Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1087
Peer-review started: January 19, 2024
First decision: February 5, 2024
Revised: February 18, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: April 27, 2024
Processing time: 94 Days and 3.3 Hours
Acute liver failure (ALF) is a common cause of postoperative death in patients with hepatocellular carcinoma (HCC) and is a serious threat to patient safety. The neutrophil-to-lymphocyte ratio (NLR) is a common inflammatory indicator that is associated with the prognosis of various diseases, and the albumin-bilirubin score (ALBI) is used to evaluate liver function in liver cancer patients. Therefore, this study aimed to construct a predictive model for postoperative ALF in HCC tumor integrity resection (R0) based on the NLR and ALBI, providing a basis for clini
To construct an ALF prediction model after R0 surgery for HCC based on NLR and ALBI.
In total, 194 patients with HCC who visited The First People’s Hospital of Lian
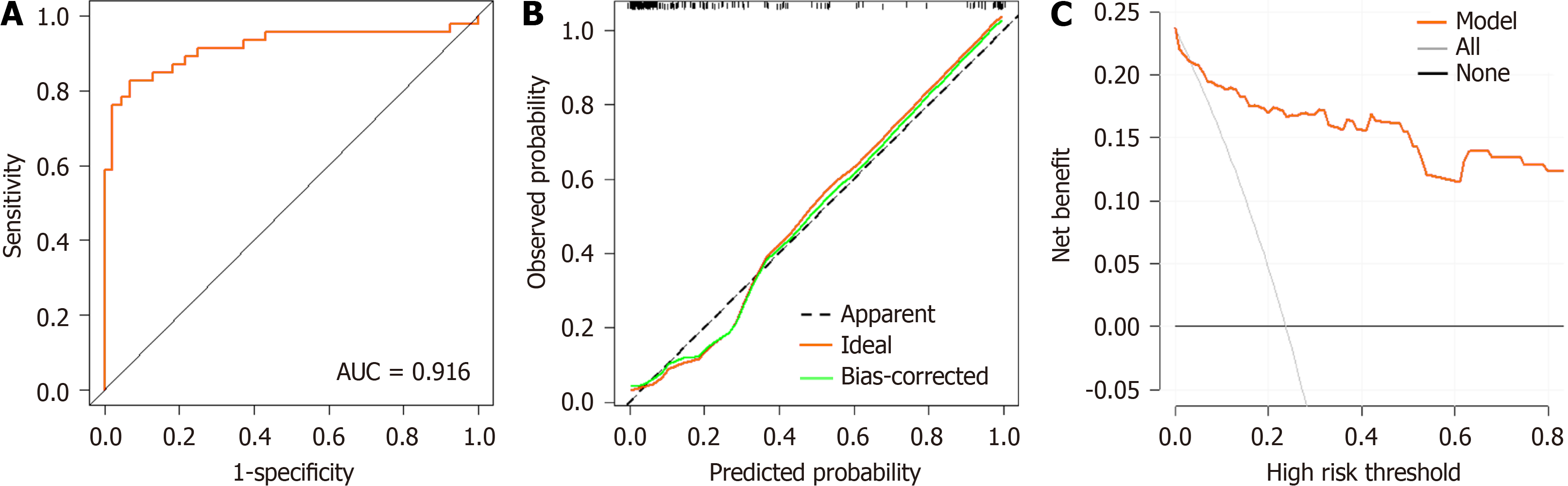
Among 194 patients with HCC who met the standard inclusion criteria, 46 cases of ALF occurred after R0 (23.71%). There were significant differences in the NLR and ALBI between the two groups (P < 0.05). The univariate analysis showed that alpha-fetoprotein (AFP) and blood loss volume (BLV) were significantly higher in the ALF group compared with the non-ALF group (P < 0.05). The multifactorial analysis showed that NLR, ALBI, AFP, and BLV were independent risk factors for ALF after R0 surgery in HCC. The predictive efficacy of NLR, ALBI, AFP, and BLV in predicting the occurrence of ALT after R0 surgery for HCC was average [area under the curve (AUC)NLR = 0.767, AUCALBI = 0.755, AUCAFP = 0.599, AUCBLV = 0.718]. The prediction model for ALF after R0 surgery for HCC based on NLR and ALBI had a better predictive efficacy (AUC = 0.916). The calibration curve and actual curve were in good agreement. DCA showed a high net gain and that the model was safer compared to the curve in the extreme case over a wide range of thresholds.
The prediction model based on NLR and ALBI can effectively predict the risk of developing ALF after HCC R0 surgery, providing a basis for clinical prevention of developing ALF after HCC R0 surgery.
Core Tip: This study aimed to identify independent risk factors associated with acute liver failure (ALF) after complete tumor resection (R0) for hepatocellular carcinoma (HCC) and to investigate their efficacy in predicting the occurrence of ALF after R0 for HCC. The results showed that the prediction model of ALF after R0 surgery for HCC, constructed based on the neutrophil-to-lymphocyte ratio and albumin-bilirubin score, had a good predictive efficacy and is expected to be a promising predictive tool in future clinical work.
- Citation: Li XP, Bao ZT, Wang L, Zhang CY, Yang W. Construction of a predictive model for acute liver failure after hepatectomy based on neutrophil-to-lymphocyte ratio and albumin-bilirubin score. World J Gastrointest Surg 2024; 16(4): 1087-1096
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1087.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1087
The liver is one of the most important and indispensable organs in the human body. Normal liver cells have a strong ability to self-replicate; however, persistent chronic inflammation can permanently impair liver repair and regeneration, leading to fibrosis, cirrhosis, liver failure, and even liver cancer. According to the latest global cancer data released in 2020, the incidence of liver cancer ranks fifth in the incidence of malignant tumors worldwide with an increasing trend each year[1]. Currently, primary liver cancer is a malignant tumor with high morbidity and mortality rates in China, accounting for more than two-thirds of the total number of liver cancers in China[1]. Complete tumor resection (R0) is the most direct and effective method for treating liver tumors and is the most important factor contributing to postoperative mortality. However, R0 resection often causes a variety of complications, among which the most difficult to manage and life-threatening is liver failure, which is the leading cause of death in postoperative patients. Thus, it is important to search for possible factors causing acute liver failure (ALF) after hepatectomy, predict liver failure in advance, assist clinicians in choosing appropriate treatment options, and improve the prognosis of patients with hepatocellular car
The serum neutrophil-to-lymphocyte ratio (NLR) is a common indicator of inflammation that can determine the in
In summary, the authors concluded that the NLR and ALBI might be associated with the occurrence of ALF after R0 surgery. To study the relationship between these two factors and the occurrence of ALF, this study aimed to construct a nomogram prediction model for the occurrence of ALF after R0 surgery for HCC by retrospectively analyzing the po
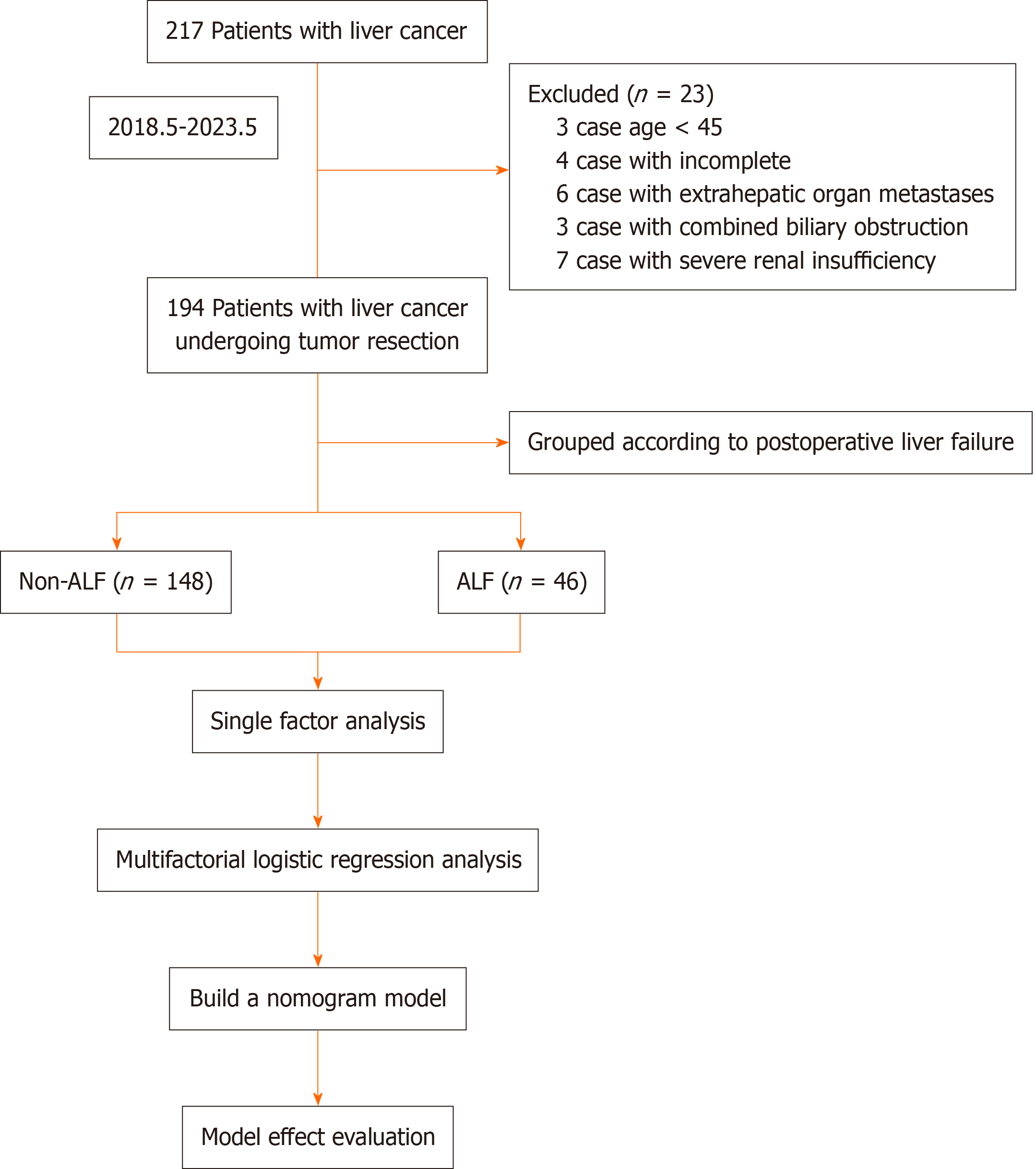
A total of 217 patients with HCC who visited The First People’s Hospital of Lianyungang for treatment between May 2018 and May 2023 were assessed; 23 patients were excluded, and 194 patients with HCC who underwent R0 were included in the study and were categorized into the ALF group (n = 46) and non-ALF group (n = 148), according to whe
The inclusion criteria were as follows: Age 45–80 years; patients underwent radical hepatectomy for HCC and met the criteria for R0, i.e., resection of all liver tumors visible to the naked eye; HCC was confirmed by postoperative patho
The exclusion criteria were as follows: Patients with preoperative rupture and bleeding of HCC; patients with metastasis to extrahepatic organs detected during intraoperative exploration; combined with biliary obstruction; combined with serious insufficiency of heart, lungs, kidneys, and other important organs; and patients with missing clinical data.
Referring to the relevant criteria proposed by the International Group for Hepatic Surgery in 2018, the diagnosis of liver failure was confirmed when a patient developed bilirubin levels > 50 mmol/L and an international normalized ratio > 1.7 on or 5 d after hepatectomy; biliary obstruction was excluded[10].
Information on factors associated with the development of ALF after R0 was collected, including gender, age, body mass index (BMI), hypertension, diabetes mellitus, history of hepatitis B, cirrhosis, pericardial integrity, type of tumor, number of tumors, surgical procedure, portal vein cancer occlusion, alpha-fetoprotein (AFP), platelets (PLT), hemoglobin (Hb), white blood cells (WBC), direct bilirubin (DBIL), total bilirubin (TBil), plasminogen time (PT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and blood loss volume (BLV), during the procedure.
The patient's peripheral venous blood (6 mL) was collected before surgery, centrifuged rapidly at 2000 rpm for 15 min, and the upper layer of serum was separated for use. NLR, Hb, PLT, and WBC counts were determined using a fully automatic blood cell analyzer (Mindray: BC-6800). TBil, DBIL, AST, and ALT levels were measured using an automatic biochemical analyzer (Beckman, AU5821). AFP was detected using a fully automatic biochemical immunoassay analyzer (Roche, cobas®8000). PT was detected using an automated coagulation analyzer (Werfen: ACL Top 700). NLR = mono
Data were analyzed with SPSS 26.0 statistical software. Comparisons of measurement data conforming to normal distribution between the two groups were performed with the t-test and expressed as the mean ± SD; comparisons of the count data between the two groups were performed using the χ2 test and expressed as [n (%)]. Variables in which there was a statistically significant difference (P < 0.05) were subjected to binary logistic regression analysis, and the risk factors affecting the occurrence of ALF after R0 surgery for HCC were screened out. R software was applied to establish the nomogram model and to plot the subjects' receiver operating characteristic curve (ROC). The nomogram model was validated for predictive performance using Bootstrap equal-volume with put-back repetitive sampling 1000 times, and calibration plots were plotted. Decision curve analysis (DCA) was also performed. Differences were statistically signi
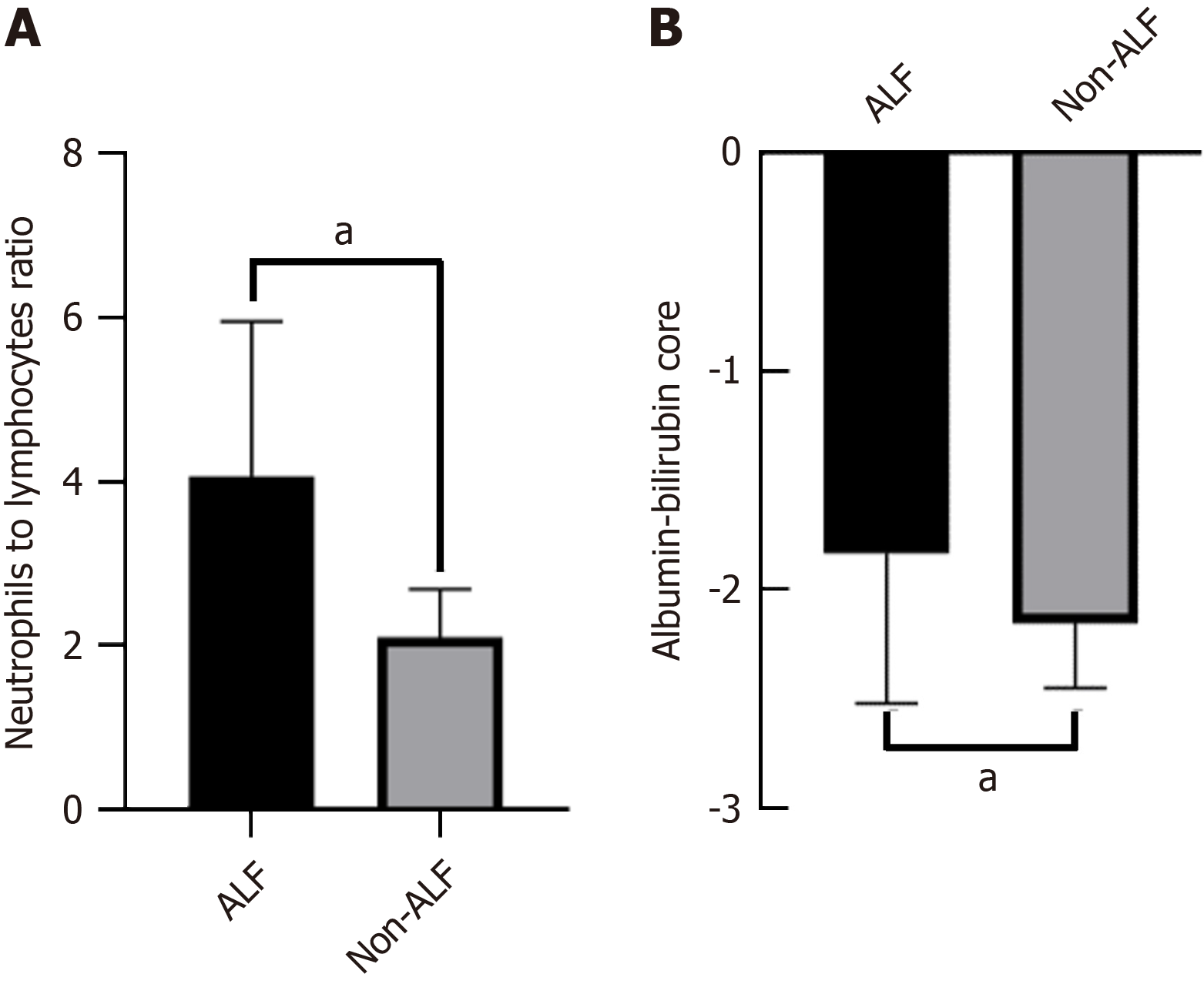
The NLR and ALBI were significantly higher in the ALF group than in the non-ALF group (P < 0.05; Figure 2).
A comparison of the general data showed that there was no statistically significant difference between the ALF and non-ALF groups in terms of sex, BMI, hypertension, diabetes mellitus, pericardial integrity, tumor type, number of tumors, and portal vein cancer screening (P > 0.05). However, there was a significant difference in terms of age, history of hepa
| Factor | Non-ALF | ALF | t/z/χ2 | P value |
| n = 148 | n = 46 | |||
| Gender | 0.382 | 0.537 | ||
| Male | 88 (59.5) | 24 (52.2) | ||
| Female | 60 (40.5) | 22 (47.8) | ||
| Age | 49.0 ± 4.0 | 51.1 ± 5.9 | -2.719 | 0.007 |
| BMI (kg/m2) | 23.454 ± 1.29 | 23.830 ± 1.424 | -1.620 | 0.110 |
| Hypertension | 0.65 | 0.42 | ||
| Yes | 52 (35.1) | 12 (26.1) | ||
| None | 96 (64.9) | 34 (73.9) | ||
| Diabetes | 0.394 | 0.53 | ||
| Yes | 42 (28.4) | 10 (21.7) | ||
| None | 106 (71.6) | 36 (78.3) | ||
| History of hepatitis | 23.454 ± 1.29 | 23.830 ± 1.424 | -2.026 | 0.043 |
| Yes | 103 (69.6) | 39 (84.8) | ||
| None | 45 (30.4) | 7 (15.2) | ||
| Liver cirrhosis | -2.026 | 0.043 | ||
| Yes | 103 (69.6) | 39 (84.8) | ||
| None | 45 (30.4) | 7 (15.2) | ||
| Envelope integrity | -1.548 | 0.122 | ||
| Complete | 127 (85.8) | 35 (76.1) | ||
| Incomplete | 21 (14.2) | 11 (23.9) | ||
| Tumor type | -0.331 | 0.74 | ||
| Isolated | 119 (80.4) | 38 (82.6) | ||
| Nodal fusion | 29 (19.6) | 8 (17.4) | ||
| Number of tumors | -0.975 | 0.33 | ||
| Single | 125 (84.5) | 36 (78.3) | ||
| Multiple | 23 (15.5) | 10 (21.7) | ||
| Surgical procedure | -2.269 | 0.023 | ||
| Open | 85 (57.4) | 35 (76.1) | ||
| Abdominal | 63 (42.6) | 11 (23.9) | ||
| Portal vein cancer plug | -1.804 | 0.071 | ||
| Negative | 136 (91.9) | 38 (8236) | ||
| Positive | 12 (8.1) | 8 (17.4) | ||
| BLV (mL) | 343.8 ± 97.9 | 418.2 ± 72.7 | -5.554 | 0.000 |
Furthermore, a comparison of laboratory data showed that there was no statistically significant difference between the ALF and non-ALF groups in terms of Hb, WBC, TBil, AST, and ALT levels (P > 0.05), while there was a significant difference in AFP, PLT, PT, and DBIL levels (P < 0.05), as shown in Table 2.
| Factor | Non-ALF | ALF | t/z/χ2 | P value |
| n = 148 | n = 46 | |||
| AFP | -2.6 | 0.009 | ||
| ≤ 400 (ng/mL) | 113 (76.4) | 26 (56.5) | ||
| > 400 (ng/mL) | 35 (23.6) | 20 (43.5) | ||
| Hb (g/L) | 134.47 ± 21.418 | 129.74 ± 19.509 | 1.336 | 0.183 |
| PLT (× 109/L) | 155.107 ± 31.693 | 135.661 ± 27.468 | 3.746 | 0 |
| WBC (× 109/L) | 5.669 ± 0.956 | 5.613 ± 0.970 | 0.345 | 0.73 |
| PT (s) | 12.2 ± 1.2 | 12.9 ± 1.2 | -3.337 | 0.001 |
| TBil (μmol/L) | 16.778 ± 7.345 | 19.152 ± 7.472 | -1.907 | 0.058 |
| DBIL (μmol/L) | 4.0 ± 2.8 | 5.0 ± 2.6 | -2.221 | 0.027 |
| ALT (u/L) | 50.692 ± 15.477 | 48.687 ± 14.367 | 0.78 | 0.436 |
| AST (u/L) | 43.562 ± 14.151 | 45.339 ± 13.180 | 0.756 | 0.451 |
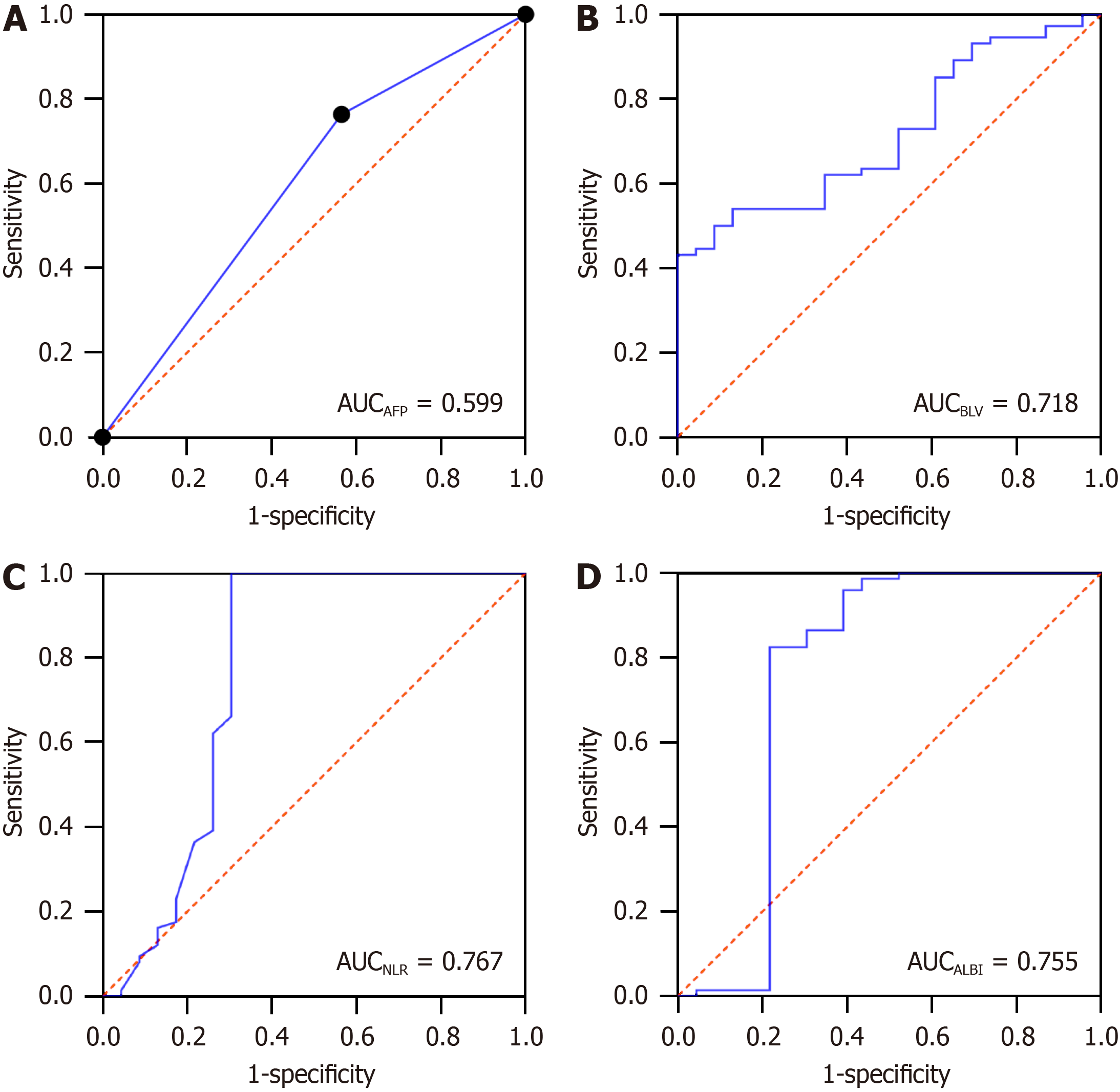
Indicators with significant differences in the NLR and ALBI scores, and the univariate analysis, were included in the multifactorial logistic regression analysis, in which AFP less than or equal to 400 ng/mL was assigned the value of "0”, and greater than 400 ng/mL was assigned the value of "1”. The results showed that AFP, NLR, ALBI, and BLV were independent risk factors for ALF after R0 surgery for HCC (Table 3). The values of the indicators were assessed using ROC curves, and the results showed that AFP, BLV, NLR, and ALBI had a certain predictive value for ALF after R0 surgery for HCC (P < 0.05), and the predictive values of NLR and ALBI were better than those of AFP and BLV, as shown in Table 4 and Figure 3.
| Factor | β | SE | OR value | 95%CI | Wald value | P value |
| AFP | 1.401 | 0.539 | 4.058 | 1.411-11.670 | 6.755 | 0.009 |
| BLV | 0.009 | 0.003 | 1.009 | 1.003-1.016 | 9.166 | 0.002 |
| NLR | 1.464 | 0.277 | 4.318 | 2.511-7.426 | 27.969 | 0.000 |
| ALBI | 2.157 | 0.665 | 8.646 | 2.350-31.816 | 10.531 | 0.001 |
| Indicator | Cut-off value | AUC | 95%CI | Specificity | Sensitivity | P value |
| AFP | 400 | 0.599 | 0.643-0.793 | - | - | 0.042 |
| BLV | 302.850 | 0.718 | 0.643-0.793 | 1.000 | 0.432 | 0.000 |
| NLR | 3.150 | 0.767 | 0.659-0.875 | 1.000 | 0.696 | 0.000 |
| ALBI | -1.942 | 0.755 | 0.640-0.870 | 0.824 | 0.783 | 0.000 |
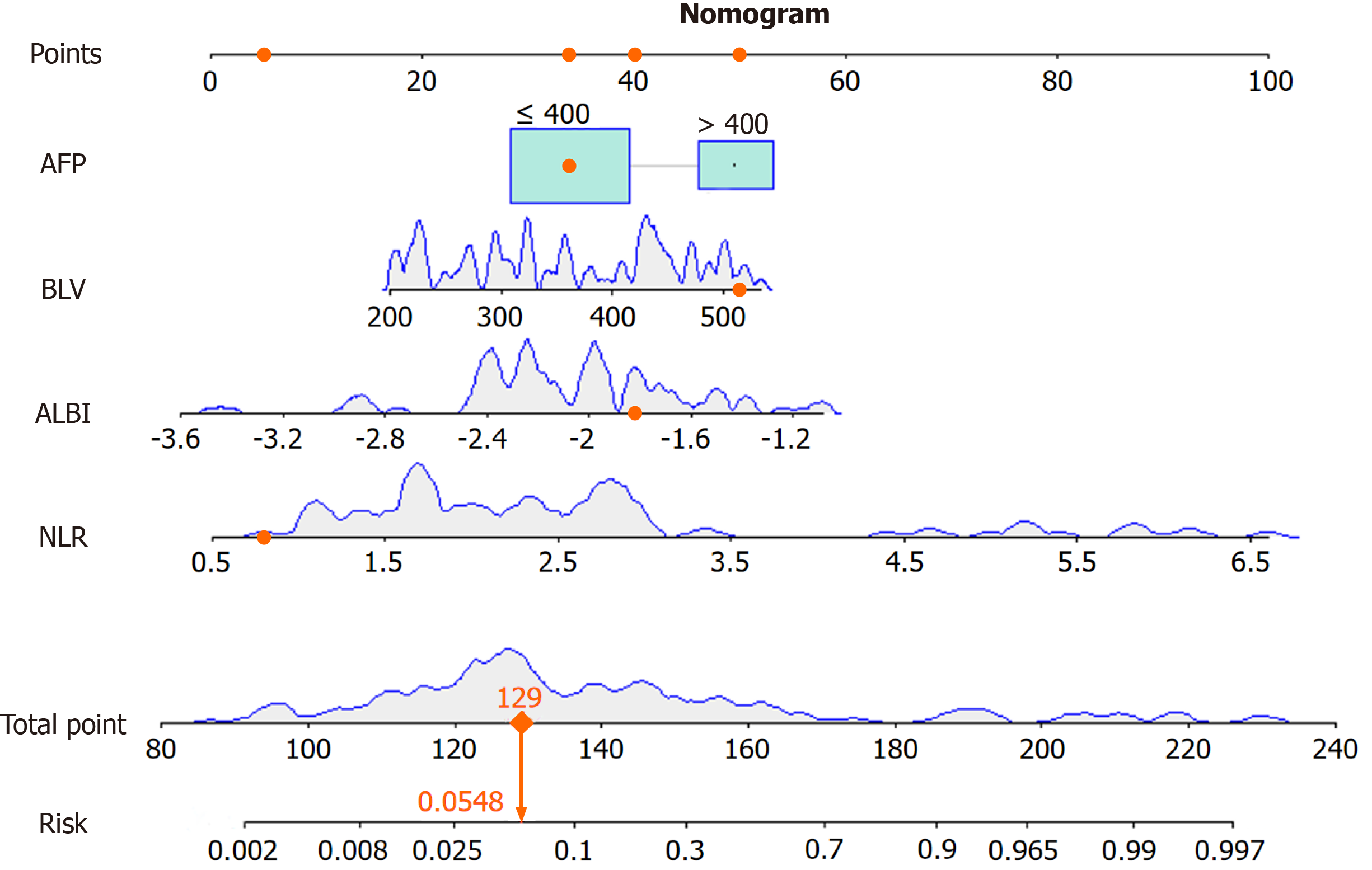
A column-line graph model was constructed based on the indicators screened using the multifactor logistic regression analysis (Figure 4). The predictive probability of the model was calculated by adding the corresponding scores of each indicator to obtain the total score. Internal validation was performed by bootstrap sampling 1000 times, and the areas under the curve (AUC), DCA curve, and calibration curve were used to evaluate the effectiveness of the column plot. The AUC was 0.916, sensitivity was 0.826, specificity was 0.932 (P = 0.000, 95% confidence interval: 0.854–0.978). The ROC cur
In recent years, immunotherapy and molecular targeting have gradually become hotspots of clinical and scientific research, R0 is still the main modality for the treatment of HCC and occupies an indispensable position. However, residual liver tissue regeneration is impaired after R0, and excessive apoptosis of liver cells will lead to the imbalance between liver regeneration and injury, resulting in ALF after hepatectomy[11].
Impaired immune system function, excessive release of inflammatory factors, and sustained inflammatory responses play important roles in exacerbating hepatocyte injury and promoting an imbalance between hepatocyte regeneration and injury. Neutrophils are the first responders to inflammation and infection in the body and are important cellular components of the immune response. In the peripheral blood, the NLR is used to reflect the inflammatory and immune status of the organism and is associated with a poor prognosis in patients with colorectal[12], gastric[13,14], breast[15], prostate[16], and lung[17] cancers. This study reviewed the relevant studies on ALF after HCC R0 surgery, and found that the serum NLR before surgery was higher in the ALF group, suggesting that preoperative NLR has a certain pre
ALBI is a hotly researched scoring model for predicting the efficacy after liver transplantation in recent years, which was first analyzed by Johnson et al[18] on the survival of 1313 patients with HCC with better accuracy due to the exclu
AFP is considered a diagnostic and prognostic tumor marker for HCC[23]. The level of AFP in normal human serum is low. However, the expression of AFP increases in HCC, and its serum level increases sharply with the deterioration of the disease[24]. Our study categorized AFP levels as less than or equal to 400 ng/mL and greater than 400 ng/mL, and it was shown that AFP levels were associated with the development of ALF after R0 surgery for HCC. In addition, its efficacy as a predictor was fair (AUCAFP = 0.599).
In this study, intraoperative BLV was considered an independent risk factor for the occurrence of ALF after R0 for HCC. Albumin is the most important protein in human plasma, accounting for approximately 50% of the total human plasma proteins, and is the basic physiological substance for maintaining the nutrition of the body. Some studies have shown that nutritional status affects disease prognosis[25]. Intraoperative massive blood loss in HCC R0 resection leads to a consequent massive loss of serum albumin, resulting in nutritional deficiencies and reduced immunity, which ulti
The prognostic efficacy of a single factor to predict the disease has some limitations. To avoid this problem, we constructed a nomogram prediction model using NLR, ALBI, AFP, and BLV as predictors for the occurrence of ALF after R0 surgery for HCC in this research. After model validation, we found that the calibration curves fit the ideal curves to a high degree, the predictive efficacy of the nomogram prediction model was better (AUC = 0.916), and the efficacy of the combined prediction was much higher than that of the single-factor prediction. It has been shown that ALBI combined with residual liver volume can be used to predict ALF in patients with HBV-associated primary HCC (AUC = 0.890)[26]. However, this requires three-dimensional reconstruction of preoperative abdominal computed tomography imaging data to measure the residual liver volume in patients with HCC, which often requires a skillful base for two-dimensional image reading. In addition, the reconstruction results vary from person to person, with instability and other shortco
In summary, the prediction model of ALF after R0 surgery for HCC based on NLR combined with ALBI has good predictive value and is expected to be a promising predictive tool in future clinical work. This was a clinical retrospective study, which was limited by the sample size. Therefore, the value of constructing a prediction model of ALF after R0 surgery for HCC based on NLR combined with ALBI needs to be further verified in a larger sample size or prospective clinical cohort study.
The construction of a prediction model for ALF after R0 surgery for HCC based on NLR and ALBI had good predictive value and is expected to be a promising predictive tool in future clinical work.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68628] [Article Influence: 13725.6] [Reference Citation Analysis (201)] |
| 2. | Mouchli M, Reddy S, Gerrard M; Boardman L; Rubio M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma." Review article. Ann Hepatol. 2021;22:100249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Hung HC, Lee JC, Wang YC, Cheng CH, Wu TH, Wu TJ, Chou HS, Chan KM, Lee WC, Lee CF. Living-Donor Liver Transplantation for Hepatocellular Carcinoma: Impact of the MELD Score and Predictive Value of NLR on Survival. Curr Oncol. 2022;29:3881-3893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Wang S, Zhang X, Chen Q, Jin ZC, Lu J, Guo J. A Novel Neutrophil-to-Lymphocyte Ratio and Sarcopenia Based TACE-Predict Model of Hepatocellular Carcinoma Patients. J Hepatocell Carcinoma. 2023;10:659-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Minici R, Siciliano MA, Ammendola M, Santoro RC, Barbieri V, Ranieri G, Laganà D. Prognostic Role of Neutrophil-to-Lymphocyte Ratio (NLR), Lymphocyte-to-Monocyte Ratio (LMR), Platelet-to-Lymphocyte Ratio (PLR) and Lymphocyte-to-C Reactive Protein Ratio (LCR) in Patients with Hepatocellular Carcinoma (HCC) undergoing Chemoembolizations (TACE) of the Liver: The Unexplored Corner Linking Tumor Microenvironment, Biomarkers and Interventional Radiology. Cancers (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Wang N, He S, Zheng Y, Wang L. The value of NLR versus MLR in the short-term prognostic assessment of HBV-related acute-on-chronic liver failure. Int Immunopharmacol. 2023;121:110489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 7. | Li Z, Jiao D, Han X, Si G, Li Y, Liu J, Xu Y, Zheng B, Zhang X. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided microwave ablation in the treatment of small hepatocellular carcinoma. Cancer Imaging. 2020;20:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Zhong X, Tang H, Lu B, You J, Piao J, Yang P, Li J. Differentiation of Small Hepatocellular Carcinoma From Dysplastic Nodules in Cirrhotic Liver: Texture Analysis Based on MRI Improved Performance in Comparison Over Gadoxetic Acid-Enhanced MR and Diffusion-Weighted Imaging. Front Oncol. 2019;9:1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kong FH, Miao XY, Zou H, Xiong L, Wen Y, Chen B, Liu X, Zhou JJ. End-stage liver disease score and future liver remnant volume predict post-hepatectomy liver failure in hepatocellular carcinoma. World J Clin Cases. 2019;7:3734-3741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JN, Conrad C, Hugh TJ, Garden OJ, Fan ST, Crawford M, Makuuchi M, Yokoyama Y, Büchler M, Padbury R. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford). 2018;20:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Sparrelid E, Olthof PB, Dasari BVM, Erdmann JI, Santol J, Starlinger P, Gilg S. Current evidence on posthepatectomy liver failure: comprehensive review. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 12. | Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2021;10:5983-5997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 14. | Mellor KL, Powell AGMT, Lewis WG. Systematic Review and Meta-Analysis of the Prognostic Significance of Neutrophil-Lymphocyte Ratio (NLR) After R0 Gastrectomy for Cancer. J Gastrointest Cancer. 2018;49:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Grassadonia A, Graziano V, Iezzi L, Vici P, Barba M, Pizzuti L, Cicero G, Krasniqi E, Mazzotta M, Marinelli D, Amodio A, Natoli C, Tinari N. Prognostic Relevance of Neutrophil to Lymphocyte Ratio (NLR) in Luminal Breast Cancer: A Retrospective Analysis in the Neoadjuvant Setting. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Kumano Y, Hasegawa Y, Kawahara T, Yasui M, Miyoshi Y, Matsubara N, Uemura H. Pretreatment Neutrophil to Lymphocyte Ratio (NLR) Predicts Prognosis for Castration Resistant Prostate Cancer Patients Underwent Enzalutamide. Biomed Res Int. 2019;2019:9450838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Platini H, Ferdinand E, Kohar K, Prayogo SA, Amirah S, Komariah M, Maulana S. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2172] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 19. | Wang J, Zhang Z, Yan X, Li M, Xia J, Liu Y, Chen Y, Jia B, Zhu L, Zhu C, Huang R, Wu C. Albumin-Bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig Liver Dis. 2019;51:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Shao L, Han B, An S, Ma J, Guo X, Romeiro FG, Mancuso A, Qi X. Albumin-to-bilirubin score for assessing the in-hospital death in cirrhosis. Transl Gastroenterol Hepatol. 2017;2:88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Ito T, Ishigami M, Morooka H, Yamamoto K, Imai N, Ishizu Y, Honda T, Nishimura D, Tada T, Yasuda S, Toyoda H, Kumada T, Fujishiro M. The albumin-bilirubin score as a predictor of outcomes in Japanese patients with PBC: an analysis using time-dependent ROC. Sci Rep. 2020;10:17812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Song Y, Yang H, Lin L, Jiang K, Liu WT, Wang BM, Lin R. [Albumin-to-bilirubin scores for assessing the prognosis in autoimmune hepatitis-related cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:2439-2446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 24. | Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 437] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 25. | Moisey LL, Merriweather JL, Drover JW. The role of nutrition rehabilitation in the recovery of survivors of critical illness: underrecognized and underappreciated. Crit Care. 2022;26:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 26. | Zou H, Wen Y, Yuan K, Miao XY, Xiong L, Liu KJ. Combining albumin-bilirubin score with future liver remnant predicts post-hepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018;38:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katila T, Finland; Victor D, United States S-Editor: Fan JR L-Editor: A P-Editor: Xu ZH