Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2808
Revised: July 2, 2024
Accepted: July 22, 2024
Published online: September 27, 2024
Processing time: 130 Days and 23.6 Hours
Gastric cancer is a kind of malignant tumor which is prevalent all over the world. Although some progress has been made in the treatment of gastric cancer, its prognosis is still not optimistic, so it is of great significance to find reliable prog
To explore the relationship between serum levels of five biomarkers [carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, CA72-4, CA24-2, and ferritin] and prognosis in patients with gastric cancer.
This study included 200 patients with gastric adenocarcinoma, and conducted an in-depth analysis of their baseline characteristics, relationship between tumor markers and staging, and prognosis. The study found that CA19-9 has a signi
This study reveals that there is a significant correlation between the expression levels of serum tumor markers CEA, CA19-9, CA72-4, CA24-2 and ferritin in patients with gastric cancer and prognosis, and can be used as important indicators for prognostic evaluation of gastric cancer. In particular, markers that appear abnormally elevated initially may help identify gastric cancer patients with poor prognosis.
Serum CEA and CA19-9 play an important role in the prognosis assessment of gastric cancer, and are effective tools to guide clinical practice and optimize individualized treatment strategies for gastric cancer patients.
Core Tip: In this study, the aim is to establish a scientific basis for tailoring personalized treatment strategies and evaluating the prognosis of individuals affected by gastric cancer. This study demonstrates a significant association between the expression levels of serum tumor markers carcinoembryonic antigen, carbohydrate antigen (CA) 19-9, CA72-4, CA24-2 and ferritin in patients with gastric cancer and their prognosis. These markers can serve as important indicators for prognostic evaluation of gastric cancer. Notably, abnormally elevated initial marker levels may aid in identifying gastric cancer patients with poor prognosis.
- Citation: Zhu JW, Gong LZ, Wang QW. Serum tumor markers (carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4, carbohydrate antigen 24-2, ferritin) and gastric cancer prognosis correlation. World J Gastrointest Surg 2024; 16(9): 2808-2814
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2808.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2808
Gastric cancer is a common malignant tumor worldwide[1-3]. Its prognosis depends on many factors, including the clinical stage of the tumor, histological type, treatment method, and the patient's overall health status[4-7]. Although some progress has been made in the treatment of gastric cancer, its prognosis is still poor, especially in the case of late diagnosis or recurrence, and the survival rate of patients is significantly reduced[8-11]. Therefore, finding reliable prognostic indicators is of great significance to guide the treatment and management of gastric cancer patients[11-15].
As a simple, non-invasive detection method, serum tumor markers have been widely used in the diagnosis, treatment response monitoring and prognosis assessment of gastric cancer[16-18]. In clinical practice, commonly used serum tumor markers include carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, CA72-4, CA24-2, and ferritin. The detection of these markers can not only help doctors confirm the diagnosis, but also provide information about the biological characteristics and prognosis of the tumor. However, the exact role of these tumor markers in the prognosis assessment of gastric cancer is still controversial, and the results of relevant studies are inconsistent[19]. As a non-invasive detection method, serum tumor markers play an important role in early screening, auxiliary diagnosis, treatment monitoring and prognosis assessment of various cancers. Among them, CEA, CA19-9, CA72-4, CA24-2 and ferritin have been widely studied and considered to have certain clinical value in gastric cancer. CEA was originally discovered in colon cancer, but its abnormal expression in gastric cancer also suggests potential prognostic value[20]. CA19-9, CA72-4 and CA24-2 mainly reflect glycosylation changes on the surface of tumor cells and are often used for monitoring digestive system tumors[21-23]. Ferritin is an indicator that reflects the body's iron metabolism. In recent years, studies have pointed out that it is associated with tumor proliferation, invasion, metastasis, and poor prognosis in gastric cancer.
CEA is a glycoprotein expressed in various tumors of the digestive system. In patients with gastric cancer, high levels of serum CEA are often associated with tumor progression, recurrence, and poor prognosis[24]. CA19-9 is a CA that also shows certain predictive value in gastric cancer, especially for lymph node metastasis and prognosis assessment of tumors. CA72-4 is a glycoprotein antigen that has certain clinical application prospects in the early diagnosis and prognosis assessment of gastric cancer[25]. CA24-2 is a mucus glycoprotein, and its level is closely related to the stage and prognosis of gastric cancer. As an iron metabolism-related protein, ferritin also plays an important role in the occurrence and development of gastric cancer, and its level is related to tumor anemia and prognosis[18].
Although many studies have explored the relationship between these serum tumor markers and the prognosis of gastric cancer, the existing evidence is not sufficient to draw consistent conclusions due to limitations in sample size, differences in study design, and heterogeneity of patient populations. Therefore, this study aims to further explore the relationship between serum tumor markers CEA, CA19-9, CA72-4, CA24-2 and ferritin and the prognosis of gastric cancer by retrospectively analyzing a large sample size of gastric cancer patient data, so as to provide more information for clinical practice. Reliable basis for prognostic assessment.
This study selected a total of 200 gastric cancer patients who received treatment in our hospital from January 2018 to December 2020 and completed follow-up. All patients were diagnosed with gastric adenocarcinoma by histopathological examination, and complete medical history records and laboratory test data were completed. Detailed information such as the patient's age, gender, tumor location, tumor node metastasis (TNM) stage, treatment method, and follow-up information were completely collected. This study adopted a retrospective cohort study design, followed the STROBE Statement (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, and conducted a comprehensive clinical data analysis of the included patients. The research focuses on exploring the relationship between the levels of serum tumor markers CEA, CA19-9, CA72-4, CA24-2 and ferritin and the prognosis of gastric cancer patients.
Inclusion criteria: Pathologically confirmed diagnosis of gastric adenocarcinoma; All patients have not received any treatment for gastric cancer before enrollment; Patients completed the detection of serum tumor markers at the first visit, and the data are complete and reliable; Patients agreed to participate in the study and signed informed consent form; Patient able to complete at least 1 year of follow-up as planned.
Exclusion criteria: The presence of concurrent or past medical history of other types of malignant tumors; The presence of radiotherapy, chemotherapy or other anti-tumor treatments for gastric cancer before enrollment; The presence of systemic diseases that seriously affect the determination of serum tumor markers, such as infection, liver and kidney severe functional impairment, etc.; Patients with incomplete data records or inability to obtain key clinical information; Patients who dropped out of the study due to subjective reasons or failed to complete at least 1 year of follow-up.
According to the normal reference range and critical value of each tumor marker in the patient's preoperative serum, the patients were divided into high expression group and low expression group. For example, for CEA, it is divided into an elevated CEA group (> 5 ng/mL) and a normal CEA group (≤ 5 ng/mL). By analogy, CA19-9, CA72-4, CA24-2 and ferritin are set respectively. corresponding cutoff value.
This study was an observational study and did not involve specific medical interventions. All patients' treatment plans (including surgery, chemotherapy, radiotherapy, etc.) follow the clinical practice guidelines and expert consensus at the time, and are formulated by the attending physician based on the patient's condition and individual differences.
The main outcome measure is the overall survival (OS) of patients with gastric cancer, which is the time from the date of diagnosis of gastric cancer to death from any cause or the last follow-up. Secondary observation indicators include disease-free survival (DFS) and other clinical characteristics (such as TNM stage, lymph node metastasis, etc.).
Data analysis was performed using statistical product and service solutions 26.0 software. Count data were expressed as frequencies and percentages, continuous variables were expressed as mean ± SD or median (interquartile range), and t-test or Mann-Whitney U test was used to compare between two groups. Survival curves were drawn using the Kaplan-Meier method, and survival differences under different marker expression levels were compared using the Log-rank test. Multifactor analysis was performed using the Cox proportional hazards model to explore the impact of each serum tumor marker on the prognosis of gastric cancer patients. P < 0.05 were considered statistically significant.
After strict inclusion and exclusion criteria, a total of 200 eligible gastric adenocarcinoma patients were finally included in this study, including 143 males and 57 females, with an average age of 62.3 ± 9.8 years. See Table 1 for details.
| Group | Age | CEA | CA19-9 | CA24-2 | CA72-4 | Fer |
| Male (n = 143) | 63.71 ± 9.07 | 31.00 ± 118.37 | 184.37 ± 716.08 | 139.68 ± 541.49 | 76.97 ± 762.42 | 259.39 ± 369.56 |
| Female (n = 57) | 57.35 ± 12.19 | 26.51 ± 106.57 | 167.06 ± 761.65 | 207.97 ± 1093.29 | 8.72 ± 24.59 | 209.23 ± 352.88 |
| F/t | 4.04 | 0.25 | 0.15 | -0.59 | 0.67 | 0.88 |
| P value | < 0.01 | 0.80 | 0.88 | 0.56 | 0.50 | 0.38 |
Among them, there is a clear correlation between CA19-9 and tumor stage, and the difference between different stages is statistically significant; In addition, the average levels of CA24-2, CEA, and CA72-4 were increased in tumor patients with different stages. Though relatively higher frequency with elevated level of ferritin was observed in phase 3 and phase 4 tumor patients, there was no marked change (Table 2).
| Group | CEA | CA199 | CA24-2 | CA72-4 | Fer | F | P value | |
| Phase 1 | Mean value | 58.17 | 1.49 | 16.74 | 8.31 | 2.66 | 2.14 | 0.10 |
| Number of cases | 30 | 30 | 30 | 30 | 30 | |||
| Standard deviation | 12.21 | 0.37 | 22.50 | 22.73 | 3.90 | |||
| Phase 2 | Mean value | 61.00 | 2.55 | 61.07 | 139.01 | 6.09 | 27.00 | 0.00 |
| Number of cases | 51 | 51 | 51 | 51 | 51 | |||
| Standard deviation | 11.26 | 0.32 | 251.26 | 740.53 | 10.94 | |||
| Phase 3 | Mean value | 63.00 | 8.00 | 234.47 | 174.40 | 10.13 | 1.95 | 0.12 |
| Number of cases | 92 | 92 | 92 | 92 | 92 | |||
| Standard deviation | 9.48 | 6.28 | 965.73 | 886.66 | 25.40 | |||
| Phase 4 | Mean value | 63.96 | 186.44 | 396.25 | 312.73 | 377.09 | 0.83 | 0.48 |
| Number of cases | 27 | 27 | 27 | 27 | 27 | |||
| Standard deviation | 9.02 | 267.09 | 739.39 | 569.28 | 1748.74 | |||
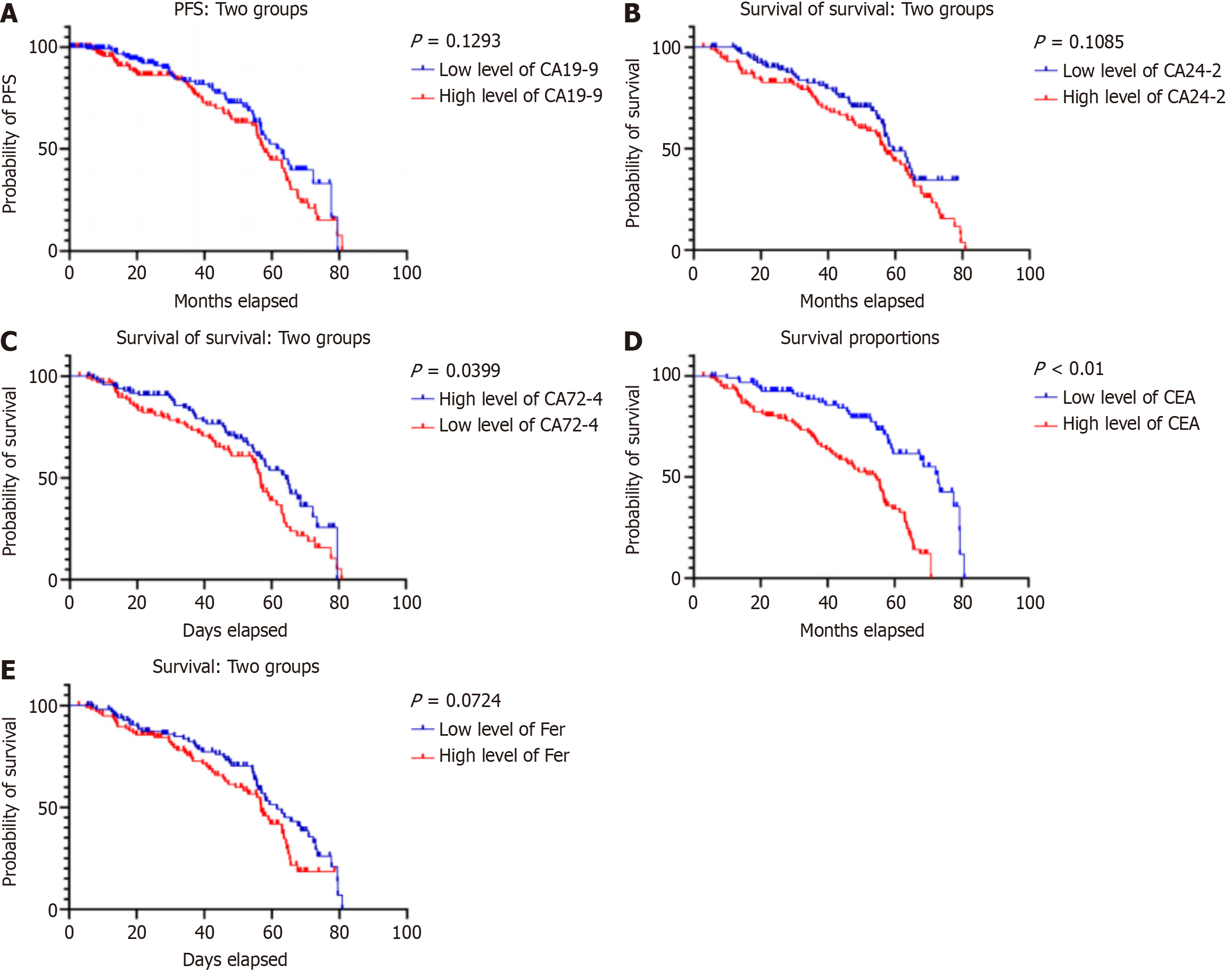
OS analysis: Kaplan-Meier survival curve method was used to evaluate the relationship between different serum tumor marker levels and patient OS. The results showed that compared with the normal CEA and CA72-4 groups, the OS time of patients in the elevated CEA group was significantly shorter (P < 0.001, Log-rank test). Similarly, the OS of patients with elevated CA19-9, elevated CA24-2, and elevated ferritin also showed significant disadvantages (Figure 1).
The Cox proportional hazards regression model was used to perform multifactor analysis on multiple factors that may affect the prognosis of gastric cancer patients. The results showed that elevated serum CA72-4 levels were an inde
| Group | B | SE | P value | OR | 95%CI | |
| Low limit | Upper limit | |||||
| Gender | 0.38 | 0.27 | 0.17 | 1.46 | 0.85 | 2.50 |
| Age | 0.02 | 0.01 | 0.15 | 1.02 | 0.99 | 1.04 |
| CEA | 0.00 | 0.00 | 0.15 | 1.00 | 1.00 | 1.00 |
| CA199 | 0.00 | 0.00 | 0.98 | 1.00 | 1.00 | 1.00 |
| CA24-2 | 0.00 | 0.00 | 0.56 | 1.00 | 1.00 | 1.00 |
| CA72-4 | 0.00 | 0.00 | 0.00 | 1.00 | 1.00 | 1.00 |
| Fer | 0.00 | 0.00 | 0.16 | 1.00 | 1.00 | 1.00 |
This study retrospectively analyzed the clinical data of 200 patients with gastric adenocarcinoma, focusing on the relationship between the levels of serum tumor markers CEA, CA19-9, CA72-4, CA24-2, and ferritin and the prognosis of gastric cancer. The results showed that elevated serum CEA and CA19-9 levels were significantly associated with shorter OS and DFS in patients with gastric cancer, and were confirmed as independent prognostic factors in multivariate analysis.
First, the prognostic value of CEA in gastric cancer patients is supported by this study. Consistent with many previous studies, this study found that elevated CEA was significantly associated with poor prognosis in gastric cancer patients. As a broad-spectrum tumor marker, the increase in CEA during the occurrence and development of gastric cancer may reflect adverse prognostic factors such as increased tumor load, strong invasiveness, or the presence of micrometastases. Therefore, preoperative CEA level can be used as an important indicator to evaluate the prognosis of gastric cancer patients. Secondly, the prognostic value of CA19-9 in this study was also verified. Although CA19-9 is not a specific marker for gastric cancer, its increase in gastric cancer patients is often related to the depth of tumor invasion, lymph node metastasis, and distant metastasis. Our results show that CA19-9 levels are closely related to the OS and DFS of patients with gastric cancer, suggesting that it may play an important role in the progression of gastric cancer. This discovery not only enriches the theoretical basis for CA19-9 in the prognosis assessment of gastric cancer, but also provides strong evidence for the rational use of this marker in clinical practice.
Although CA72-4, CA24-2 and ferritin showed a certain association with prognosis in univariate analysis, they did not reach statistical significance in multivariate analysis. This may be related to factors such as sample size, disease heterogeneity, and measurement error related. Previous studies have had mixed views on the role of these markers in the prognosis of gastric cancer, and further large-scale prospective studies are needed to verify and improve them. It is worth noting that although serum tumor markers have certain value in assessing the prognosis of gastric cancer, they are not a decisive factor. Comprehensive judgment must be made based on clinical pathological characteristics, molecular biology indicators and other factors. Future research should explore how to integrate serum tumor markers with other prognostic factors to build a more accurate prognostic model, in order to achieve personalized treatment and management of gastric cancer patients.
In summary, this study highlights the important role of serum CEA and CA19-9 in the prognosis assessment of gastric cancer, and is expected to become an effective tool to guide clinical practice and optimize individualized treatment strategies for gastric cancer patients. However, more clinical research is still needed to verify and promote these findings, and to explore more potential prognostic markers to better serve the diagnosis, treatment and improve the quality of life of gastric cancer patients.
Serum CEA and CA19-9 play an important role in the prognosis assessment of gastric cancer, and are effective tools to guide clinical practice and optimize individualized treatment strategies for gastric cancer patients.
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3308] [Article Influence: 551.3] [Reference Citation Analysis (6)] |
| 2. | Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol. 2022;29:1559-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 3. | Engstrand L, Graham DY. Microbiome and Gastric Cancer. Dig Dis Sci. 2020;65:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Bessède E, Mégraud F. Microbiota and gastric cancer. Semin Cancer Biol. 2022;86:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Mukkamalla SKR, Recio-Boiles A, Babiker HM. Gastric Cancer. 2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 6. | Qiu X, Shen C, Zhao W, Zhang X, Zhao D, Zhu Y, Li G, Yang L. Prognostic Value of the Combination of HB (hemoglobin) and CEA in Resectable Gastric Cancer. J Cancer. 2022;13:2246-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Shibata C, Nakano T, Yasumoto A, Mitamura A, Sawada K, Ogawa H, Miura T, Ise I, Takami K, Yamamoto K, Katayose Y. Comparison of CEA and CA19-9 as a predictive factor for recurrence after curative gastrectomy in gastric cancer. BMC Surg. 2022;22:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Zhang S, Li JZ, Du T, Li HQ, Hu RH, Ma CY, Cui XM, Song C, Jiang XH. The Modified Glasgow Prognostic Score Predicts Survival in Gastric Cancer Patients with Normal CEA and CA19-9. Can J Gastroenterol Hepatol. 2022;2022:3953004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Yuan Y, Zhang X, Du K, Zhu X, Chang S, Chen Y, Xu Y, Sun J, Luo X, Deng S, Qin Y, Feng X, Wei Y, Fan X, Liu Z, Zheng B, Ashktorab H, Smoot D, Li S, Xie X, Jin Z, Peng Y. Circ_CEA promotes the interaction between the p53 and cyclin-dependent kinases 1 as a scaffold to inhibit the apoptosis of gastric cancer. Cell Death Dis. 2022;13:827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Mo TM, Tian L, Chen JQ. Gastrin-17 Combined with CEA, CA12-5 and CA19-9 Improves the Sensitivity for the Diagnosis of Gastric Cancer. Int J Gen Med. 2021;14:8087-8095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Ruan J, Wang W, Lu Y, Wang H, Yu X, Wang H, Teng L. Prognostic Value of the Combination of CEA and Fibrinogen/Albumin Ratio in Resectable Gastric Cancer. Cancer Manag Res. 2020;12:2767-2775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Di T, Lai YR, Luo QY, Chen ZG, Du Y, Lin RD, Yang LQ, Zhang L, Sun J. A novel nomogram integrated with PDL1 and CEA to predict the prognosis of patients with gastric cancer. Clin Transl Oncol. 2023;25:2472-2486. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Miki Y, Yashiro M, Kuroda K, Okuno T, Togano S, Masuda G, Kasashima H, Ohira M. Circulating CEA-positive and EpCAM-negative tumor cells might be a predictive biomarker for recurrence in patients with gastric cancer. Cancer Med. 2021;10:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Culcu S, Yuksel C, Aydin F, Bakirarar B, Aksel B, Dogan L. The effect of CEA/Albumin ratio in gastric cancer patient on prognostic factors. Ann Ital Chir. 2022;93:447-452. [PubMed] |
| 15. | Jing R, Cui M, Ju S, Pan S. The Changes and Clinical Significance of Preoperative and Postoperative Serum CEA and CA19-9 in Gastric Cancer. Clin Lab. 2020;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Yuan H, Xu F, Wang S, Liu D, Zhang H, Zhang J, Shi M, Yan C, Zhu Z. Analysis of circulating tumor DNA identifies distinct therapeutic response to intraperitoneal and intravenous paclitaxel plus S-1 in gastric cancer patients with peritoneal metastasis. Ther Adv Med Oncol. 2024;16:17588359231225038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Luo K, Zhao C, Luo Y, Pan C, Li J. Electrochemical sensor for the simultaneous detection of CA72-4 and CA19-9 tumor markers using dual recognition via glycosyl imprinting and lectin-specific binding for accurate diagnosis of gastric cancer. Biosens Bioelectron. 2022;216:114672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 18. | Wang H, Jin W, Wan C, Zhu C. Diagnostic value of combined detection of CA72-4, CA19-9, and carcinoembryonic antigen comparing to CA72-4 alone in gastric cancer: a systematic review and meta-analysis. Transl Cancer Res. 2022;11:848-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 19. | Song XH, Liu K, Yang SJ, Zhang WH, Chen XL, Zhao LY, Chen XZ, Yang K, Zhou ZG, Hu JK. Prognostic Value of Changes in Preoperative and Postoperative Serum CA19-9 Levels in Gastric Cancer. Front Oncol. 2020;10:1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Yin HH, Xu MQ, Liu BZ, Tao L, Ma YJ, Li F, Zhang WJ. Combination of preoperative CA19-9 levels, cell differentiation, and age predicts survival for patients with gastric cancer before surgery. Medicine (Baltimore). 2021;100:e28017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Yu J, Zhang S, Zhao B. Differences and correlation of serum CEA, CA19-9 and CA72-4 in gastric cancer. Mol Clin Oncol. 2016;4:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Zhuang PY, Zhu MJ, Wang JD, Zhou XP, Quan ZW, Shen J. Xanthogranulomatous cholecystitis: a clinicopathological study of its association with gallbladder carcinoma. J Dig Dis. 2013;14:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 24. | Maruyama T, Akashi Y, Hakoda H, Sako A, Ueda K, Kato S, Azuma K, Kaneko Y, Ikeguchi A, Nagai S, Oda T. Preoperative CA19-9 is a prognostic factor in pT3N0 gastric cancer patients undergoing curative resection. Langenbecks Arch Surg. 2022;407:2273-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 25. | Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16:2685-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/