Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2620
Revised: July 15, 2024
Accepted: July 22, 2024
Published online: August 27, 2024
Processing time: 60 Days and 3.6 Hours
Acute non-variceal upper gastrointestinal bleeding (ANVUGIB) represents a sig
To assess the prognostic value of the Rockall risk score in a Chinese cohort of patients with ANVUGIB.
A retrospective analysis of 168 ANVUGIB patients’ medical records was condu
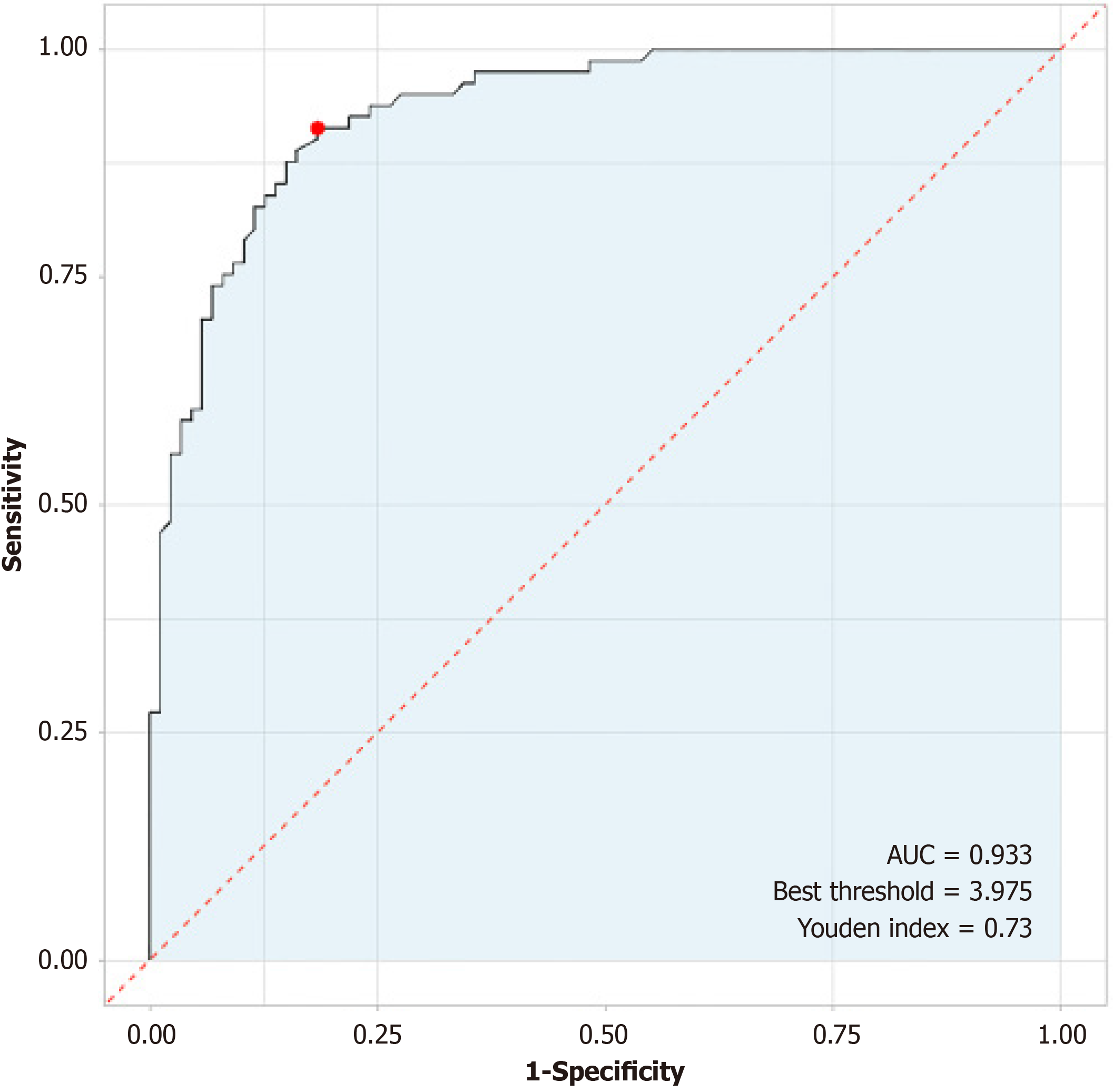
Significant associations were found between the Rockall score and various clinical outcomes. High Rockall scores were significantly associated with rebleeding events (r = 0.735, R2 = 0.541, P < 0.001) and strongly positively correlated with adverse outcomes. Low hemoglobin levels (t = 2.843, P = 0.005), high international normalized ratio (t = 3.710, P < 0.001), active bleeding during endoscopy (χ2 = 7.950, P = 0.005), large ulcer size (t = 6.348, P < 0.001), and requiring blood transfusion (χ2 = 6.381, P = 0.012) were all significantly associated with rebleeding events. Furthermore, differences in treatment and management strategies were identified between patients with and without rebleeding events. ROC analysis indicated the excellent discriminative power (sensitivity: 0.914; specificity: 0.816; area under the curve: 0.933; Youden index: 0.730) of the Rockall score in predicting rebleeding events within 3 months.
This study provides valuable insights into the prognostic value of the Rockall risk score for ANVUGIB in the Chinese population. The results underscore the potential of the Rockall score as an effective tool for risk stratification and prognostication, with implications for guiding risk-appropriate management strategies and optimizing care for patients with ANVUGIB.
Core Tip: This retrospective clinical study aimed to assess the prognostic value of the Rockall risk score in a Chinese cohort of patients with acute non-variceal upper gastrointestinal bleeding (ANVUGIB). The conclusion of this study provides valuable insights into the prognostic value of the Rockall risk score for ANVUGIB in the Chinese population. The results underscore the potential of the Rockall score as an effective tool for risk stratification and prognostication, with implications for guiding risk-appropriate management strategies and optimizing care for patients with ANVUGIB.
- Citation: Han DP, Gou CQ, Ren XM. Predictive utility of the Rockall scoring system in patients suffering from acute nonvariceal upper gastrointestinal hemorrhage. World J Gastrointest Surg 2024; 16(8): 2620-2629
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2620.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2620
Acute non-variceal upper gastrointestinal bleeding (ANVUGIB) refers to nonvariceal gastrointestinal bleeding occurring above the suspensory ligament of the duodenum[1-3]. Its common causes include peptic ulcer disease, gastrointestinal tumors, and acute gastric mucosal lesions, which make it a prevalent medical emergency in internal medicine[4,5]. The mortality rate of upper gastrointestinal bleeding (UGIB) ranges from 2% to 10%, which is associated with substantial treatment costs. In recent years, the application of proton pump inhibitors (PPIs) and the advancement of techniques, such as gastrointestinal endoscopy, have improved the treatment outcomes of UGIB[6,7]. However, no significant decline has been observed in the incidence and mortality rates[8]. Therefore, early identification of high-risk bleeding and poor prognosis is particularly important.
The timely assessment of ANVUGIB severity and the accurate prognostication of patient outcomes are crucial to guide clinical management and optimize patient care[9-11]. In this context, risk stratification tools, such as the Rockall risk score, have been developed to aid in UGIB evaluation[12-14]. The Rockall score incorporates clinical and endoscopic parameters to stratify patients into different risk categories and allow for risk-appropriate management strategies[7,15,16]. This system has been extensively utilized in clinical practice, and numerous studies have investigated its application in adult populations with UGIB in various geographical locations[10,17,18]. However, these studies have produced conflicting findings on the correlation degree between the score and certain morbidity and mortality outcomes[2,19-21].
Nurses play a crucial role in the care and management of patients with ANVUGIB. They often serve as frontline healthcare providers responsible for patient assessment, monitoring, and support during diagnostic and therapeutic interventions. Given the potential severity and clinical complexity of ANVUGIB, nurses are essential in the prompt recognition of deteriorating clinical conditions, early intervention for potential hemorrhage, and ongoing patient education and support. Moreover, they are pivotal in the coordination of care among multidisciplinary teams, including facilitating timely endoscopic procedures, administering medications, and monitoring patients’ response to treatment. To date, no study has validated the Rockall score for ANVUGIB in China. Therefore, this retrospective clinical research aims to evaluate the prognostic value of the Rockall risk score in a Chinese cohort of patients presenting with acute ANVUGIB. Understanding the prognostic value of risk stratification tools, such as the Rockall risk score, in ANVUGIB is of para
This study adopted a retrospective clinical design. Data from the medical records of patients with ANVUGIB were utilized to evaluate the prognostic value of the Rockall risk score.
This study comprised a cohort of 104 patients diagnosed with ANVUGIB who presented to our hospital between December 2020 and December 2022. Patients were divided into without rebleeding (n = 87) and rebleeding (n = 81) groups according to the occurrence of rebleeding events within 3 months after the Rockall risk score assessment. This study was approved by the Ethics Committee of Chengyang District People’s Hospital. Given its retrospective nature, this research solely utilized de-identified patient data. Thus, informed consent was waived because of the lack of potential for harm or influence on patient care.
The inclusion criteria were as follows: Aged 18-70 years and confirmed diagnosis with ANVUCGIB based on clinical symptoms, endoscopic findings, or other relevant diagnostic criteria as per the established medical guidelines and institutional practices[22-25].
The exclusion criteria were as follows: (1) Variceal bleeding, which refers to gastrointestinal bleeding from enlarged veins in the esophagus, stomach, or duodenum; (2) Incomplete or insufficient documentation of clinical data, including incomplete Rockall risk score components, missing laboratory parameters, or incomplete endoscopic findings; (3) Known gastrointestinal conditions that could substantially influence the interpretation of the Rockall risk score and clinical outcomes, such as inflammatory bowel disease, malignancies, or severe coagulopathies; and (4) Concurrent severe illnesses or conditions that could independently impact prognosis or confound the interpretation of rebleeding events, such as advanced organ failure or life-threatening systemic conditions.
The Rockall scoring system is a widely utilized clinical tool for predicting the risk of rebleeding and mortality. Scores of ≥ 5 indicate high risk, 3-4 represent moderate risk, and 0-2 indicate low risk. Patients with documented Rockall risk scores were included in this study. The Rockall score was calculated based on clinical and endoscopic parameters[16,26].
Patients whose comprehensive clinical data, including demographic information, endoscopic findings, and treatment details, were available in their medical records were included in this study.
For blood testing, 5 mL of fasting blood sample was collected from the antecubital vein in the morning and measured using the Beckman DxH800 hematology analyzer.
Various statistical tests, such as t-test and χ2 test, were performed to assess the relationship between Rockall score and clinical outcomes, including rebleeding events. Statistical analysis was conducted using SPSS 29.0 software (SPSS Inc, Chicago, IL, United States). Categorical data were presented as n (%) and analyzed by χ2 test. The normality of continuous variables was assessed using the Shapiro-Wilk method. Continuous variables following a normal distribution were expressed as (± SD) and analyzed using a corrected variance t-test. A two-tailed P < 0.05 was considered statistically significant.
Spearman correlation and receiver operating characteristic (ROC) analyses were performed. The authors discussed any statistical adjustments or corrections made for potential confounding variables.
This study included 168 patients with ANVUGIB, of which 87 were in the without rebleeding group and 81 were in the rebleeding group (Table 1). The mean age of patients was 52.84 years (SD = 8.15) in the without rebleeding group and 54.15 years (SD = 7.48) in the rebleeding group. The difference in age between the two groups was not statistically significant (t = 1.087, P = 0.279). In terms of gender distribution, the without rebleeding group had 25 males and 30 females, and the rebleeding group had 30 males and 19 females (χ2 = 0.310, P = 0.577). The prevalence of smoking, drinking, hypertension, diabetes and hyperlipidemia was not significantly different between the two groups (P > 0.05). For comorbidities, a trend toward a high prevalence of renal failure (t = 3.005, P = 0.083) and pneumonia (t = 1.984, P = 0.159) was observed in the rebleeding group. No significant difference in the prevalence of septicemia (χ2 = 0, P = 1.000) and the use of nonsteroidal anti-inflammatory drugs (χ2 = 2.095, P = 0.148), or anticoagulants (χ2 = 2.188, P = 0.139) was found between the two groups.
| Parameter | Without rebleeding group (n = 87) | Rebleeding group (n = 81) | t/χ2 | P value |
| Age (years) | 52.84 ± 8.15 | 54.15 ± 7.48 | 1.087 | 0.279 |
| Gender (M/F) | 35 (40.23)/52 (59.77) | 37 (45.68)/44 (54.32) | 0.310 | 0.577 |
| BMI (kg/m2) | 23.39 ± 1.31 | 23.65 ± 1.23 | 1.283 | 0.201 |
| Smoking history (Yes/No) | 11 (12.64)/76 (87.36) | 10 (12.35)/71 (87.65) | 0.000 | 1.000 |
| Alcohol history (Yes/No) | 20 (22.99)/67 (77.01) | 19 (23.46)/62 (76.54) | 0.000 | 1.000 |
| Hypertension (Yes/No) | 9 (10.34)/78 (89.66) | 9 (11.11)/72 (88.89) | 0.000 | 1.000 |
| Diabetes (Yes/No) | 7 (8.05)/80 (91.95) | 6 (7.41)/75 (92.59) | 0.000 | 1.000 |
| Hyperlipidemia (Yes/No) | 8 (9.2)/79 (90.8) | 7 (8.64)/74 (91.36) | 0.000 | 1.000 |
| Renal failure (Yes/No) | 8 (9.2)/79 (90.8) | 16 (19.75)/65 (80.25) | 3.005 | 0.083 |
| Pneumonia (Yes/No) | 6 (6.9)/81 (93.1) | 12 (14.81)/69 (85.19) | 1.984 | 0.159 |
| Septicemia (Yes/No) | 14 (16.09)/73 (83.91) | 14 (17.28)/67 (82.72) | 0.000 | 1.000 |
| NSAID use (Yes/No) | 43 (49.43)/44 (50.57) | 50 (61.73%)/31 (38.27) | 2.095 | 0.148 |
| Anticoagulant use (Yes/No) | 29 (33.33)/58 (66.67) | 37 (45.68)/44 (54.32) | 2.188 | 0.139 |
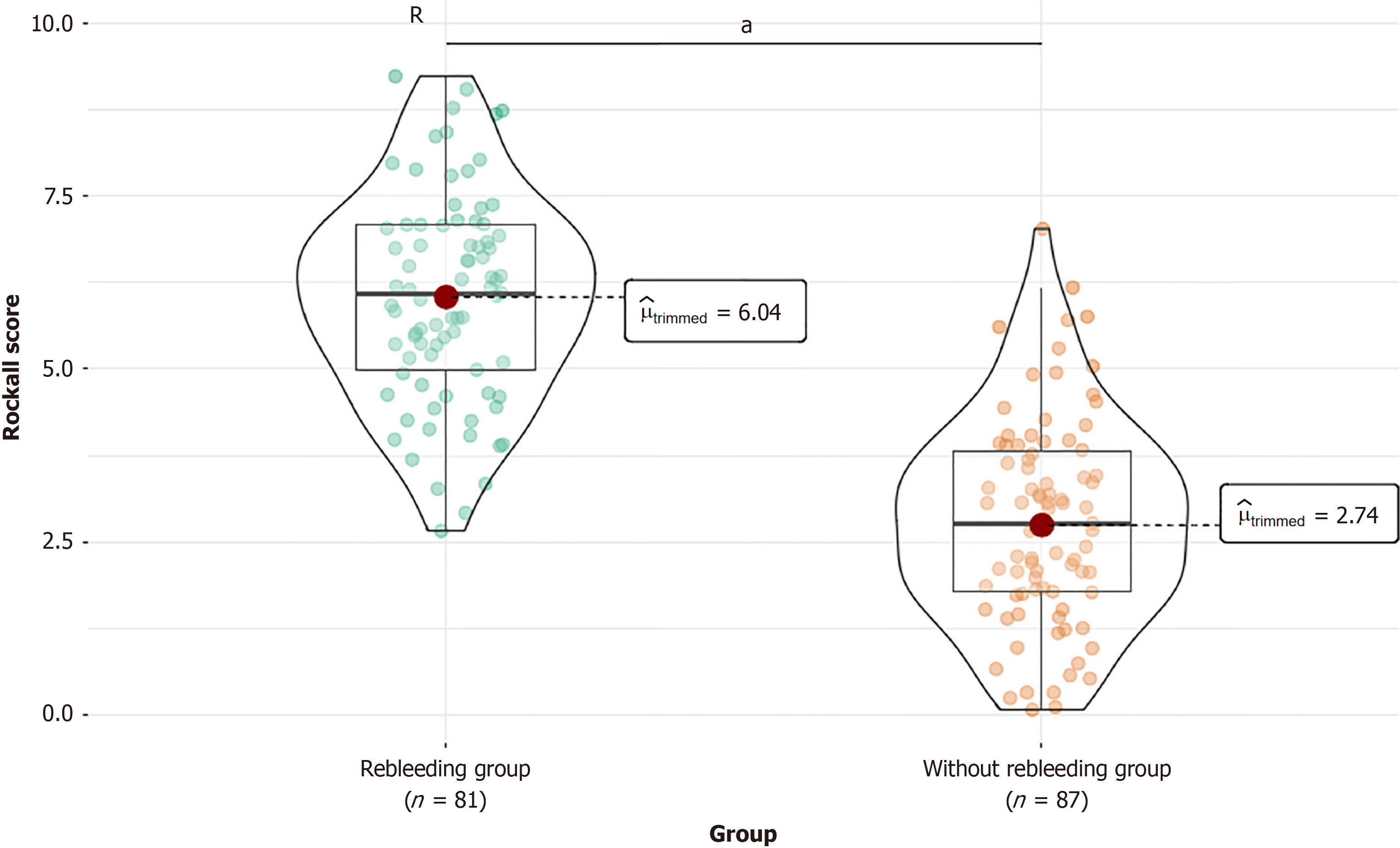
Comparison of medication use revealed significant differences in the Rockall score (t = 13.984, P < 0.001) between the two groups (Figure 1). The without rebleeding group had a mean score of 2.8 (SD = 1.5), and the rebleeding group had a mean score of 6.03 (SD = 1.5). This finding indicates a clear association between high Rockall scores and rebleeding events in patients with ANVUGIB.
Comparison of laboratory parameters revealed significant differences in hemoglobin levels (t = 2.843, P = 0.005) between the two groups (Table 2). The rebleeding group had a mean of 113.95 g/L (SD = 11.44), and the rebleeding group had a mean of 109.14 g/L (SD = 10.48). In addition, the international normalized ratio (INR) was significantly higher in the rebleeding group (mean score of 1.48, SD = 0.29) than in the without rebleeding group (mean score of 1.34, SD = 0.18, t = 3.710, P < 0.001). No significant differences in platelet count (t = 1.344, P = 0.181) and albumin levels (t = 1.168, P = 0.244) were observed between the two groups. These findings suggest that low hemoglobin levels and high INR values are associated with rebleeding events in patients with ANVUGIB.
| Parameter | Without rebleeding group (n = 87) | Rebleeding group (n = 81) | t value | P value |
| Hemoglobin (g/L) | 113.95 ± 11.44 | 109.14 ± 10.48 | 2.843 | 0.005 |
| Platelet count (× 104/microliter) | 24.48 ± 8.15 | 22.97 ± 6.37 | 1.344 | 0.181 |
| INR | 1.34 ± 0.18 | 1.48 ± 0.29 | 3.710 | < 0.001 |
| Albumin (g/dL) | 3.91 ± 0.59 | 3.79 ± 0.67 | 1.168 | 0.244 |
Comparison of endoscopic findings revealed significant differences in active bleeding (Table 3) between the without rebleeding (37 cases) and with rebleeding (53 cases) groups (χ2 = 7.950, P = 0.005), indicating a clear association between active bleeding during endoscopy and rebleeding events. In addition, the average ulcer size was significantly larger in the rebleeding group (mean size of 1.8 cm, SD = 0.65) than in the without rebleeding group (mean size of 1.19 cm, SD = 0.58)
| Parameter | Without rebleeding group (n = 87) | Rebleeding group (n = 81) | t/χ2 | P value |
| Active bleeding (Yes/No) | 37 (42.53)/50 (57.47) | 53 (65.43)/28 (34.57) | 7.950 | 0.005 |
| Average ulcer size (cm) | 1.19 ± 0.58 | 1.8 ± 0.65 | 6.348 | < 0.001 |
| Number of ulcers (≤ 2/> 2) | 49 (56.32)/38 (43.68) | 30 (37.04)/51 (62.96) | 5.512 | 0.019 |
| Ulcer location (gastric/duodenal) | 35 (40.23)/52 (59.77) | 38 (46.91)/43 (53.09) | 0.515 | 0.473 |
Comparison of treatment and management strategies revealed significant differences between the two groups (Table 4). Endoscopic therapy was significantly more common in the without rebleeding group (60 patients) than in the rebleeding group (30 patients) (χ2 = 15.933, P < 0.001). Moreover, blood transfusion was significantly more frequent in the rebleeding group (6 patients) than in the without rebleeding group (39 patients) (χ2 = 6.381, P = 0.012). Although the prevalence of PPI therapy was higher in the without rebleeding group compared with that in the rebleeding group, the difference was not statistically significant (χ2 = 0.255, P = 0.613). These findings suggest that endoscopic therapy and blood transfusion are associated with rebleeding events in patients with ANVUGIB.
| Parameter | Without rebleeding group (n = 87) | Rebleeding group (n = 81) | χ2 | P value |
| PPI therapy (Yes/No) | 85 (97.7)/2 (2.3) | 77 (95.06)/4 (4.94) | 0.255 | 0.613 |
| Endoscopic therapy (Yes/No) | 60 (68.97)/27 (31.03) | 30 (37.04)/51 (62.96) | 15.933 | < 0.001 |
| Blood transfusion (Yes/No) | 39 (44.83)/48 (55.17) | 53 (65.43)/28 (34.57) | 6.381 | 0.012 |
Spearman correlation analysis revealed several significant relationships in patients with ANVUGIB (Table 5). The Rockall score exhibited a strong positive correlation with rebleeding events (r = 0.735, R2 = 0.541, P < 0.001), indicating its pre
| r value | R2 value | P value | |
| Rockall score | 0.735 | 0.541 | < 0.001 |
| Hemoglobin (g/L) | -0.215 | 0.046 | 0.005 |
| INR | 0.281 | 0.079 | < 0.001 |
| Average ulcer size (cm) | 0.443 | 0.197 | < 0.001 |
| Renal failure (Yes/No) | 0.151 | 0.023 | 0.051 |
| Active bleeding (Yes/No) | 0.229 | 0.053 | 0.003 |
| Number of ulcers (≤ 2/>2) | -0.193 | 0.037 | 0.012 |
| Endoscopic therapy (Yes/No) | -0.32 | 0.102 | < 0.001 |
| Blood transfusion (Yes/No) | 0.207 | 0.043 | 0.007 |
Multivariate logistic regression analysis (Table 6) revealed that the Rockall score was a significant independent predictor of outcomes in patients with ANVUGIB, with an odds ratio (OR) of 1.134 [95% confidence interval (CI): 1.106-1.164, P < 0.001]. Although significant in the univariate analysis (OR: 0.96, 95%CI: 0.932-0.988, P = 0.007), the hemoglobin level did not retain its significance in the multivariate model (OR: 0.998, 95%CI: 0.994-1.002, P = 0.317). The INR (OR: 1.142, 95%CI: 0.926-1.409, P = 0.214) and impact of blood transfusion necessity (OR: 1.055, 95%CI: 0.955-1.166, P = 0.29) also lost their significance in the multivariate analysis. Meanwhile, the average ulcer size (OR: 1.156, 95%CI: 1.069-1.25, P < 0.001) and presence of active bleeding at the time of endoscopy (OR: 1.117, 95%CI: 1.007-1.24, P = 0.036) retained their significance. The number of ulcers (OR: 0.91, 95%CI: 0.824-1.006, P = 0.065) and application of endoscopic therapy (OR: 0.908, 95%CI: 0.817-1.01, P = 0.075) did not reach statistical significance in the multivariate analysis. These results highlight the Rockall score, average ulcer size, and active bleeding as crucial prognostic indicators in this clinical setting.
| Sensitivities | Specificities | AUC | Youden index | |
| Rockall score | 0.914 | 0.816 | 0.933 | 0.730 |
| Hemoglobin (g/L) | 0.519 | 0.736 | 0.641 | 0.255 |
| INR | 0.494 | 0.874 | 0.668 | 0.368 |
| Average ulcer size (cm) | 0.864 | 0.552 | 0.752 | 0.416 |
| Active bleeding (Yes/No) | 0.654 | 0.575 | 0.615 | 0.229 |
| Number of ulcers (≤ 2/>2) | 0.63 | 0.563 | 0.596 | 0.193 |
| Endoscopic therapy (Yes/No) | 0.63 | 0.69 | 0.66 | 0.32 |
| Blood transfusion (Yes/No) | 0.654 | 0.552 | 0.603 | 0.206 |
ROC analysis (Table 7) demonstrated the high predictive utility of the Rockall score for rebleeding events within 3 months in patients with ANVUGIB (Figure 2). The sensitivity was 0.914, and the specificity was 0.816, with an area under the curve (AUC) of 0.933, indicating excellent discriminative power. The calculated Youden index of 0.730 underscores the strong overall performance of the Rockall score in predicting rebleeding events, further supporting its clinical relevance in risk assessment for this patient population.
| Factor | Univariate regression | Multivariate regression | ||||||
| OR | 95%CI | β | P value | OR | 95%CI | β | P value | |
| Rockall score | 3.891 | 2.729-6.057 | 1.359 | < 0.001 | 1.134 | 1.106-1.164 | 0.126 | 0 |
| Hemoglobin (g/L) | 0.96 | 0.932-0.988 | -0.04 | 0.007 | 0.998 | 0.994-1.002 | -0.002 | 0.317 |
| INR | 11.647 | 3.092-48.723 | 2.455 | < 0.001 | 1.142 | 0.926-1.409 | 0.133 | 0.214 |
| Average ulcer size (cm) | 5.013 | 2.826-9.577 | 1.612 | < 0.001 | 1.156 | 1.069-1.25 | 0.145 | 0 |
| Active bleeding (Yes/No) | 2.558 | 1.378-4.822 | 0.939 | 0.003 | 1.117 | 1.007-1.24 | 0.111 | 0.036 |
| Number of ulcers (≤ 2/>2) | 0.456 | 0.244-0.843 | -0.785 | 0.013 | 0.91 | 0.824-1.006 | -0.094 | 0.065 |
| Endoscopic therapy (Yes/No) | 0.265 | 0.138-0.497 | -1.329 | < 0.001 | 0.908 | 0.817-1.01 | -0.096 | 0.075 |
| Blood transfusion (Yes/No) | 2.33 | 1.257-4.382 | 0.846 | 0.008 | 1.055 | 0.955-1.166 | 0.054 | 0.29 |
ANVUGIB is a critical medical emergency with significant morbidity and mortality rates[2,15,16]. The timely assessment of its severity and accurate prognostication of patient outcomes are crucial for guiding clinical management and optimi
The findings provide important insights into the prognostic value of the Rockall risk score for ANVUGIB. The study population consisted of 168 patients with ANVUGIB, offering a substantial sample size for evaluating the performance of the Rockall score in this patient population. Results revealed significant associations between the Rockall score and various clinical outcomes including rebleeding events, laboratory parameters, endoscopic findings, and treatment stra
One of the key findings of this study is the significant association between high Rockall scores and rebleeding events in patients with ANVUGIB. Comparison of Rockall scores between the without rebleeding and rebleeding groups de
In addition to rebleeding events, the study identified significant associations between the Rockall score and various laboratory parameters, endoscopic findings, and treatment strategies. Low hemoglobin levels and high INR were found to be associated with rebleeding events, indicating the potential use of these parameters as prognostic indicators in patients with ANVUGIB. Furthermore, active bleeding during endoscopy, large ulcer size, and a high number of ulcers were significantly associated with rebleeding events, emphasizing their importance in risk assessment and clinical decision-making. These findings are consistent with previous literature highlighting the role of endoscopic parameters in risk stratification and prognostication for patients with UGIB[26,30,31]. Moreover, this study identified significant differences in treatment and management strategies between patients with and without rebleeding events, with implications for risk-appropriate therapeutic interventions in patients with ANVUGIB.
This study contributes to the ongoing discourse regarding the use of risk stratification tools in ANVUGIB management. Although the Rockall score has been extensively utilized in clinical practice for risk assessment in patients with UGIB, its application for ANVUGIB, particularly in the Chinese population, is rarely researched. The findings offer valuable insights into the performance of the Rockall score in predicting adverse outcomes in patients with ANVUGIB, thus filling a significant gap in literature. By demonstrating the significant associations of the Rockall score with rebleeding events, laboratory parameters, endoscopic findings, and treatment strategies, this study provides a comprehensive evaluation of the prognostic value of this score in the Chinese perspective and thereby enhances our understanding of its clinical utility in this patient population.
The use of the Rockall risk score as a prognostic tool can guide nurses in identifying patients at high risk of rebleeding events, thereby prompting vigilant monitoring, timely intervention, and close collaboration with the healthcare team to optimize patient outcomes. Furthermore, the identified significant associations of laboratory parameters, endoscopic findings, and treatment strategies with rebleeding events provide nurses with valuable clinical indicators to consider in the assessment and care planning for patients with ANVUGIB. This knowledge can aid nursing practices in terms of individualizing patient care, facilitating shared decision-making, and enabling timely interventions, such as advocating for endoscopic therapy or coordinating blood transfusion as part of comprehensive patient care. As such, the integration of the study findings into nursing practices can enhance the quality of care provided to patients with ANVUGIB and ultimately contribute to improved patient outcomes and experiences.
Despite the compelling findings of this study, several limitations should be acknowledged. First, its retrospective design may have introduced inherent biases related to data collection and patient selection. In addition, its single-center nature may limit the generalizability of the findings to other patient populations in different clinical settings. The absence of a validation cohort and the retrospective nature of the data analysis may limit the robustness of the findings and their applicability to prospective clinical practice. Future prospective studies involving large, multicenter cohorts are war
This retrospective clinical study provides valuable insights into the prognostic value of the Rockall risk score in Chinese patients with ANVUGIB. The Rockall score was found to be significantly associated with rebleeding events, laboratory parameters, endoscopic findings, and treatment strategies, highlighting its potential as an effective tool for risk stratification and prognostication in this patient population. The findings contribute to the growing body of evidence surrounding risk assessment and clinical decision-making for patients with ANVUGIB, emphasizing the potential of the Rockall score to guide risk-appropriate management strategies and optimize patient care in the Chinese context. Despite this study’s limitations, its findings underscore the significance of risk stratification tools in ANVUGIB management and provide a foundation for further research in this critical area of clinical practice.
| 1. | Alzoubaidi D, Lovat LB, Haidry R. Management of non-variceal upper gastrointestinal bleeding: where are we in 2018? Frontline Gastroenterol. 2019;10:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Kim MS, Moon HS, Kwon IS, Park JH, Kim JS, Kang SH, Sung JK, Lee ES, Kim SH, Lee BS, Jeong HY. Validation of a new risk score system for non-variceal upper gastrointestinal bleeding. BMC Gastroenterol. 2020;20:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (36)] |
| 4. | Martino A, Di Serafino M, Orsini L, Giurazza F, Fiorentino R, Crolla E, Campione S, Molino C, Romano L, Lombardi G. Rare causes of acute non-variceal upper gastrointestinal bleeding: A comprehensive review. World J Gastroenterol. 2023;29:4222-4235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 5. | Zhong C, Tan S, Ren Y, Lü M, Peng Y, Fu X, Tang X. Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Guo CLT, Wong SH, Lau LHS, Lui RNS, Mak JWY, Tang RSY, Yip TCF, Wu WKK, Wong GLH, Chan FKL, Lau JYW, Sung JJY. Timing of endoscopy for acute upper gastrointestinal bleeding: a territory-wide cohort study. Gut. 2022;71:1544-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Meier B, Wannhoff A, Denzer U, Stathopoulos P, Schumacher B, Albers D, Hoffmeister A, Feisthammel J, Walter B, Meining A, Wedi E, Zachäus M, Pickartz T, Küllmer A, Schmidt A, Caca K. Over-the-scope-clips versus standard treatment in high-risk patients with acute non-variceal upper gastrointestinal bleeding: a randomised controlled trial (STING-2). Gut. 2022;71:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Bhutia KD, Lamtha SC. Retrospective study of etiology of non variceal acute gastrointestinal bleeding in Eastern Himalayan region of india in Sikkim. J Family Med Prim Care. 2019;8:573-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Chapman W, Siau K, Thomas F, Ernest S, Begum S, Iqbal T, Bhala N. Acute upper gastrointestinal bleeding: a guide for nurses. Br J Nurs. 2019;28:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Dertli R, Toka B, Asıl M, Kayar Y, Karakarcayıldız A, Göktepe MH, Bıyık M, Konur Ş, Ataseven H. Can neutrophil-lymphocyte ratio predict mortality in acute non-variceal upper gastrointestinal bleeding? Ulus Travma Acil Cerrahi Derg. 2022;28:626-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Pioppo L, Bhurwal A, Reja D, Tawadros A, Mutneja H, Goel A, Patel A. Incidence of Non-variceal Upper Gastrointestinal Bleeding Worsens Outcomes with Acute Coronary Syndrome: Result of a National Cohort. Dig Dis Sci. 2021;66:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Hajiagha Mohammadi AA, Reza Azizi M. Prognostic factors in patients with active non-variceal upper gastrointestinal bleeding. Arab J Gastroenterol. 2019;20:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Huang J, Liao F, Tang J, Shu X. Development of a model for predicting acute cerebral infarction induced by non-variceal upper gastrointestinal bleeding. Clin Neurol Neurosurg. 2023;235:107992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kate V, Sureshkumar S, Gurushankari B, Kalayarasan R. Acute Upper Non-variceal and Lower Gastrointestinal Bleeding. J Gastrointest Surg. 2022;26:932-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Zhu Y, Yang W, Zhang Y, Ye L, Hu B. The value of endoscopically-placed metal clips for transcatheter arterial embolization in the treatment of recurrent acute non-variceal upper gastrointestinal bleeding. BMC Gastroenterol. 2023;23:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Wang X, Yang M, Xu J, Kuai Y, Sun B. Risk analysis of 30-day rebleeding in acute non-variceal upper gastrointestinal bleeding. Arab J Gastroenterol. 2023;24:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Marmo R, Soncini M, Bucci C, Zullo A; GISED. Comparison of assessment tools in acute upper gastrointestinal bleeding: which one for which decision. Scand J Gastroenterol. 2022;57:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Lakatos L, Gonczi L, Lontai L, Izbeki F, Patai A, Racz I, Gasztonyi B, Varga-Szabo L, Ilias A, Lakatos PL. Incidence, Predictive Factors, Clinical Characteristics and Outcome of Non-variceal Upper Gastrointestinal Bleeding - A Prospective Population-based Study from Hungary. J Gastrointestin Liver Dis. 2021;30:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Shalimar, Vaishnav M, Elhence A, Kumar R, Mohta S, Palle C, Kumar P, Ranjan M, Vajpai T, Prasad S, Yegurla J, Dhooria A, Banyal V, Agarwal S, Bansal R, Bhattacharjee S, Aggarwal R, Soni KD, Rudravaram S, Singh AK, Altaf I, Choudekar A, Mahapatra SJ, Gunjan D, Kedia S, Makharia G, Trikha A, Garg P, Saraya A. Outcome of Conservative Therapy in Coronavirus disease-2019 Patients Presenting With Gastrointestinal Bleeding. J Clin Exp Hepatol. 2021;11:327-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Khoury T, Darawsheh F, Daher S, Yaari S, Katz L, Mahamid M, Kadah A, Mari A, Sbeit W. Predictors of endoscopic intervention in upper gastrointestinal bleeding patients hospitalized for another illness: a multi-center retrospective study. Panminerva Med. 2020;62:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Jono F, Iida H, Fujita K, Kaai M, Kanoshima K, Ohkuma K, Nonaka T, Ida T, Kusakabe A, Nakamura A, Koyama S, Nakajima A, Inamori M. Comparison of computed tomography findings with clinical risks factors for endoscopic therapy in upper gastrointestinal bleeding cases. J Clin Biochem Nutr. 2019;65:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Alali AA, Barkun AN. An update on the management of non-variceal upper gastrointestinal bleeding. Gastroenterol Rep (Oxf). 2023;11:goad011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (36)] |
| 23. | Cañamares-Orbís P, Lanas Arbeloa Á. New Trends and Advances in Non-Variceal Gastrointestinal Bleeding-Series II. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 24. | Haddad FG, El Imad T, Nassani N, Kwok R, Al Moussawi H, Polavarapu A, Ahmed M, El Douaihy Y, Deeb L. In-hospital acute upper gastrointestinal bleeding: What is the scope of the problem? World J Gastrointest Endosc. 2019;11:561-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Martino A, Di Serafino M, Amitrano L, Orsini L, Pietrini L, Martino R, Menchise A, Pignata L, Romano L, Lombardi G. Role of multidetector computed tomography angiography in non-variceal upper gastrointestinal bleeding: A comprehensive review. World J Gastrointest Endosc. 2022;14:739-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 26. | Ebrahimi Bakhtavar H, Morteza Bagi HR, Rahmani F, Shahsavari Nia K, Ettehadi A. Clinical Scoring Systems in Predicting the Outcome of Acute Upper Gastrointestinal Bleeding; a Narrative Review. Emerg (Tehran). 2017;5:e36. [PubMed] |
| 27. | Wang CH, Hung MS, Wu KH, Chen YC. Comparison of Two Scoring Systems in Predicting Outcomes in Non-Variceal Upper Gastrointestinal Bleeding in Taiwanese Population. J Acute Med. 2017;7:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Botianu A, Matei D, Tantau M, Acalovschi M. Mortality and need of surgical treatment in acute upper gastrointestinal bleeding: a one year study in a tertiary center with a 24 hours/day-7 days/week endoscopy call. Has anything changed? Chirurgia (Bucur). 2013;108:312-318. [PubMed] |
| 29. | Stanley AJ, Laine L, Dalton HR, Ngu JH, Schultz M, Abazi R, Zakko L, Thornton S, Wilkinson K, Khor CJ, Murray IA, Laursen SB; International Gastrointestinal Bleeding Consortium. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 30. | Laursen SB, Leontiadis GI, Stanley AJ, Møller MH, Hansen JM, Schaffalitzky de Muckadell OB. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: a nationwide cohort study. Gastrointest Endosc. 2017;85:936-944.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 31. | Thanapirom K, Ridtitid W, Rerknimitr R, Thungsuk R, Noophun P, Wongjitrat C, Luangjaru S, Vedkijkul P, Lertkupinit C, Poonsab S, Ratanachu-ek T, Hansomburana P, Pornthisarn B, Thongbai T, Mahachai V, Treeprasertsuk S. Prospective comparison of three risk scoring systems in non-variceal and variceal upper gastrointestinal bleeding. J Gastroenterol Hepatol. 2016;31:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/