Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2255
Revised: June 16, 2024
Accepted: July 1, 2024
Published online: July 27, 2024
Processing time: 88 Days and 1.7 Hours
Cost analyses of patients undergoing esophagectomy is valuable for identifying modifiable expenditure drivers to target and curtail costs while improving the quality of care. We aimed to define the cost-complication relationship after eso
To assess the relationship between the hospital costs and potential cost drivers post esophagectomy and investigate the relationship between the cost-driving variables (predicting variables) and hospital costs (dependent variable).
In this retrospective single center study, the severity of complications was graded using the Clavien-Dindo (CD) classification system. Key esophagectomy complications were categorized and defined according to consensus guidelines. Raw costing data included the in-hospital costs of the index admission and any unplanned admission within 30 postoperative days. We used correlation analysis to assess the relationship between key clinical variables and hospital costs (in United States dollars) to identify cost drivers. A mediation model was used to investigate the relationship between these variables and hospital costs.
A total of 110 patients underwent primary esophageal resection. The median admission cost was $47822.7 (interquartile range: 35670.2-68214.0). The total effects on costs were $13593.9 (95%CI: 10187.1-17000.8, P < 0.001) for each increase in CD severity grade, $4781 (95%CI: 3772.7-5789.3, P < 0.001) for each increase in the number of complications, and $42552.2 (95%CI: 8309-76795.4, P = 0.015) if a key esophagectomy complication developed. Key esophagectomy complications drove the costs directly by $11415.7 (95%CI: 992.5-21838.9, P = 0.032).
The severity and number of complications, and the development of key esophagectomy complications significantly contributed to total hospital costs. Continuous institutional initiatives and strategies are needed to enhance patient outcomes and minimize costs.
Core Tip: Our findings show that complications following esophagectomy are common, with most patients experiencing at least one complication, and over 40% of patients developing a major complication. Moreover, we have demonstrated that the severity, number of complications and the presence of esophagectomy key complications significantly contributed to total hospital costs. Reoperation, prolonged intensive care stay and hospital stay were major drivers of hospital costs. This study highlights the importance of a continuous institutional quality review to prevent and mitigate complications, and the need for improved intervention strategies to enhance patient outcomes and minimize costs.
- Citation: Buchholz V, Lee DK, Liu DS, Aly A, Barnett SA, Hazard R, Le P, Kioussis B, Muralidharan V, Weinberg L. Cost burden following esophagectomy: A single centre observational study. World J Gastrointest Surg 2024; 16(7): 2255-2269
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2255.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2255
Esophagectomy is a complex surgical procedure and the keystone of multimodal treatment for locally advanced esophageal cancer. It is associated with significantly high postoperative morbidity. The delivery of high-standard and innovative treatment, particularly in the context of esophagectomy, can drive steep increases in health expenditures, undermining the economic sustainability of cancer healthcare systems. Simultaneously, healthcare systems strive to maintain performance standards without compromising cancer treatment outcomes[1].
Postoperative complications are indicators of surgical quality and performance. Their effect on the surgical cost of care has been established in previous studies assessing the costs of major procedures[2-4]. Consequently, cost analysis of patients undergoing esophagectomy is valuable for identifying modifiable expenditure drivers to target and curtail costs while improving the quality of care.
Although a few studies have explored the economic effects of post-esophagectomy complications[5-8], the literature on this topic remains scarce and inconsistent in relation to data sourcing, definitions of complications, and severity grading. Therefore, we aimed to define the cost-complication relationship after esophagectomy and delineate the incremental contributions to costs.
This study was conducted at Austin Health, a university-affiliated tertiary referral center for upper gastrointestinal conditions. The Human Research Ethics Committee of Austin Hospital approved this retrospective observational study as a clinical audit, and the protocol was registered with the Australian New Zealand Clinical Trials Registry. Given that this was a retrospective observational audit, trial registration of this study was undertaken after ethics approval and data collection. There were no changes to the original study protocol at any stage. Data analysis was only undertaken after trial registration. The key timelines for the study are as follows: (1) September 23, 2019: Study protocol approved by the Austin Health Office for Human Research (approval No. Audit/19/Austin/103); (2) October 10, 2019: Data collection following ethics approval; (3) November 19, 2020: Data collection completed; and (4) December 22, 2020: Retrospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12620001377921). The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies[9,10].
We included patients aged ≥ 18 years who underwent esophagectomy between January 2010 and December 2019. Patients were identified using the International Statistical Classification of Diseases and Related Health Problems 10th Revision and codes specific to esophagectomy. Surgical procedures consisted of two- or three-stage esophagectomy performed using an open, laparoscopic, or hybrid approach for esophageal cancer, benign tumors, and motility disorders. All surgical procedures were performed by eight surgeons from the upper gastrointestinal and thoracic surgery units.
All patients, independent of the treating surgical unit, underwent an enhanced recovery after surgery (ERAS) program aligned with international guidelines[11], which included a comprehensive pre-optimization program for smoking and alcohol cessation. As part of our institution’s diabetes discovery initiative, all patients with an HbA1c level of 8.3% (67 mmol/mol) had a personalized plan for glycemia and were managed according to the hospital’s perioperative guidelines for patients with diabetes, with an inpatient blood glucose target of 5-10 mmol/L based on the Australian Diabetes Society guidelines. All participants underwent a comprehensive multidisciplinary assessment, with optimization of nutrition, medical comorbidities, and hemoglobin levels, based on the National Blood Authority of Australia’s Patient Blood Management Initiative[12]. Standard perioperative care included strict transfusion practices following these guidelines. General anesthesia was managed using an ERAS protocol designed to standardize care.
Postoperatively, all patients were admitted to the intensive care unit (ICU) for at least one overnight stay and discharged to a dedicated surgical ward by a multidisciplinary team of surgeons, anesthetists, perioperative physicians, and pain clinicians. Analgesia was optimized by an acute pain service that reviewed all patients twice daily.
All data were sourced directly by the authors using prospectively recorded patient variables from the hospital’s electronic health records (Cerner Millennium, KS, United States). Preoperative patient parameters included demographic information, body mass index, history of smoking and alcohol abuse, American Society of Anesthesiologists (ASA) score, comorbidities, Carlson comorbidity index (CCI), history of previous abdominal or thoracic surgery, preoperative blood values, pathological diagnosis, and neoadjuvant treatment.
Intraoperative parameters included the type of surgery (open or minimally invasive laparoscopy & thoracoscopy), the surgical approach (transthoracic, 3-stage or transhiatal), the operative time, the volume of transfused crystalloids, colloids, and blood products, the use of vasoactive medications, and the intraoperative complications. Postoperative variables included ICU admission and care duration, postoperative blood values, blood product transfusion, histopathology, American Joint Committee on Cancer pathologic stage group (8th edition)[13], length of hospital stay, discharge destination, and readmissions (30 days, 90 days, and one year). Postoperative complications were derived directly from patient files.
The severity of complications was graded using the Clavien-Dindo (CD) classification system[14]. Major complications were defined as CD III-IV. Key complications of esophagectomy (anastomotic leak, conduit necrosis, chyle leak, and vocal cord palsy) were categorized and defined according to the Esophagectomy Complications Consensus Group (ECCG) definitions[15]. Raw costing data were provided by the hospital’s clinical informatics and costing center and included the in-hospital costs of the index admission under the surgical service and the costs of any unplanned admission within 30 days. The clinical-based cost buckets included anesthesia, ICU, medical emergency team call, operating theater (including endoscopy), allied health, pharmacy, radiology, pathology, medical consult, blood product, and ward costs (e.g., costs of the hospital bed, nursing, and catering). Costs were inflated to June 30, 2022 values based on the end-of-fiscal-quarter Australian Consumer Price Index and converted to United States dollars (USD) based on the market rate on June 30, 2022.
Statistical analysis was performed using the R software (version 4.2.1; 2022, R Core Team, Vienna, Austria). The normality of continuous variables was evaluated using the Shapiro-Wilk test and a visual check of the Q-Q plot. Data are presented as mean ± SD, median [interquartile range (IQR)], (minimum, maximum), or number (percentile). An unadjusted cost analysis was performed using Wilcoxon and Kruskal-Wallis rank-sum tests. Pairwise comparisons using the Wilcoxon rank-sum test with continuity correction were also performed. The P value was adjusted using the method of Benjamini and Hochberg during multiple pairwise comparisons.
Correlation analysis was used to assess the relationship between the measured variables and hospital costs and identify potential cost drivers. The correlations among the likely cost drivers were visualized as a correlation matrix (R package “Performance Analytics,” ver. 2.0.4)[16]. The complex relationship between the hospital cost and measured variables was investigated using a correlation data frame network plot with various coefficient limits and the incorporated function of R package “corrr” ver. 0.4.4[17].
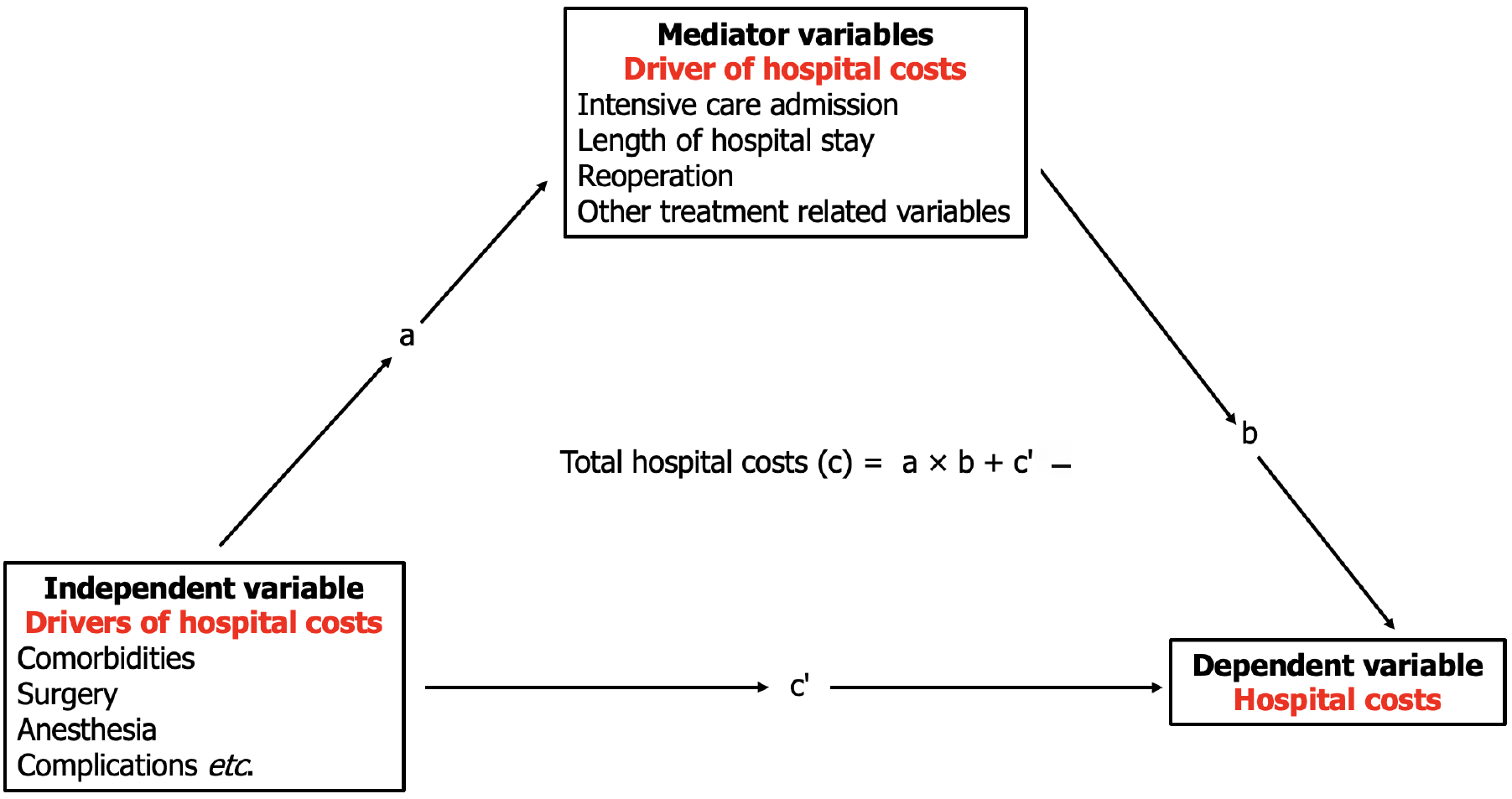
We used a mediation model to investigate the relationship between the cost-driving variables (predicting variables) and hospital costs (dependent variable). The model clarifies situations where a total exposure-outcome effect is identified, but a direct causal effect between the predictor and dependent variables is not apparent. The mediation model suggests that the mediating variable transmits the effect of the predicting variable on the dependent variable[18,19]. The mediator variable’s transmittance effect (indirect effect) is then quantified and can be complete or partial (Figure 1).
Multiple mediation effect analyses were performed to evaluate the direct and indirect effects of the severity and number of complications and the presence of key esophagectomy complications on hospital costs using generalized linear models (R package “mma” ver. 10.6-1)[20]. The 95%CI of the estimated effects were calculated using the nonparametric bootstrap method with 1000 repetitions. The expected mortality was estimated using Kaplan-Meier survival analysis. Statistical significance was determined using a two-sided P value below 0.05.
A total of 110 patients underwent primary esophageal resection for benign and malignant diseases during the study period. Baseline patient characteristics are summarized in Table 1. The study population was predominantly male (83%), with a mean age of 64.5 years. The mean CCI score was 4.4. Eighty (72.7%) patients were current or past smokers with an average smoking history of 20 pack years. Ninety-four patients (85.4%) underwent open surgery, and 16 (14.5%) underwent hybrid or minimally invasive esophagectomy. The most prevalent procedure was the two-stage esopha
| Variable | n = 110 |
| Demographics | |
| Sex | |
| Male | 91 (82.7) |
| Female | 19 (17.3) |
| Age (years) | 64.47 ± 9.694 |
| Body mass index (kg/m2) | 27.0 ± 4.9 |
| Smoking | |
| Never | 30 (27.3) |
| Active | 12 (10.9) |
| Quit < 6 weeks prior to surgery | 5 (4.5) |
| Quit 6 weeks to 90 days prior to surgery | 4 (3.6) |
| Quit > 90 days prior to surgery | 59 (53.6) |
| Pack year history | 20 (0-40) |
| Alcohol consumption > 4 standard drinks | 13 (11.8) |
| Risk classification | |
| ASA | |
| 1 | 2 (1.8) |
| 2 | 35 (31.8) |
| 3 | 68 (61.8) |
| 4 | 5 (4.5) |
| ECOG | |
| 0 | 78 (70.9) |
| 1 | 28 (25.5) |
| 2 | 3 (2.7) |
| ACCI (median) | 4 (3-5) |
| Comorbidities | |
| Coronary artery disease | 3 (2.7) |
| Myocardial infarction | 6 (5.5) |
| Congestive heart failure | 1 (0.9) |
| Peripheral vascular disease | 7 (6.4) |
| Cerebrovascular accident | 5 (4.5) |
| Chronic pulmonary disease | 11 (10) |
| Diabetes mellitus (uncomplicated) | 13 (11.8) |
| Diabetes mellitus (end-organ damage) | 2 (1.8) |
| Moderate to severe renal disease | 1 (0.9) |
| Synchronous malignancy (solid tumor) | 2 (1.8) |
| Past malignancy | 16 (14.5) |
| Previous laparotomy | 13 (11.8) |
| Previous thoracotomy | 8 (7.3) |
| Previous hiatal operation | 4 (3.6) |
| Laboratory tests | |
| Hemoglobin (g/L) | 132 (90, 176) |
| White cell ( 109/L) | 6.6 (3, 13) |
| Platelet ( 109/L) | 231.5 (110, 541) |
| Creatinine (mmol/L) | 79.5 (44, 72) |
| eGFR (mL/minute/1.73 m2) | 87 (33, 91) |
| Albumin (g/L) | 38 (27, 44) |
| Principal diagnosis (indication for surgery) | |
| Malignant | 104 (94.5) |
| Benign | 6 (5.5) |
| Surgical approach | |
| Open | 94 (85.4) |
| Minimally invasive (laparoscopy & thoracoscopy) | 3 (2.7) |
| Hybrid (chest or abdomen) | 13 (11.8) |
| Conversion to open | 5 (4.5) |
| Anastomosis site | |
| Chest | 68 (61.8) |
| Neck | 42 (38.2) |
| Esophageal conduit | |
| Stomach | 107 (97.3) |
| Colon | 3 (2.7) |
| AJCC staging (8th edition) | |
| I | 36 (32.7) |
| II | 14 (12.7) |
| IIIA | 10 (9) |
| IIIB | 27 (24.5) |
| IVA | 13 (11.8) |
| IVB | 3 (2.7) |
| Resection margin | |
| R0: Negative | 97 (88.1) |
| R1: Microscopic positive | 10 (9) |
| R2: Macroscopic positive | 1 (0.9) |
| Admission details | |
| ICU length of stay (days) n = 108 | 2.7 (1.6-6.3) |
| HDU length of stay (days) n = 17 | 0.5 (0.4-0.7) |
| Length of hospital stay | 18 (13-27) |
| Discharge destination | |
| Home | 84 (76.4) |
| Hospital at home | 5 (4.5) |
| Rehabilitation facility/subacute care | 19 (17.3) |
| Death | 2 (1.8) |
| Readmission | |
| 30-day readmission | 26 (23.6) |
| 90-day readmission | 47 (42.7) |
Of the 104 patients (94.5%) who underwent surgery for esophageal malignancy, 76 (69%) received neoadjuvant chemotherapy or chemoradiotherapy before the operation, and four (3.6%) underwent salvage esophagectomy more than 12 months after completing definitive chemoradiotherapy. The median length of stay in the ICU was 2.7 days (1.6-6.3), and the median length of hospital stay was 18 days (13-27). In total, 89 patients (80%) were discharged home, and 19 patients (17.3%) were transferred to a rehabilitation or nursing facility.
In total, 658 complications were recorded. All but two patients had at least one complication. Sixty-two (56.3%) patients had minor complications, while 46 (41.8%) experienced major complications (Table 2). Two patients died during the index admission (1.8%). A detailed breakdown of complications is summarized in the Supplementary Table 2. The most common complications were electrolyte imbalance (n = 93, 84.5%), hypotension requiring intervention (n = 67, 60.9%), atrial fibrillation (n = 37, 33.6%), pneumonia (n = 36, 32.7%), and anemia requiring transfusion (n = 31, 28.2%). Key complications of esophagectomy (anastomotic leak, conduit necrosis, chyle leak, and recurrent nerve palsy) occurred in 47 (42.7%) patients. Twenty patients (18%) experienced an anastomotic leak, with severity varying from CD-II to CD-IVb. Three patients experienced conduit necrosis; all three required surgery, and one required diversion surgery (Table 3).
| Clavien-Dindo highest grade | n = 110 |
| None | 2 (1.8) |
| I | 4 (3.6) |
| II | 58 (52.7) |
| IIIa | 9 (8.2) |
| IIIb | 13 (11.8) |
| IVa | 19 (17.3) |
| IVb | 3 (2.7) |
| V | 2 (1.8) |
| Number of complications per patient | |
| 0-2 | 10 (9) |
| 3-6 | 58 (52.7) |
| > 7 | 42 (38.1) |
| Complications per patient (mean SD) | 6.0 ± 2.9 |
| Complications | Grade | n = 110 |
| Anastomotic leak: Full-thickness GI defect involving esophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification | Type I: Local defect requiring no change in therapy or treated medically or with dietary modification | 11 (10.0) |
| Type II: Localized defect requiring interventional but not surgical therapy | 5 (4.5) | |
| Type III: Localized defect requiring surgical therapy | 4 (3.64) | |
| Subtotal | 20 (18.2) | |
| Conduit necrosis/failure: Postoperative identification of conduit necrosis | Type I: Focal conduit necrosis identified endoscopically requiring monitoring or non-surgical therapy | 0 (0) |
| Type II: Focal conduit necrosis focal identified endoscopically and not associated with free anastomotic or conduit leak, requiring surgical therapy without esophageal diversion | 2 (1.8) | |
| Type III: Conduit necrosis extensive requiring with conduit resection with diversion | 1 (0.9) | |
| Subtotal | 3 (2.7) | |
| Chyle leak: Milky discharge upon initiation of enteric feeds and/or pleural fluid analysis demonstrating triglyceride level > 100 mg/dL and/or chylomicrons in pleural fluid | Type Ia: < 1 L output, Treatment-enteric dietary 3 modifications | 3 (2.7) |
| Type Ib: > 1 L output, treated with enteric dietary modifications | 0 (0) | |
| Type IIa: < 1 L output, treated with total parenteral nutrition | 1 (0.9) | |
| Type IIb: > 1 L output, treated with total parenteral nutrition | 0 (0) | |
| Type IIIa: < 1 L output, treated with interventional or surgical therapy | 2 (1.8) | |
| Type IIIb: > 1 L output, treated with interventional or surgical therapy | 5 (4.5) | |
| Subtotal | 11 (10) | |
| Type Ia: Unilateral injury transient injury requiring no therapy (dietary modification aloud) | 5 (4.5) | |
| Type Ib: Bilateral injury transient injury requiring no therapy (dietary modification aloud) | 0 (0) | |
| Type IIa: Unilateral injury requiring elective surgical procedure, for example thyroplasty or medialization procedure | 3 (2.7) | |
| Type IIb: Unilateral injury requiring elective surgical procedure for example thyroplasty or medialization procedure | 0 (0) | |
| Type IIIa: Unilateral injury requiring acute surgical intervention (due to aspiration or respiratory issues), for example, thyroplasty or medialization procedure | 2 (1.8) | |
| Type IIIb: Bilateral Injury requiring acute surgical intervention (due to aspiration or respiratory issues), for example, thyroplasty or medialization procedure | 3 (2.7) | |
| Subtotal | 13 (11.8) | |
| Total | 47 (42.7) |
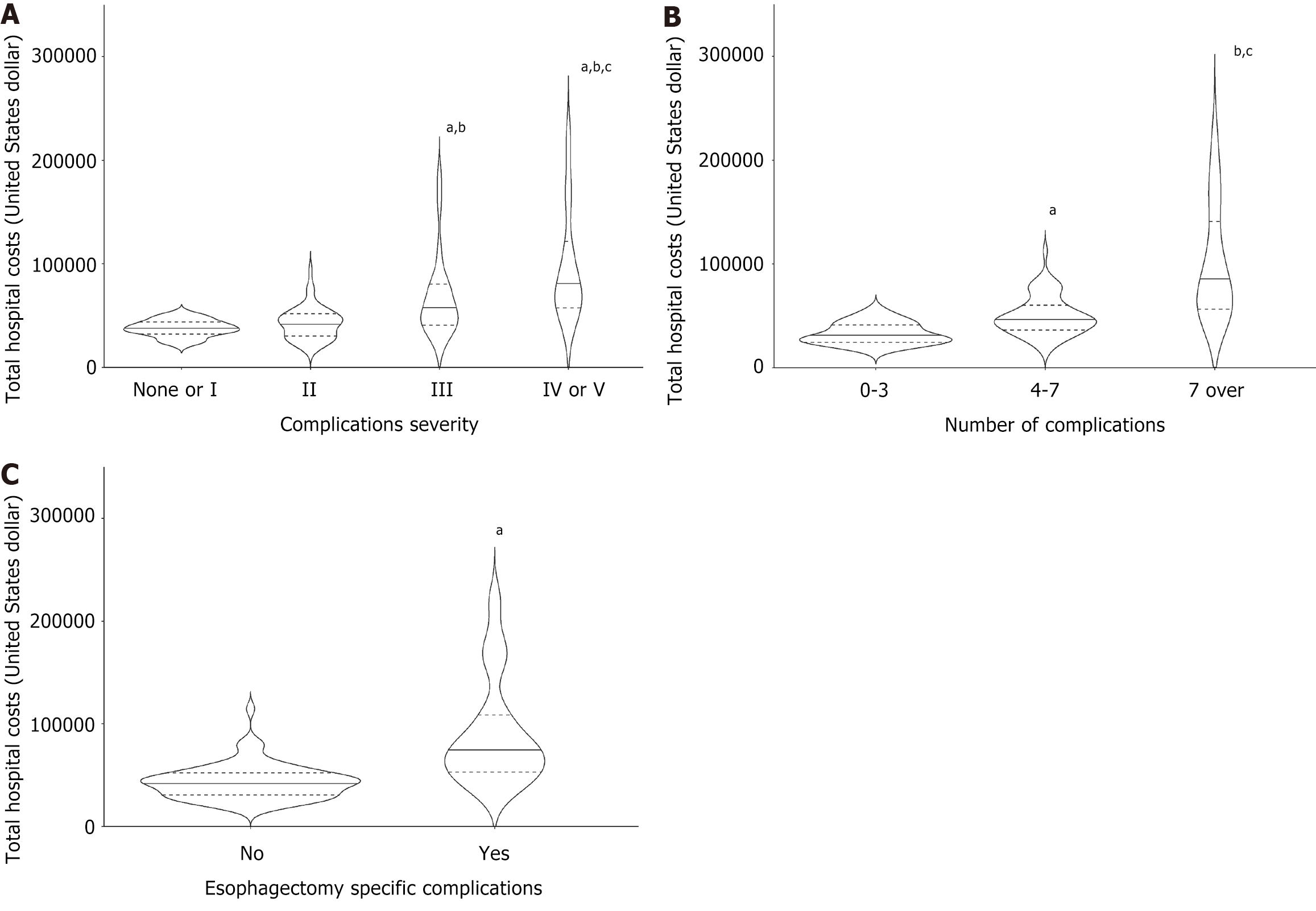
The median admission cost was USD 47822.7 (IQR 35670.2-68214.0). The highest expenditures were for the ICU stay, the operating theater, and ward care (Supplementary Table 3). An unadjusted analysis of complications and hospital costs demonstrated a significant association between the severity of complications and cost increments, with cost increments increasing as the CD severity grade advanced. For example, hospital costs doubled for patients with CD grades IV-V compared with patients without complications or with CD grade I (Figure 2A and Table 4).
| Median cost USD (IQR) | |
| CD grade | |
| No complication or CD I | 37427.94 (34277.96-42283.38) |
| CD II | 43174.77 (29114.99-49366.61) |
| CD III | 54454.79 (43421.93-78679.18)a,b |
| CD IV & V | 76 063.38 (61 579.73-114 664.51)a,b,c |
| Number of complications | |
| 0-3 | 29629.58 (25592.76-41683.1) |
| 4-7 | 46666.14 (36410.33-58611.02)d |
| > 7 | 75516.55 (56456.86-136612.02)d,e |
| Esophagectomy key complication | |
| No | 42937.5 (29992.1-49629.0) |
| Yes | 75516.6 (53544.1-101343.0)f |
The number of complications per patient similarly influenced admission costs. The median admission cost for patients with seven or more complications of any grade was 2.5 times that for patients with 0-3 complications (Figure 2B and Table 4). Likewise, key complications of esophagectomy were associated with high additional costs. The median overall admission cost for patients who experienced any of the four key complications was USD 75517.0 compared to USD 42937.5 for patients without esophagectomy-specific complications (Figure 2C and Table 4).
Spearman’s correlation analysis was performed to identify the relationships between various perioperative parameters, complications, and total costs (Supplementary Table 4). The severity (CD grade) of complications, the number of complications, and the presence of esophagectomy key complications were moderately or highly related to the total hospital cost [Spearman’s correlation coefficient Ρ = 0.585, 0.670, and 0.576, respectively; P < 0.001, 0.001, and 0.004 (P < 0.001), respectively].
The duration of ICU or high-dependency unit (HDU) stay, length of hospital stay, emergency reoperation, ASA classification, CCI score, history of previous laparotomy, surgery time, postoperative lowest albumin level, postoperative creatinine level, total red blood cell units given during the admission, and readmission within 90 days after discharge were significantly correlated with the hospital cost as well as the CD severity grade and the number of complications. Using a network matrix of the correlation data frame, we identified several of the parameters listed above as potential mediator variables in the relationship between complications and costs (Supplementary Figure 1). These variables were related to each other over the hospital cost and complications. The visual checking of a network plot of a correlation data frame showed the possible mediation effects of several variables listed above from postoperative complications to the hospital cost (Supplementary Figure 2).
We first established that the complication severity grade, number of complications, and key esophagectomy complications were significant predictors of costs (linear regression coefficients: USD 19176, 95%CI: 12972.6-25379.4; USD 7648, 95%CI: 5809.5-9486.5; USD 40086, 95%CI: 28206.4-51965.6, respectively; all P < 0.001). These results imply that all three variables have direct, indirect, and combined effects on costs.
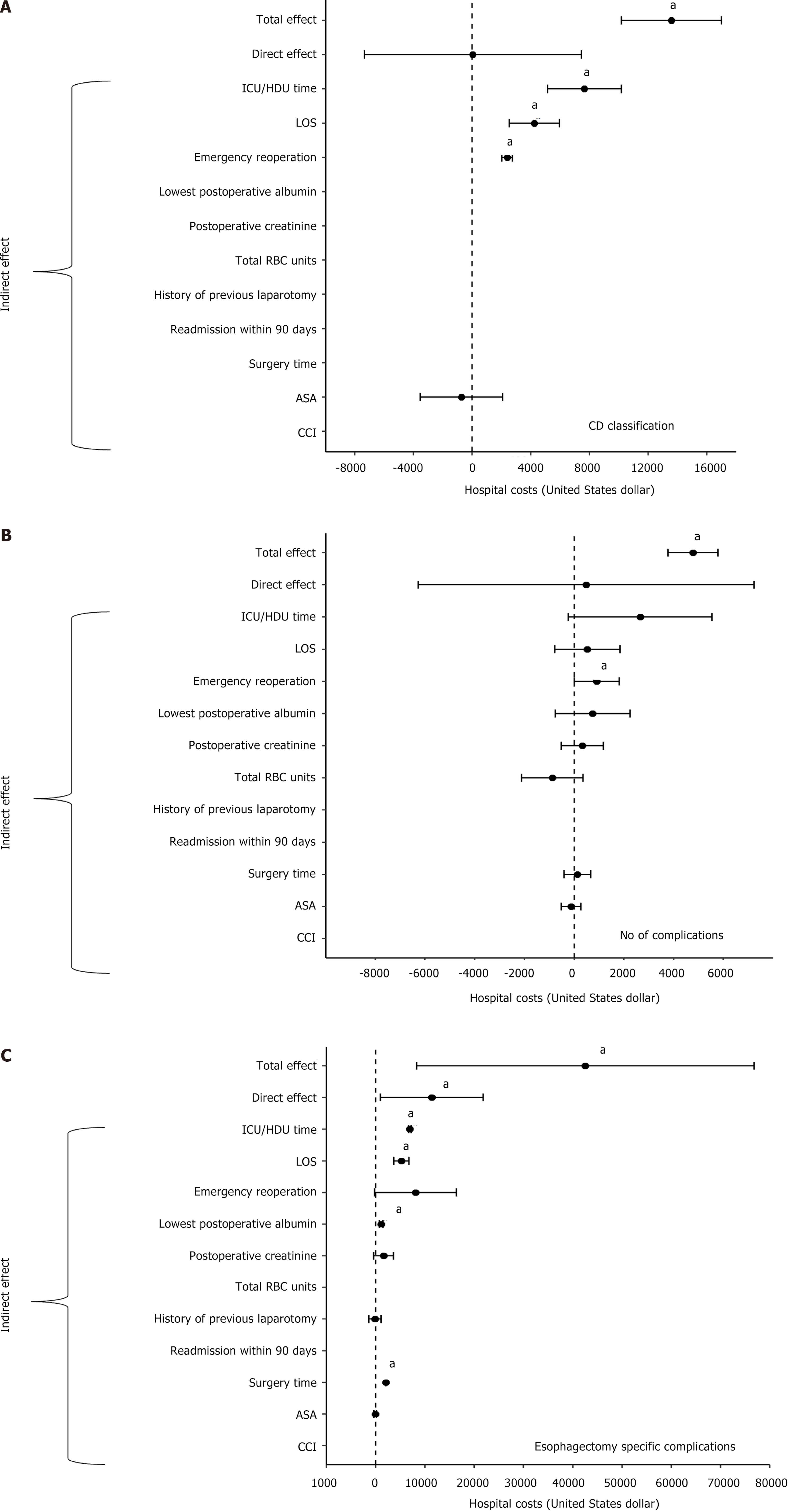
Multiple mediation effect analyses using the generalized linear model revealed that the complications’ CD severity grade (Figure 3A), the number of complications (Figure 3B), and the presence of esophagectomy key complications (Figure 3C) had significant total effects on admission cost (Supplementary Table 5). The total effects on costs were USD 13593.9 (95%CI: 10187.1-17000.8, P < 0.001) for each increase in CD severity grade, USD 4781 (95%CI: 3772.7-5789.3, P < 0.001) for each increase in the number of complications, and USD 42552.2 (95%CI: 8309.0-76795.4, P = 0.015) for key esophagectomy complications.
The severity of complications did not have a significant direct effect on costs (P = 0.991). The total effect was partially mediated by ICU/HDU stay time (USD 7658.9, 95%CI: 5130.3-10187.6, P < 0.001), length of hospital stay (USD 4239.6, 95%CI: 2520.2-5959.0, P < 0.001), and emergency reoperation (USD 2381.7, 95%CI: 2011.4-2752.0, P < 0.001). Similarly, the direct effect of the number of complications on costs was insignificant (P = 0.889), and the total effect of each complication number increase on costs was partially mediated by the emergency reoperation variable (USD 911.6, 95%CI: 6.4-1816.9, P = 0.048).
In contrast, key esophagectomy complications significantly drove the costs directly by USD 11415.7 (95%CI: 992.5-21838.9, P = 0.032). Additionally, the total effect on costs was partially mediated by ICU/HDU stay time (USD 6951, 95%CI: 6703.9-7198.2, P < 0.001), length of hospital stay (USD 5248.1, 95%CI: 3701.4-6794.7, P < 0.001), surgery time (USD 2056.6, 95%CI: 2045.7-2067.4, P < 0.001), and the postoperative lowest albumin level (USD 1122.4, 95%CI: 782.8-1462.0, P < 0.001; Figure 3C).
This study presents a detailed cost analysis of the postoperative costs associated with esophagectomy and demonstrates the economic burden of complications on hospital costs. We found a high incidence of complications following esophagectomy, with almost all patients experiencing at least one complication and over 40% developing a major complication. In addition, one-third of the patients experienced esophagectomy-specific complications, most of which required intervention. However, despite the high rate of complications, in-hospital mortality remained low at 1.8% and below international standards[21], indicating that esophagectomy is a safe procedure when performed in a specialized center.
Our study’s high overall complication rate likely reflects our meticulous perusal of patient medical records. We used a comprehensive rather than selective approach for data collection, including minor complications often neglected in other studies, and adhered strictly to the CD classification. The lack of consensus regarding the assessment of complications hampers the comparison of our results with those of previous studies. First, the studies followed a selected or elaborate repertoire of complications. For example, Carrott et al[22] tracked a list of 29 complications, whereas Low et al[21], in their multicenter benchmark study, followed a list of 48 different complications[21]. We tracked all the reported complications. Thus, we provided an accurate assessment of patients’ postoperative course.
Second, studies differ in their choice of complication severity grading system. Such severity grading systems include the accordion system[22], the Society of Thoracic Surgeons consensus guidelines[23], and the CD grading system[5-7,21]. We followed the widely used and validated therapy-oriented CD grading system in alignment with the international ECCG[15]. Lastly, the authors differed in their categorization of minor and major complications. While Goense et al[7]defined major complications as CD grade IIIb and above, we and others[5,6], considered CD grade IIIa the cutoff for major complications. Our major complication rate (41.8%) is similar to that reported in recent studies that applied the same grading system and severity criteria[5,11].
The total admission cost for esophagectomy at our institution is comparable to the cost data reported by other international centers[5,7,22]. We used an activity-based costing methodology to underline the proportional expense components. Operating theater, intensive care, and ward care were the top contributors to total costs. These findings align with those of previous publications[5-7], and reflect the surgical and anaesthetic complexity of the operation and the high level of intensive care and ward care support required following esophagectomy.
We found that hospital costs escalate with the increasing severity of complications. Most studies have used dichotomized cost analysis to compare the costs of minor and major complications. An exception is a study by Carrott et al[22], which explored the gradual cost increase for each accordion severity grade. We applied a similar high-resolution approach and demonstrated that cumulative costs increase with each CD severity grade. We showed that cost increments were profoundly higher as the severity grade advanced, with the most substantial cost addition noted between CD grades III and IV-V. Likewise, an increasing number of complications induced a marked cost escalation, particularly in patients with seven or more complications. The rise in cost associated with the increased number of complications has been explored in other major procedures[2-4], but has not yet been quantified in the context of esophagectomy.
We applied mediation analysis to expose the underlying mechanism by which severity and the number of complications (independent variables) drive costs (dependent variable)[18]. Mediation analysis decomposes the effect of the severity and number of complications into their direct effect on costs and the indirect effect through a mediator cost driver variable. Our results suggest that the severity of complications does not have a significant direct effect on total costs. Instead, the effect is indirect and mediated by ICU/HDU stay, length of hospital stay, and emergency reoperation. Similarly, the cumulative effect of each increase in the number of complications on total admission costs was indirectly mediated by emergency reoperation.
Several factors could explain our findings. First, the post-esophagectomy complication rate is high, and many patients experience major complications. Therefore, the cost increase is generated by the consecutive economic burden of the medical activity required for their treatment: Readmission to the ICU, reoperation, and prolonged hospital stay. Further, reoperation and intensive care were the most significant contributors to the overall cost, and as others showed previously, expenditure for both rose drastically in patients with major complications[9-11]. Finally, as demonstrated by Goense et al[7], the length of stay is significantly prolonged once a patient experiences a complication, leading to further expenses for multiple medical activities.
The documentation of four key complications is considered essential for quality and outcome monitoring in centers performing esophagectomy[15]. Each of the four complications was individually evaluated in previous cost analyses. Complications found to significantly increase costs (in both univariate and multivariate analyses) included anastomotic leak[7,22], chyle leak[7], and laryngeal nerve palsy[6]. Our study examined all four complications as a group. We illustrated the substantial contribution of esophagectomy-specific complications to total hospital costs, adding USD 46417 to the mean cost for patients without these key complications. Our mediation analysis confirmed that key esophagectomy complications directly increase the total costs while also partially driving costs through various mediator variables, mainly ICU/HDU time and length of hospital stay and, to a lesser extent, surgery time and lowest albumin levels. These findings indicate that preventing esophagectomy-specific complications is an important target when developing a cost-effective intervention strategy.
Our findings highlight the need to monitor and optimize the outcomes of patients undergoing esophageal resection. To achieve this objective, it is necessary to minimize the number of complications and mitigate their severity. Complications were linked to reduced quality of life and survival[6,22,24]. Therefore, targeting complications will diminish expenditure while promoting better short- and long-term outcomes. Proactive interventions to improve patients’ ability to withstand surgical stress and modify postoperative morbidity include smoking cessation, optimization of background comorbi
The benefits of ERAS programs in reducing morbidity are now established across multiple surgical procedures, including esophagectomy[26]. A meta-analysis from 2017 reviewing ERAS for esophagectomy showed reduced non-surgical complications and length of stay but not reduced surgical complications[27]. The ERAS Society Guidelines statement from 2019 addressed these issues and provided evidence-based recommendations for preoperative, operative, and postoperative care[11]. A recent study showed that the successful application of the revised ERAS reduced the number and severity of complications (both surgical and medical), the reoperation rate, and the length of stay. However, adherence to the protocol was most affected by postoperative complications[26]. The development of ERAS protocol modifications for patients with complications may help moderate their consequences.
Prevention and management of the four key esophagectomy complications are challenging. The optimization of surgical techniques can reduce their prevalence. In addition, high suspicion, early diagnosis, and prompt intervention can preclude patient decompensation[27]. Prevention and intervention strategies have been thoroughly discussed in the literature. Examples include avoidance or judicious use of vasopressors, operative optimization of conduit blood supply, tension-free anastomosis to reduce anastomotic leak and conduit necrosis[28], thoracic anastomosis to reduce recurrent laryngeal nerve injury and early medialization to prevent aspiration[29], and selective thoracic duct ligation for patients with positive intraoperative provocative chyle leak test[30]. Studies investigating superior techniques and technique optimization continue to be paramount.
Surgical volume is linked to a reduced complication rate and severity when comparing high-volume units to low-volume units and high-volume vs low-volume surgeons within a high-volume unit[31,32]. Most centers in Australia, including ours, are medium-volume centers based on international standards. Therefore, reduced morbidity and consequent cost savings may be achieved by centralizing esophagectomy.
Applying actual financial costs using hospital data and activity-based methodology rather than registers or insurance claims[6], provided an accurate account of the financial burden associated with all treatment components of esophagectomy and allowed us to identify the costliest medical interventions. We applied the widely used and validated CD complication severity grading system[14], and extracted data directly from patient files to ensure accuracy in the classification of severity. Lastly, we provide a unique insight into the complex effect of complications on admission costs, being the first to use mediation analysis.
The limitations of our study include its retrospective design and the relatively small sample size of a single center setup. A multicenter analysis could validate our findings and guide future interventions. Most of the procedures were open, and a comparative analysis of the minimally invasive approach was not performed. Additionally, we focused on short-term costs and outcomes during index admission or readmission within 30 days. Therefore, the financial implications of late- or long-term clinical and economic outcomes were not assessed and are areas for future research.
Complications after esophagectomy are common, resulting in a heavy financial burden and compromising patient outcomes. Our findings demonstrated that the severity and number of complications and the presence of key complications of esophagectomy significantly contributed to total hospital costs. We showed that the effect of the severity and the number of complications on admission cost was mediated through the costs of reoperation, prolonged ICH/HDU stay, and prolonged hospital stay, namely, activities known for their highest resource use. The effect of the presence of esophagectomy key complications was partially mediated but retained a significant direct effect on costs. This study highlights the importance of a continuous institutional quality review to prevent and mitigate complications, and the need for improved intervention strategies to enhance patient outcomes and minimize costs. Additionally, our analysis of cost drivers may shed insights into standardizing the way cost outcomes should be measured.
| 1. | Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3375] [Article Influence: 210.9] [Reference Citation Analysis (0)] |
| 2. | Cosic L, Ma R, Churilov L, Debono D, Nikfarjam M, Christophi C, Weinberg L. The financial impact of postoperative complications following liver resection. Medicine (Baltimore). 2019;98:e16054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Johnston SA, Louis M, Churilov L, Ma R, Marhoon N, Bui A, Christophi C, Weinberg L. The financial burden of complications following rectal resection: A cohort study. Medicine (Baltimore). 2020;99:e20089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Short MN, Aloia TA, Ho V. The influence of complications on the costs of complex cancer surgery. Cancer. 2014;120:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Browning AF, Chong L, Read M, Hii MW. Economic burden of complications and readmission following oesophageal cancer surgery. ANZ J Surg. 2022;92:2901-2906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Löfgren A, Åkesson O, Johansson J, Persson J. Hospital costs and health-related quality of life from complications after esophagectomy. Eur J Surg Oncol. 2021;47:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Goense L, van Dijk WA, Govaert JA, van Rossum PS, Ruurda JP, van Hillegersberg R. Hospital costs of complications after esophagectomy for cancer. Eur J Surg Oncol. 2017;43:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Park MG, Haro G, Mabeza RM, Sakowitz S, Verma A, Lee C, Williamson C, Benharash P. Association of frailty with clinical and financial outcomes of esophagectomy hospitalizations in the United States. Surg Open Sci. 2022;9:80-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3438] [Cited by in RCA: 7226] [Article Influence: 380.3] [Reference Citation Analysis (1)] |
| 10. | Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 2030] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 11. | Low DE, Allum W, De Manzoni G, Ferri L, Immanuel A, Kuppusamy M, Law S, Lindblad M, Maynard N, Neal J, Pramesh CS, Scott M, Mark Smithers B, Addor V, Ljungqvist O. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations. World J Surg. 2019;43:299-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 12. | National Blood Authority. Blood Management Guidelines: Module 2, Perioperative. 2012. [cited 23 June 2024]. Available from: https://www.blood.gov.au/sites/default/files/documents/2024-06/Patient%20Blood%20Management%20Guidelines%20%20Module%202%20-%20Perioperative.PDF. |
| 13. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 568] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 14. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26103] [Article Influence: 1186.5] [Reference Citation Analysis (2)] |
| 15. | Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, DʼJourno XB, Griffin SM, Hölscher AH, Hofstetter WL, Jobe BA, Kitagawa Y, Kucharczuk JC, Law SY, Lerut TE, Maynard N, Pera M, Peters JH, Pramesh CS, Reynolds JV, Smithers BM, van Lanschot JJ. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015;262:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 896] [Article Influence: 81.5] [Reference Citation Analysis (1)] |
| 16. | Peterson BG, Boudt K, Bennett R, Ulrich J, Zivot E, Cornilly D, Hung E, Lestel M, Balkissoon K, Wuertz D, Christidis AA, Martin, Zeheng RD, Zhou ZZ, Shea JM. Performance Analytics: Econometric Tools for Performance and Risk Analysis. R package version 2.0.4. Feb 6, 2020. [cited 23 June 2024]. Available from: https://cran.r-project.org/web/packages/PerformanceAnalytics/index.html, 2020. |
| 17. | Kuhn M, Jackson S, Cimentada J. corrr: Correlations in R, version 0.4.4. 2022. [cited 23 June 2024]. Available from: https://corrr.tidymodels.org. |
| 18. | Rijnhart JJM, Lamp SJ, Valente MJ, MacKinnon DP, Twisk JWR, Heymans MW. Mediation analysis methods used in observational research: a scoping review and recommendations. BMC Med Res Methodol. 2021;21:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 19. | MacKinnon DP, Pirlott AG. Statistical approaches for enhancing causal interpretation of the M to Y relation in mediation analysis. Pers Soc Psychol Rev. 2015;19:30-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Yu QZ, Li B. mma: Multiple Mediation Analysis. R package version 10.6-1. “mma” is an R package for mediation analysis with multiple mediators that produces complex mediation models and functioans to identify mediators and statistical inference on mediation effects. 2022. [cited 23 June 2024]. Available from: https://cran.r-project.org/web/packages/mma/index.html. |
| 21. | Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G, Davies A, D'Journo XB, Gisbertz SS, Griffin SM, Hardwick R, Hoelscher A, Hofstetter W, Jobe B, Kitagawa Y, Law S, Mariette C, Maynard N, Morse CR, Nafteux P, Pera M, Pramesh CS, Puig S, Reynolds JV, Schroeder W, Smithers M, Wijnhoven BPL. Benchmarking Complications Associated with Esophagectomy. Ann Surg. 2019;269:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 597] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 22. | Carrott PW, Markar SR, Kuppusamy MK, Traverso LW, Low DE. Accordion severity grading system: assessment of relationship between costs, length of hospital stay, and survival in patients with complications after esophagectomy for cancer. J Am Coll Surg. 2012;215:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Geller AD, Zheng H, Gaissert H, Mathisen D, Muniappan A, Wright C, Lanuti M. Relative Incremental Cost of Postoperative Complications of Esophagectomy. Semin Thorac Cardiovasc Surg. 2019;31:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Rutegård M, Lagergren P, Rouvelas I, Mason R, Lagergren J. Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur J Surg Oncol. 2012;38:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Halliday LJ, Doganay E, Wynter-Blyth VA, Hanna GB, Moorthy K. The Impact of Prehabilitation on Post-operative Outcomes in Oesophageal Cancer Surgery: a Propensity Score Matched Comparison. J Gastrointest Surg. 2021;25:2733-2741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Puccetti F, Klevebro F, Kuppusamy M, Han S, Fagley RE, Low DE, Hubka M. Analysis of Compliance with Enhanced Recovery After Surgery (ERAS) Protocol for Esophagectomy. World J Surg. 2022;46:2839-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Pisarska M, Małczak P, Major P, Wysocki M, Budzyński A, Pędziwiatr M. Enhanced recovery after surgery protocol in oesophageal cancer surgery: Systematic review and meta-analysis. PLoS One. 2017;12:e0174382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Fabian T. Management of Postoperative Complications After Esophageal Resection. Surg Clin North Am. 2021;101:525-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Friedman AD, Burns JA, Heaton JT, Zeitels SM. Early versus late injection medialization for unilateral vocal cord paralysis. Laryngoscope. 2010;120:2042-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Lin Y, Li Z, Li G, Zhang X, Deng H, Yang X, Liu L. Selective En Masse Ligation of the Thoracic Duct to Prevent Chyle Leak After Esophagectomy. Ann Thorac Surg. 2017;103:1802-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Munasinghe A, Markar SR, Mamidanna R, Darzi AW, Faiz OD, Hanna GB, Low DE. Is It Time to Centralize High-risk Cancer Care in the United States? Comparison of Outcomes of Esophagectomy Between England and the United States. Ann Surg. 2015;262:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Dolan D, White A, Lee DN, Mazzola E, Polhemus E, Kucukak S, Wee JO, Swanson SJ. Short and Long-term Outcomes Among High-Volume vs Low-Volume Esophagectomy Surgeons at a High-Volume Center. Semin Thorac Cardiovasc Surg. 2022;34:1340-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/