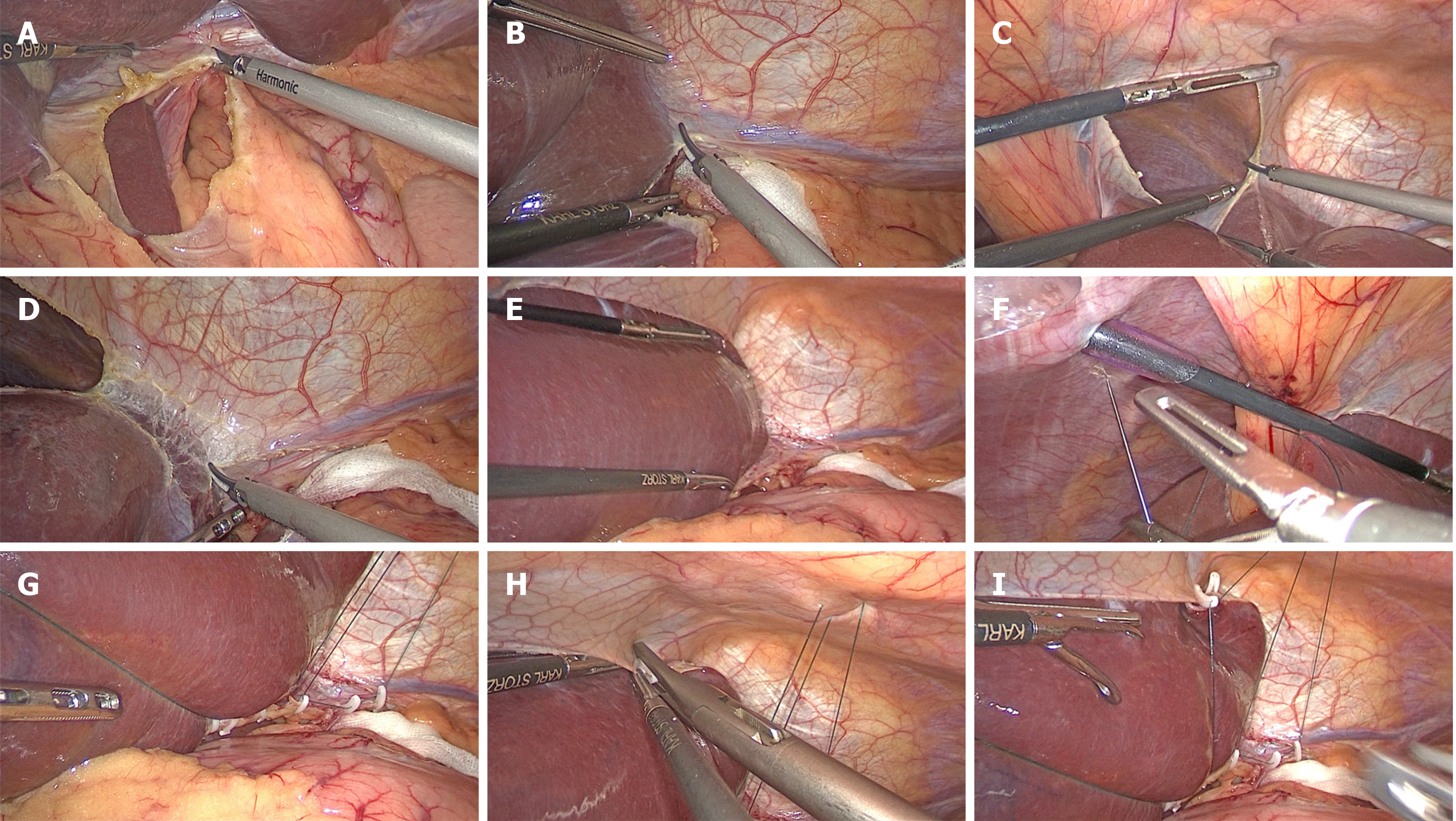
The procedures for the modified hepatic left lateral lobe inversion were as follows. First, the hepatogastric ligament was opened (Figure 1A), and the left deltoid ligament was disconnected (Figure 1B). The falciform ligament in the avascular area was subsequently dissected with an ultrasound knife or electric hook on the cephalic round ligament (Figure 1C), and the left coronary ligament was continuously dissected (Figure 1D), avoiding damaging the veins from the diaphragm. After confirming the activity of the hepatic left lateral lobe, it was pushed through the dissected falciform ligament gap to the right superior hepatic space and inverted (Figure 1E). In cases of difficult inversion, the accessory left hepatic artery was clamped and disconnected after opening the hepatogastric ligament at the lower edge of the liver. A 2-0 purse-string suture needle was subsequently inserted into the abdominal cavity from the left side of the falciform ligament, closely adhering to the xiphoid process caudally, followed by further insertion into the abdominal cavity close to the puncture point after perforation of the cavity, passing through the round ligament near the liver inside the cavity and then perforation of the cavity from the right side of the falciform ligament (Figure 1F). The purse-string suture was fixed to the upper edge of the opened hepatogastric ligament via an absorbable clip below the liver (Figure 1G), and the inverse hepatic left lateral lobe was fixed to the upper edge of the dissected falciform ligament via an absorbable clip (Figure 1H). Afterward, the purse-string suture was tightened outside the body to obstruct the inverse hepatic left lateral lobe between the abdominal wall and the liver (Figure 1I). After surgery, the external purse-string suture was released, the fixed purse-string suture inside the body was cut, the absorbable clips were removed, and the hepatic left lateral lobe was carefully repositioned (Video).
All the patients were treated with modified hepatic left lateral lobe inversion during LPG, with an operative time of 12.1 (11.1-13.7) minutes and an overall surgical duration of 195.0 (172.0-219.0) minutes. The patients did not require liver re-retraction or alteration of the retraction method and had no obstruction of the esophageal hiatus by the liver, and no pliers to assist in exposure were needed. Three patients underwent accessory left hepatic artery ligation, and no patient experienced hepatic bleeding, vein injury or congestion during LPG. The average postoperative length of hospital stay was 8 (7-12) days. Within 30 days after LPG, no Clavien-Dindo grade ≥ II complications in the digestive system occurred, and only one patient experienced pulmonary infection. On day 1 post-LPG, some patients presented elevated ALT and AST levels. The elevations were more obvious in two patients who underwent accessory left hepatic artery ligation and then decreased significantly on day 3, and both decreased to normal preoperative levels on day 7 (Table 2).
| Number | Overall surgical duration (min) | Hepatic left lateral lobe inversion time (min) | Length of hospital stay (d) | Postoperative complications | ALT on the 1st day after surgery (U/L) | ALT on the 3rd day after surgery (U/L) | ALT on the 7th day after surgery (U/L) | AST on the 1st day after surgery (U/L) | AST on the 3rd day after surgery (U/L) | AST on the 7th day after surgery (U/L) |
| 1 | 172.0 | 11.1 | 9 | None | 26.3 | 19.8 | 10.8 | 28.7 | 18.2 | 15.9 |
| 2 | 175.0 | 11.2 | 9 | None | 23.5 | 4 | 14.5 | 41.3 | 20.4 | 4.2 |
| 3 | 185.0 | 11.4 | 8 | None | 22.2 | 20.8 | 46.2 | 21.4 | 17.2 | 32.8 |
| 4 | 186.0 | 11.5 | 11 | None | 11.5 | 4.1 | 21.8 | 22.8 | 12.1 | 21.8 |
| 5 | 190.0 | 11.7 | 7 | None | 74.6 | 21.6 | 36.4 | 79.6 | 21.5 | 34.4 |
| 6 | 193.0 | 11.9 | 7 | None | 28.6 | 15.7 | 12.6 | 22.9 | 10.7 | 18.6 |
| 7 | 195.0 | 12.1 | 7 | None | 11.6 | 25.2 | 6.3 | 8.2 | 30.4 | 11.6 |
| 8 | 198.0 | 12.2 | 8 | Pulmonary infection | 112.1 | 47.8 | 20.3 | 109.6 | 40.4 | 15.6 |
| 9 | 200.0 | 12.3 | 7 | None | 44.7 | 42.6 | 19.3 | 18.2 | 25.3 | 23.4 |
| 10 | 204.0 | 12.5 | 7 | None | 50.6 | 19.3 | 20.4 | 62.9 | 18.9 | 21.7 |
| 11 | 216.0 | 12.8 | 10 | None | 23.1 | 9.2 | 5.5 | 15.4 | 8.2 | 11.5 |
| 12 | 217.0 | 13.2 | 12 | None | 296.3 | 13.5 | 16.1 | 234.1 | 72.4 | 43.4 |
| 13 | 219.0 | 13.7 | 12 | None | 27.9 | 10.8 | 16 | 36 | 14.1 | 14.8 |
In laparoscopic radical gastrectomy for gastric cancer, surgical field exposure affects the surgical difficulty and process, and effective hepatic left lateral lobe obstruction is crucial for exposing the surgical field. Compared with laparoscopic distal gastrectomy (LDG), LPG requires better exposure of the esophageal hiatus, especially in cases where lower me
The modified hepatic left lateral lobe inversion used in this study involves exposing the diaphragmatic hiatus and dissecting the lower mediastinal lymph nodes during open surgery, completely isolating the hepatic left lateral lobe and providing a broad field of view. In 10 patients, the esophageal hiatus was adequately exposed, without the need for readjustment of hepatic left lateral lobe inversion or additional sutures, suspensions and puncture holes, while not affecting the hepatic branch of the vagus nerve. Therefore, hepatic left lateral lobe inversion is suitable for nerve-preserving surgery in early-onset patients. Additionally, in two cases of LPG requiring anastomosis in the lower me
In 2020, Nakamura et al[7] first proposed hepatic left lateral lobe inversion, which requires dissection of the round, falciform, coronary and left deltoid ligaments and superficial hepatogastric ligament beneath the Arantius duct and physiological adhesion of hepatic segments S3-S4. After it was modified by Harada et al[6], it only required the falciform, coronary and left deltoid ligaments to be dissected. In our study, further modifications were made, and the average operative time was 12.1 (11.1-13.7) minutes, without a significant increase. The operative time is expected to be further shortened with the learning curve.
According to the literature, liver dysfunction may occur after laparoscopic radical gastrectomy and fundoplication, which is related to liver compression or large accessory left hepatic artery ligation when the field of view is exposed[13,14]. Nakamura et al[7] revealed that hepatic left lateral lobe inversion resulted in slight abnormalities in liver function indicators and that the postoperative ALT and AST levels were significantly lower than those in patients treated with the Nathanson liver retractor. This study yielded similar results, possibly because the inverse hepatic left lateral lobe is located on the right side of the falciform ligament without external pressure or compression. Although two patients in our study underwent accessory left hepatic artery ligation, their postoperative hepatic enzymes were significantly ele
When performing hepatic left lateral lobe inversion, direct or retraction-induced damage to the left inferior phrenic vein should be avoided, and dissection of the coronary ligament should not be too deep to avoid hepatic vein damage. The operation should be gentle, and the liver should be gently pushed when using pliers to avoid direct liver com
We believe that hepatic left lateral lobe inversion is not strongly needed during LDG. The indications for hepatic left lateral lobe inversion are LPG and laparoscopic total gastrectomy, especially for cases requiring long-term maintenance of the field of view near the esophageal hiatus, such as muscle flap reconstruction (single- or double-muscle flaps) and side overlap anastomosis. The necessity and feasibility of hepatic left lateral lobe inversion should be evaluated based on its size and hardness, as well as surgical difficulty.
In summary, modified hepatic left lateral lobe inversion during LPG is a safe, effective and low-cost method for exposing the field of view with minimal impact on liver function. However, this method is still in the early stage of exploration, and larger, multicenter studies in the future are needed to further evaluate its safety and advantages.
| 1. | Bracale U, Pignata G, Lirici MM, Hüscher CG, Pugliese R, Sgroi G, Romano G, Spinoglio G, Gualtierotti M, Maglione V, Azagra S, Kanehira E, Kim JG, Song KY; Guideline Committee Of The Italian Society Of Hospital Surgeons-ACOI and Italian Hi-Tech Surgical Club-IHTSC. Laparoscopic gastrectomies for cancer: The ACOI-IHTSC national guidelines. Minim Invasive Ther Allied Technol. 2012;21:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Gojayev A, Yüksel C, Mercan Ü, Çaparlar MA, Cetindag O, Akbulut S, Ünal AE, Bayar S, Demirci S. The effect and clinical significance of using nathanson liver retractor on liver function tests in laparoscopic gastric cancer surgery. Pol Przegl Chir. 2021;94:54-61. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Yoshikawa K, Shimada M, Higashijima J, Nakao T, Nishi M, Takasu C, Kashihara H, Eto S. Combined liver mobilization and retraction: A novel technique to obtain the optimal surgical field during laparoscopic total gastrectomy. Asian J Endosc Surg. 2016;9:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Hachiya O, Sato T, Toda M, Kimura W. An Easy and Safe Method of Liver Retraction Using a Silicone Disc and Needle Forceps for Laparoscopic Gastrectomy. J Laparoendosc Adv Surg Tech A. 2019;29:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Du G, Kong D, Shi B, Jiang Z, Aniu M, Yang J, Zhang H, Gao L, Jin B. Liver retraction using n-butyl-2-cyanoacrylate glue during laparoscopic cholecystectomy. Medicine (Baltimore). 2021;100:e25879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Harada H, Hayami M, Makuuchi R, Ida S, Kumagai K, Ohashi M, Nunobe S. A sandwiching method that simplifies hepatic left lateral segment inversion to secure an optimal surgical view around the esophageal hiatus in laparoscopic and robotic gastrectomy for upper gastric and esophagogastric junction cancers. Langenbecks Arch Surg. 2023;408:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Nakamura K, Suda K, Shibasaki S, Nakauchi M, Kikuchi K, Inaba K, Uyama I. The Hepatic Left Lateral Segment Inverting Method Offering a Wider Operative Field of View During Laparoscopic Proximal Gastrectomy. J Gastrointest Surg. 2020;24:2395-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Bai JG, Lv Y, Dang CX. Adenocarcinoma of the Esophagogastric Junction in China according to Siewert's classification. Jpn J Clin Oncol. 2006;36:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today. 2016;46:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 836] [Article Influence: 278.7] [Reference Citation Analysis (2)] |
| 11. | Hosogi H, Sakaguchi M, Yagi D, Onishi R, Hashimoto Y, Sakai Y, Kanaya S. Side-overlap esophagogastric tube (SO-EG) reconstruction after minimally invasive Ivor Lewis esophagectomy or laparoscopic proximal gastrectomy for cancer of the esophagogastric junction. Langenbecks Arch Surg. 2022;407:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Culcu S, Tamam S, Azili C, Ersoz S, Morkavuk B, Unal AE, Demirci S. Liver Dysfunction After Use of Nathanson Retractor During Laparoscopic Gastrectomy for Gastric Cancer. J Laparoendosc Adv Surg Tech A. 2023;33:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, Kinoshita T, Iwasaki Y, Misawa K, Takiguchi N, Kaji M, Okitsu H, Yoshikawa T, Terashima M; Stomach Cancer Study Group of Japan Clinical Oncology Group. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 14. | Pasenau J, Mamazza J, Schlachta CM, Seshadri PA, Poulin EC. Liver hematoma after laparoscopic nissen fundoplication: a case report and review of retraction injuries. Surg Laparosc Endosc Percutan Tech. 2000;10:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/