Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2583
Revised: July 11, 2024
Accepted: July 15, 2024
Published online: August 27, 2024
Processing time: 79 Days and 3.7 Hours
Acute pancreatitis (AP) is a disease caused by abnormal activation of pancreatic enzymes and can lead to self-digestion of pancreatic tissues and dysfunction of other organs. Enteral nutrition plays a vital role in the treatment of AP because it can meet the nutritional needs of patients, promote the recovery of intestinal func
To establish and validate a predictive model for enteral nutrition aspiration during hospitalization in patients with AP.
A retrospective review was conducted on 200 patients with AP admitted to Chengdu Shangjin Nanfu Hospital, West China Hospital of Sichuan University from January 2020 to February 2024. Clinical data were collected from the electronic medical record system. Patients were randomly divided into a validation group (n = 40) and a modeling group (n = 160) in a 1:4 ratio, matched with 200 patients from the same time period. The modeling group was further categorized into an aspiration group (n = 25) and a non-aspiration group (n = 175) based on the occurrence of enteral nutrition aspiration during hospitalization. Univariate and multivariate logistic regression analyses were performed to identify factors influencing enteral nutrition aspiration in patients with AP during hospitalization. A prediction model for enteral nutrition aspiration during hospitalization was constructed, and calibration curves were used for validation. Receiver operating characteristic curve analysis was conducted to evaluate the predictive value of the model.
There was no statistically significant difference in general data between the validation and modeling groups (P > 0.05). The comparison of age, gender, body mass index, smoking history, hypertension history, and diabetes history showed no statistically significant difference between the two groups (P > 0.05). However, patient position, consciousness status, nutritional risk, Acute Physiology and Chronic Health Evaluation (APACHE-II) score, and length of nasogastric tube placement showed statistically significant differences (P < 0.05) between the two groups. Multivariate logistic regression analysis showed that patient position, consciousness status, nutritional risk, APACHE-II score, and length of nasogastric tube placement were independent factors influencing enteral nutrition aspiration in patients with AP during hospitalization (P < 0.05). These factors were incorporated into the prediction model, which showed good consistency between the predicted and actual risks, as indicated by calibration curves with slopes close to 1 in the training and validation sets. Receiver operating characteristic analysis revealed an area under the curve (AUC) of 0.926 (95%CI: 0.8889-0.9675) in the training set. The optimal cutoff value is 0.73, with a sensitivity of 88.4 and specificity of 85.2. In the validation set, the AUC of the model for predicting enteral nutrition aspiration in patients with AP patients during hospitalization was 0.902, with a standard error of 0.040 (95%CI: 0.8284-0.9858), and the best cutoff value was 0.73, with a sensitivity of 91.9 and specificity of 81.8.
A prediction model for enteral nutrition aspiration during hospitalization in patients with AP was established and demonstrated high predictive value. Further clinical application of the model is warranted.
Core Tip: The study aims to establish and verify a predictive model for the risk of enteral nutrition aspiration during hospitalization in patients with acute pancreatitis. By comprehensively analyzing patient clinical data, independent risk factors are identified to provide early risk warning, guide clinical nursing intervention, and reduce the incidence of complications. Rate, improve patient prognosis.
- Citation: Hou P, Wu HJ, Li T, Liu JB, Zhao QQ, Zhao HJ, Liu ZM. Prediction model establishment and validation for enteral nutrition aspiration during hospitalization in patients with acute pancreatitis. World J Gastrointest Surg 2024; 16(8): 2583-2591
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2583.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2583
Acute pancreatitis (AP) is a disease caused by abnormal activation of pancreatic enzymes and can lead to self-digestion of pancreatic tissues and dysfunction of other organs. AP usually occurs in adults, with an annual incidence rate of 5/100000 to 30/100000, and the incidence rate is increasing every year[1]. The main inducing factors of AP include cho
A retrospective review was conducted on 200 patients diagnosed with AP and admitted to Chengdu Shangjin Nanfu Hospital, West China Hospital of Sichuan University from January 2020 to February 2024. The inclusion criteria were as follows: (1) All patients who met the clinical diagnosis of AP; (2) Patients who received enteral nutrition for more than one week; and (3) Patients with complete clinical data. The exclusion criteria included the following: (1) Patients with other intestinal diseases; (2) Patients with mental disorders; and (3) Patients with contraindications to nasogastric feeding. This study was approved by the Medical Ethics Committee of West China Hospital, Sichuan University (No. 2023-1702), and patient informed consent was exempted.
Clinical data were collected from the electronic medical record system, and patients were randomly divided into a validation group (n = 40) and a modeling group (n = 160) in a 1:4 ratio, matched with 200 patients from the same time period. The modeling group was further categorized into an aspiration group (n = 25) and a non-aspiration group (n = 135) based on the occurrence of enteral nutrition aspiration during hospitalization. Patients were observed for one month after treatment and hospitalization, and the criteria for aspiration judgment were as follows: (1) Typical clinical manifestations, such as coughing, rapid breathing, and increased heart rate during enteral nutrition; (2) Residual enteral nutrition fluid in the patient’s oral and nasal cavity or enteral nutrition fluid found in sputum after suctioning; (3) Potential of hydrogen (pH) value monitoring of respiratory secretions, with pH less than 7; and (4) Bronchoscopy examination showing the presence of gastric contents in the patient’s respiratory tract. Any of the above criteria was considered as aspiration.
(1) The general information of patients in the validation and modeling groups was compared; (2) Univariate analysis of factors affecting enteral nutrition aspiration in patients with AP during hospitalization was conducted in the modeling group; (3) Multivariate logistic regression analysis was performed for factors affecting enteral nutrition aspiration in patients with AP during hospitalization; (4) A prediction model was established for enteral nutrition aspiration in patients with AP during hospitalization and validated with a calibration curve; and (5) The predictive value of the model was analyzed using the receiver operating characteristic (ROC) curve, and area under the curve (AUC), sensitivity, and specificity were obtained.
Experimental data collected were analyzed using SPSS 27.0. Normally distributed metric data in the experimental data were represented as “mean ± SD” and independent sample one-tailed t-test was used for comparison. Count data were represented as the number of cases or rates, and χ2 test or Fisher’s exact test was used for comparison. Univariate and binary logistic regression analyses were conducted to evaluate factors affecting enteral nutrition aspiration in patients with AP during hospitalization. ROC curve was used to evaluate the predictive value of the model for enteral nutrition aspiration in patients with AP during hospitalization, and a difference with P < 0.05 was considered statistically significant.
No statistically significant difference was found in general information between the two groups of patients (P > 0.05, Table 1).
| Validation (n = 40) | Modeling (n = 160) | t/χ2 | P value | ||
| Age (years) | 46.22 ± 5.24 | 46.18 ± 5.27 | 0.043 | 0.966 | |
| Gender | Male | 24 | 88 | 0.325 | 0.850 |
| Female | 16 | 72 | |||
| BMI (kg/m2) | 21.17 ± 2.36 | 21.21 ± 2.13 | 0.104 | 0.917 | |
| Position | Active | 20 | 84 | 0.080 | 0.961 |
| Passive | 20 | 76 | |||
| Consciousness status | Alert | 33 | 130 | 0.149 | 0.985 |
| Disturbance | 6 | 24 | |||
| Coma | 1 | 6 | |||
| Nutritional risk | Low | 33 | 127 | 0.195 | 0.907 |
| High | 7 | 33 | |||
| AP ACHE-II score | 15.19 ± 3.16 | 15.37 ± 2.45 | 0.391 | 0.696 | |
| Length of nasogastric tube placement (cm) | 47.22 ± 2.05 | 47.56 ± 2.18 | 0.892 | 0.373 | |
| Smoking history | Yes | 20 | 81 | 0.005 | 0.998 |
| No | 20 | 79 | |||
| Hypertension | Yes | 18 | 73 | 0.005 | 0.998 |
| No | 22 | 87 | |||
| Diabetes | Yes | 8 | 28 | 0.136 | 0.935 |
| No | 32 | 132 |
Age, gender, body mass index (BMI), smoking history, hypertension history, and diabetes history showed no statistically significant difference between the two groups of patients (P > 0.05). However, body position, consciousness status, nutritional risk, Acute Physiology and Chronic Health Evaluation (APACHE-II) score, and nasogastric tube insertion length were statistically significantly different (P < 0.05, Table 2).
| Aspiration group (n = 25) | Non-aspiration group (n = 135) | t/χ2 | P value | ||
| Age (years) | 46.16 ± 5.13 | 46.27 ± 5.50 | 0.093 | 0.926 | |
| Gender | Male | 13 | 75 | 0.108 | 0.948 |
| Female | 12 | 60 | |||
| BMI (kg/m2) | 21.16 ± 2.23 | 21.22 ± 1.61 | 0.160 | 0.873 | |
| Position | Active | 19 | 65 | 6.562 | 0.038 |
| Passive | 6 | 70 | |||
| Consciousness status | Alert | 8 | 122 | 56.281 | < 0.001 |
| Disturbance | 16 | 8 | |||
| Coma | 1 | 5 | |||
| Nutritional risk | Low | 5 | 122 | 63.810 | < 0.001 |
| High | 20 | 13 | |||
| AP ACHE-II score | 17.46 ± 2.25 | 13.54 ± 2.16 | 8.282 | < 0.001 | |
| Length of nasogastric tube placement (cm) | 52.16 ± 2.21 | 45.16 ± 2.23 | 14.436 | < 0.001 | |
| Smoking history | Yes | 12 | 69 | 0.082 | 0.960 |
| No | 13 | 66 | |||
| Hypertension | Yes | 10 | 63 | 0.378 | 0.828 |
| No | 15 | 72 | |||
| Diabetes | Yes | 6 | 22 | 0.867 | 0.648 |
| No | 19 | 113 |
Body position, consciousness status, nutritional risk, APACHE-II score, and nasogastric tube insertion length were assigned values (actual values were substituted) as independent variables, and enteral nutrition aspiration was used as the dependent variable (aspiration = 1, non-aspiration = 0) for analysis. The results of multivariate logistic regression analysis showed that body position, consciousness status, nutritional risk, APACHE-II score, and nasogastric tube insertion length were independent factors affecting enteral nutrition aspiration in AP patients during hospitalization (P < 0.05), as shown in Table 3.
| Risk factors | β | SE | Ward | OR | 95%CI | P value |
| Position | 1.152 | 0.445 | 6.703 | 3.165 | 1.323-7.571 | < 0.001 |
| Consciousness status | 0.903 | 0.322 | 7.871 | 2.468 | 1.313-4.639 | < 0.001 |
| Nutritional risk | 1.243 | 0.484 | 6.593 | 3.465 | 1.342-8.947 | < 0.001 |
| APACHE-II score | 1.072 | 0.362 | 8.768 | 2.921 | 1.437-5.938 | < 0.001 |
| Length of nasogastric tube placement (cm) | 1.179 | 0.418 | 7.955 | 3.251 | 1.433-7.276 | < 0.001 |
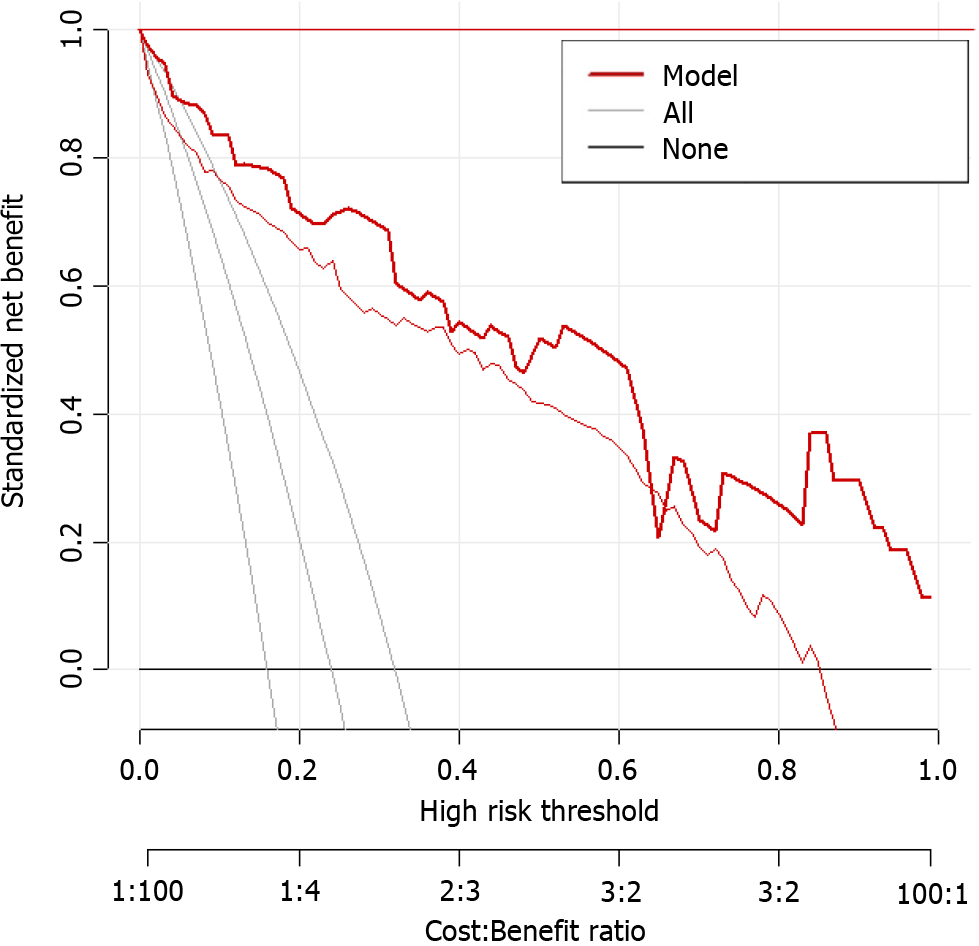
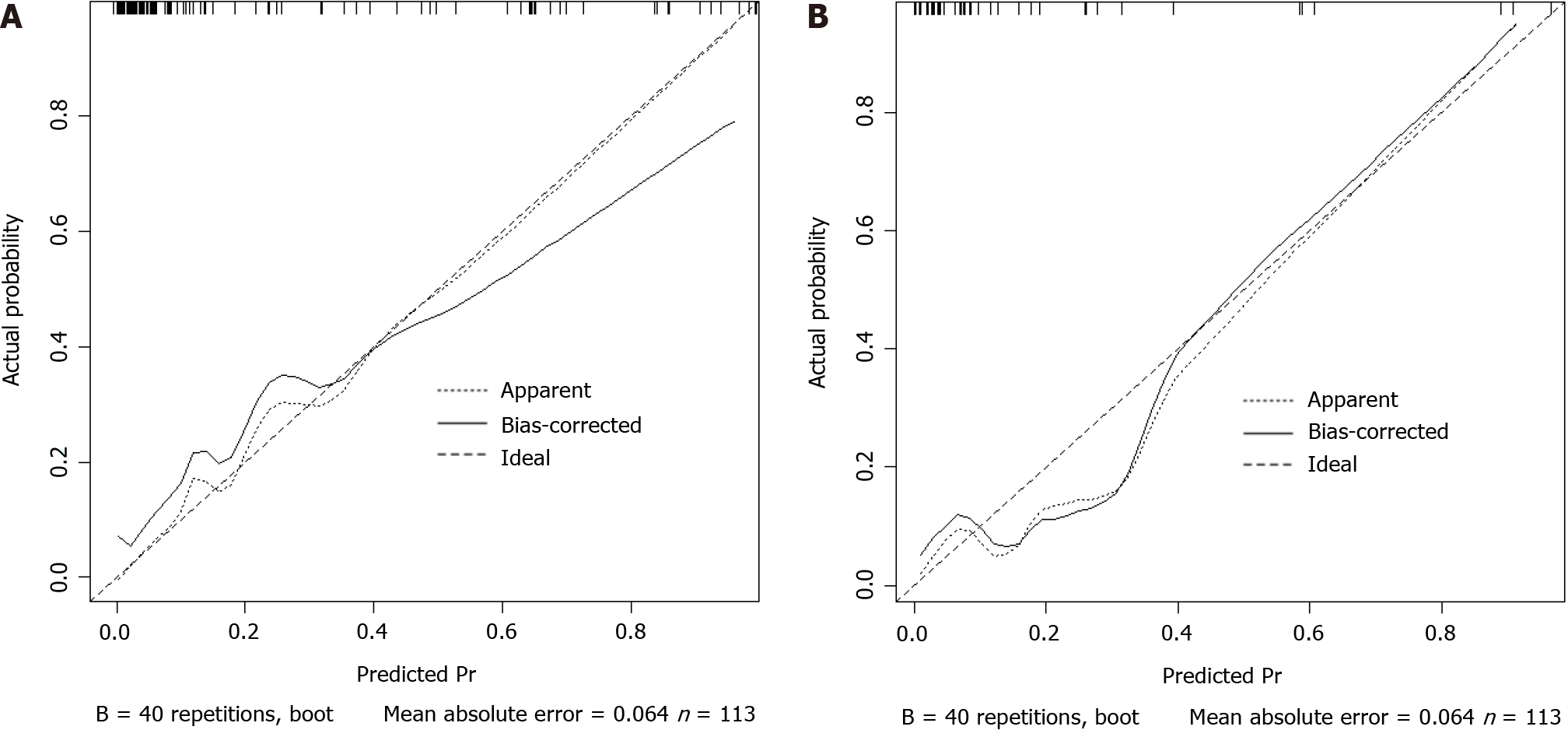
Based on the results of logistic regression analysis, body position, consciousness status, nutritional risk, APACHE-II score, and nasogastric tube insertion length were included in the prediction model for enteral nutrition aspiration in patients with AP during hospitalization (Figure 1). The calibration curve slope of this model was close to 1 in the training and validation sets, indicating good consistency between the predicted and actual risks of aspiration (Figure 2).
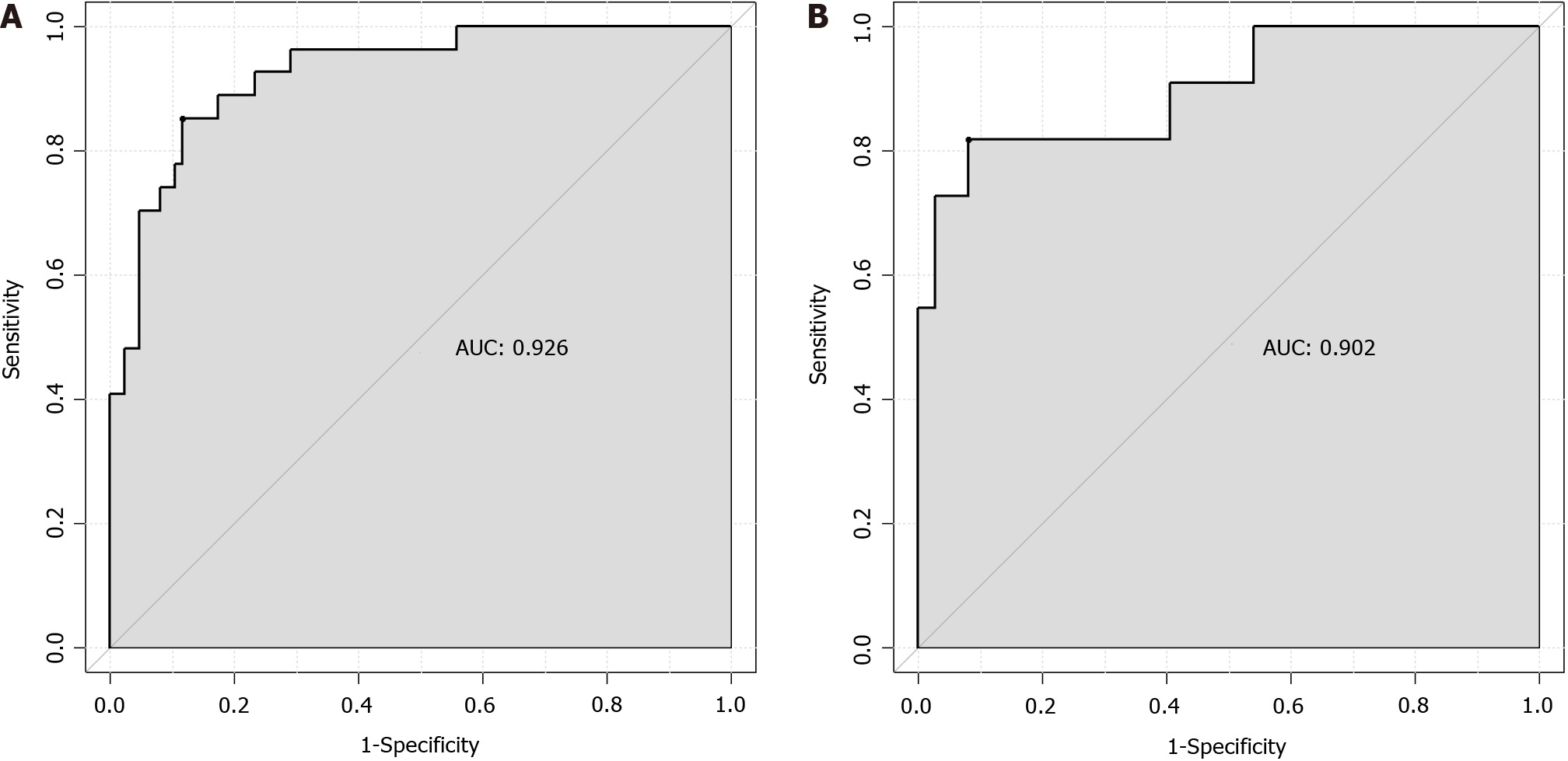
The ROC analysis results showed that the AUC of the prediction model for enteral nutrition aspiration in patients with AP during hospitalization was 0.926 (standard error = 0.034, 95%CI: 0.8889-0.9675) in the training set, with a best cutoff value of 0.73, sensitivity of 88.4, and specificity of 85.2 (Figure 3A). In the validation set, the AUC of the prediction model was 0.902 (standard error = 0.040, 95%CI: 0.8284-0.9858), with a best cutoff value of 0.73, sensitivity of 91.9, and specificity of 81.8 (Figure 3B).
Enteral nutrition aspiration during AP hospitalization is a common clinical problem that not only increases the difficulty of treatment but also prolongs hospital stay[8]. Aspiration of enteral nutrition has significant adverse effects on patients, including risk of serious complications, such as pulmonary infections and aspiration pneumonia, which can threaten the patient’s life. Aspiration can affect patient’s nutritional intake, leading to deterioration in their nutritional status and affecting the recovery process[9,10]. Furthermore, aspiration can cause psychological stress for patients, reducing their treatment confidence and quality of life. The present study investigated this issue and established a prediction model to provide clinical reference.
The results showed no statistically significant difference in age, gender, BMI, smoking history, hypertension history, or diabetes history between the two groups of patients (P > 0.05). However, statistically significant differences were found in body position, consciousness status, nutritional risk, APACHE-II score, and nasogastric tube insertion length between the two groups (P < 0.05). Further analysis using multivariate logistic regression analysis showed that body position, consciousness status, nutritional risk, APACHE-II score, and nasogastric tube insertion length were independent risk factors for enteral nutrition aspiration in patients with AP during hospitalization (P < 0.05). This finding indicates the influence of body position, consciousness status, nutritional risk, APACHE-II score, and nasogastric tube insertion length on aspiration in patients with AP. During AP treatment, when patients are in an active position, they may change position or move their bodies frequently, which may cause the nasogastric tube to shift. If the nasogastric tube shifts or becomes loose, then nutritional fluid may reflux into the esophagus or respiratory tract, thereby increasing the risk of aspiration[11]. In addition, an active position may reduce the coordination between a patient’s breathing and swallowing actions and nasogastric tube operation[12]. For example, during a change in position, a patient may not be able to effec
Based on the results of the logistic regression analysis, body position, consciousness status, nutritional risk, APACHE-II score, and nasogastric tube insertion length were determined to be the key factors for constructing a prediction model for enteral nutrition aspiration in patients with AP during hospitalization. These factors not only independently affect the risk of aspiration but also provide abundant information for the construction of the model regarding their interactions. After incorporating these factors into the model, this study established a tool that can accurately predict the risk of aspiration. The analysis of the calibration curve showed that that the slope of the curve was close to 1, which means that the predicted risk of aspiration by the model is highly consistent with the observed risk. This result fully demonstrates the effectiveness and reliability of the model. Further ROC analysis showed that the AUC of the model for predicting enteral nutrition aspiration in patients with AP during hospitalization was 0.926 in the training set, with a standard error of 0.034 (95%CI: 0.8889-0.9675); the best cut-off value was 0.73, with a sensitivity of 88.4 and a specificity of 85.2. In the validation set, the AUC of the model for predicting enteral nutrition aspiration in patients with AP during hospitalization was 0.902, with a standard error of 0.040 (95%CI: 0.8284-0.9858); the best cutoff value was 0.73, with a sensitivity of 91.9 and a specificity of 81.8.
Although the model established in this study has good predictive value, it still has limitations. The construction of the model is mainly based on the currently available clinical data and variables, which may not fully represent all the factors that affect the risk of aspiration. Other potential influencing factors may not have been included in the model, so it may have a certain impact on the predictive results. Therefore, the model needs further improvement and refinement. By continuously collecting data, optimizing the model algorithm, and adjusting it in combination with clinical practice, a more accurate, stable, and practical prediction model can be developed in the future, providing more powerful support for the treatment and recovery of patients with AP.
The prediction model established for enteral nutrition aspiration in patients with AP during hospitalization has high predictive value and can provide effective reference for clinical doctors. It can help identify high-risk patients in advance, develop personalized enteral nutrition plans, reduce the incidence of aspiration, and improve patient’s treatment effect and quality of life. Therefore, this model should be used and promoted in clinical practice.
| 1. | Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C, Jakob SM, Loudet CI, Malbrain ML, Montejo González JC, Paugam-Burtz C, Poeze M, Preiser JC, Singer P, van Zanten AR, De Waele J, Wendon J, Wernerman J, Whitehouse T, Wilmer A, Oudemans-van Straaten HM; ESICM Working Group on Gastrointestinal Function. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43:380-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 501] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 2. | Nally DM, Kelly EG, Clarke M, Ridgway P. Nasogastric nutrition is efficacious in severe acute pancreatitis: a systematic review and meta-analysis. Br J Nutr. 2014;112:1769-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Schneider H, Boyle N, McCluckie A, Beal R, Atkinson S. Acute severe pancreatitis and multiple organ failure: total parenteral nutrition is still required in a proportion of patients. Br J Surg. 2000;87:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 470] [Article Influence: 78.3] [Reference Citation Analysis (3)] |
| 5. | Ramai D, Morris JD, Fang J. Top Tips for Direct Percutaneous Endoscopic Jejunostomy (DPEJ) Tube Placement. Dig Dis Sci. 2024;69:1534-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 6. | Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C, Beyer G, Sutton R. Acute Pancreatitis: Diagnosis and Treatment. Drugs. 2022;82:1251-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 338] [Reference Citation Analysis (1)] |
| 7. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 622] [Article Influence: 124.4] [Reference Citation Analysis (1)] |
| 8. | De Lucia SS, Candelli M, Polito G, Maresca R, Mezza T, Schepis T, Pellegrino A, Zileri Dal Verme L, Nicoletti A, Franceschi F, Gasbarrini A, Nista EC. Nutrition in Acute Pancreatitis: From the Old Paradigm to the New Evidence. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Liu M, Gao C. A systematic review and meta-analysis of the effect of total parenteral nutrition and enteral nutrition on the prognosis of patients with acute pancreatitis. Ann Palliat Med. 2021;10:10779-10788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Ramírez-Maldonado E, López Gordo S, Pueyo EM, Sánchez-García A, Mayol S, González S, Elvira J, Memba R, Fondevila C, Jorba R. Immediate Oral Refeeding in Patients With Mild and Moderate Acute Pancreatitis: A Multicenter, Randomized Controlled Trial (PADI trial). Ann Surg. 2021;274:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Gopi S, Saraya A, Gunjan D. Nutrition in acute pancreatitis. World J Gastrointest Surg. 2023;15:534-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (6)] |
| 12. | Jin Z, Wang Z, Wang J. Early Enteral Nutrition Prevent Acute Pancreatitis From Deteriorating in Obese Patients. J Clin Gastroenterol. 2020;54:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Bukowski JS, Dembiński Ł, Dziekiewicz M, Banaszkiewicz A. Early Enteral Nutrition in Paediatric Acute Pancreatitis-A Review of Published Studies. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Marchetti J, Reis AMD, Santos AFD, Franzosi OS, Luft VC, Steemburgo T. High nutritional risk is associated with unfavorable outcomes in patients admitted to an intensive care unit. Rev Bras Ter Intensiva. 2019;31:326-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Wan Z, Liao D. Efficacy of enteral nutrition for patients with acute pancreatitis: A systematic review and meta-analysis of 17 studies. Exp Ther Med. 2023;25:184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Takada T, Isaji S, Mayumi T, Yoshida M, Takeyama Y, Itoi T, Sano K, Iizawa Y, Masamune A, Hirota M, Okamoto K, Inoue D, Kitamura N, Mori Y, Mukai S, Kiriyama S, Shirai K, Tsuchiya A, Higuchi R, Hirashita T. JPN clinical practice guidelines 2021 with easy-to-understand explanations for the management of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2022;29:1057-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 17. | Gaitanidis A, Breen K, Mendoza A, Fawley J, Lee J, Parks J, Kaafarani HMA, Velmahos G, Fagenholz PJ. Enteral nutrition is associated with high rates of pneumonia in intensive care unit (ICU) patients with acute pancreatitis. J Crit Care. 2022;69:154012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Engineering JOH. Retracted: Exploration of the Curative Effect of Early Enteral Nutrition Nursing on Patients with Severe Acute Pancreatitis and the Improvement of Patients' Mental Health and Inflammation Level. J Healthc Eng. 2023;2023:9895454. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/