Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.955
Peer-review started: November 26, 2023
First decision: December 15, 2023
Revised: December 16, 2023
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 27, 2024
Processing time: 116 Days and 22.5 Hours
Abdominal cocoon syndrome (ACS) represents a category within sclerosing encapsulating peritonitis, characterized by the encapsulation of internal organs with a fibrous, cocoon-like membrane of unknown origin, resulting in bowel obstruction and ischemia. Diagnosing this condition before surgery poses a cha
Three male patients, aged 53, 58, and 61 originating from Northern Thailand, arrived at our medical facility complaining of abdominal pain without any prior surgeries. Their vital signs remained stable during the assessment. The diagnosis of abdominal cocoon was confirmed through abdominal computed tomography (CT) before surgery. In the first case, the CT scan revealed capsules around the small bowel loops, showing no enhancement, along with mesenteric congestion affecting both small and large bowel loops, without a clear obstruction. The second case showed intestinal obstruction due to an encapsulated capsule on the CT scan. In the final case, a patient presented with recurring abdominal pain. Initially, the radiologist suspected enteritis as the cause after the CT scan. However, a detailed review led the surgeon to suspect encapsulating peritoneal sclerosis (ACS) and subsequently perform surgery. The surgical procedure involved complete removal of the encapsulating structure, resection of a portion of the small bowel, and end-to-end anastomosis. No complications occurred during surgery, and the patients had a smooth recovery after surgery, eventually discharged in good health. The histopathological examination of the fibrous membrane (cocoon) across all cases consistently revealed the presence of fibro-collagenous tissue, without any indications of malignancy.
Individuals diagnosed with abdominal cocoons commonly manifest vague symptoms of abdominal discomfort. An elevated degree of clinical suspicion, combined with the application of appropriate radiological evaluations, markedly improves the probability of identifying the abdominal cocoon before surgical intervention. In cases of complete bowel obstruction or ischemia, the established norm is the comprehensive removal of the peritoneal sac as part of standard care. Resection with intestinal anastomosis is advised solely when ischemia and gangrene have been confirmed.
Core Tip: Diagnosing Abdominal cocoon syndrome (ACS) poses challenges, often necessitating laparotomy for confirmation. This study presents three distinct ACS cases: one featuring bowel obstruction, another with isolated ischemia, and the last highlighting disparities between radiological findings and surgical assessments. Preoperative computed tomography scans played a crucial role in diagnosis, revealing diverse manifestations such as capsules encasing the bowels, mesenteric congestion, or ambiguous obstructions. Surgical excision of encapsulating structures led to successful recovery. Additionally, one case involved a traumatic event, requiring exploratory laparotomy a year later, where no fibrosis was found around the previously removed intestine. Early clinical suspicion, coupled with precise radiological examination, aids in identifying ACS before surgery. Complete removal of the sac during obstruction/ischemia is the established approach, recommending resection solely for confirmed ischemic complications.
- Citation: Vipudhamorn W, Juthasilaparut T, Sutharat P, Sanmee S, Supatrakul E. Abdominal cocoon syndrome-a rare culprit behind small bowel ischemia and obstruction: Three case reports. World J Gastrointest Surg 2024; 16(3): 955-965
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/955.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.955
Intra-abdominal inflammation, recognized as sclerosing encapsulating peritonitis (SEP), stands as an uncommon yet consequential condition precipitating intestinal obstruction and the peril of gangrene. This affliction delineates a scenario where abdominal organs become enveloped or encased within a dense fibrous tissue, evoking the imagery of a protective 'cocoon.' SEP, a complex entity, assumes two primary classifications: primary SEP and secondary encapsulating peritonitis.
Primary SEP, a distinctly rare manifestation, perplexes medical practitioners owing to its elusive etiology, devoid of identifiable causes. Conversely, secondary encapsulating peritonitis emerges consequent to prolonged and recurrent inflammation within the abdominal cavity, notably observed in patients undergoing peritoneal dialysis[1,2]. This dichotomy between primary and secondary forms poses diagnostic and therapeutic challenges, demanding tailored approaches for optimal management.
The diagnosis of abdominal cocoon syndrome (ACS) poses considerable challenges, necessitating a comprehensive approach involving meticulous medical history assessment, thorough physical examination, heightened clinical suspicion, and a range of both laboratory and radiological investigations[3]. Typically, symptomatic ACS presents as acute intestinal obstruction, often leading to emergent visits to healthcare facilities. Rigorous evaluation involving detailed medical and surgical histories, alongside physical examinations and diverse laboratory investigations, is crucial in excluding common causes of intestinal obstruction, predominantly attributed (80%) to postoperative adhesions. A minority of cases (approximately 6%) stem from atypical conditions such as internal hernias, substantial intussusception, and chronic idiopathic intestinal pseudo-obstruction[4].
Internal hernias represent a pertinent differential diagnosis, exhibiting similar computed tomography (CT) findings to ACS but usually lacking a membrane-like sac evident in CT scans[5]. Conversely, chronic idiopathic intestinal pseudo-obstruction manifests as bowel dilation in both small and large intestines, devoid of a membrane-like sac as seen in ACS[6]. Traditionally, ACS is diagnosed intraoperatively by the presence of a thick membrane enveloping the intestines[4]. However, recent advancements in radiological imaging have proven valuable in facilitating preoperative diagnoses.
The management approach to primary SEP remains a subject of intense debate within the medical community. While unanimity eludes consensus regarding the ideal course of action, surgical intervention typically garners favor among most surgeons, particularly when patients confront complications such as bowel obstruction. Contrarily, the therapeutic strategies for secondary SEP exhibit a spectrum of variability. Ongoing research and evolving clinical observations reflect the prevalent utilization of steroids and tamoxifen post-SEP diagnosis. Initial indications hint at the potential efficacy of steroids, tamoxifen, or Angiotensin II inhibitors, showcasing a promising avenue for further exploration and refinement of treatment modalities[7].
This distinct delineation between primary and secondary SEP necessitates a nuanced understanding, not solely for accurate diagnosis but also for devising tailored therapeutic interventions. Exploring the interplay between etiological factors, pathophysiological mechanisms, and treatment outcomes holds significant promise in enhancing the management strategies for these encapsulating peritonitis entities[8]. We present in this report 3 cases of abdominal cocoons diagnosed preoperatively by abdominal CT and managed with surgical excision of all attached membranes.
Case 1: A 58-year-old male patient presented with left lower quadrant abdominal pain persisting for eight hours.
Case 2: A 61-year-old male presented with recurrent abdominal pain and substantial weight loss for one month.
Case 3: A 53-year-old male presented with colicky periumbilical pain for ten hours.
Case 1: A 58-year-old male patient admitted to Maharaj Chiang Mai Hospital exhibited escalating left lower quadrant abdominal pain persisting for eight hours. Over two years, he intermittently experienced chronic abdominal pain, though the symptoms spontaneously resolved. Abdominal pain was not associated with nausea and vomiting.
Case 2: A 61-year-old male presented with recurrent abdominal pain and substantial weight loss, decreasing from 45 to 40 kg within a month. Upon admission to a rural hospital, he reported early satiety and abdominal distension. Following successful management like partial small bowel obstruction at the rural facility, he was referred to the General Surgery Outpatient Department at Lampang Hospital.
Case 3: A 53-year-old male. Patient endured recurrent abdominal pain persisting for the last eight years, mostly resolving spontaneously. During this period, he sought medical attention at Lampang Hospital's Emergency Room (ER) on five occasions and was admitted once five years ago. However, his symptoms were effectively managed with pain control. On this occasion, the patient experienced colicky periumbilical pain ten hours before presenting at the ER. Initial pain management led to slight symptom improvement, prompting discharge. However, the patient returned to the ER three hours later due to the persistent symptoms, leading to subsequent admission to the general surgical ward.
Case 1: His medical history included non-valvular atrial fibrillation, managed with a daily intake of 2 mg of warfarin.
Case 2: No significant past history or any illness or surgery.
Case 3: No significant past history or any illness or surgery.
The patient denied any family history of malignant tumours.
Case 1: Upon clinical examination, pronounced tenderness and guarding were observed in the left lower quadrant, alongside the identification of a palpable 5 cm × 8 cm mass characterized by firm consistency.
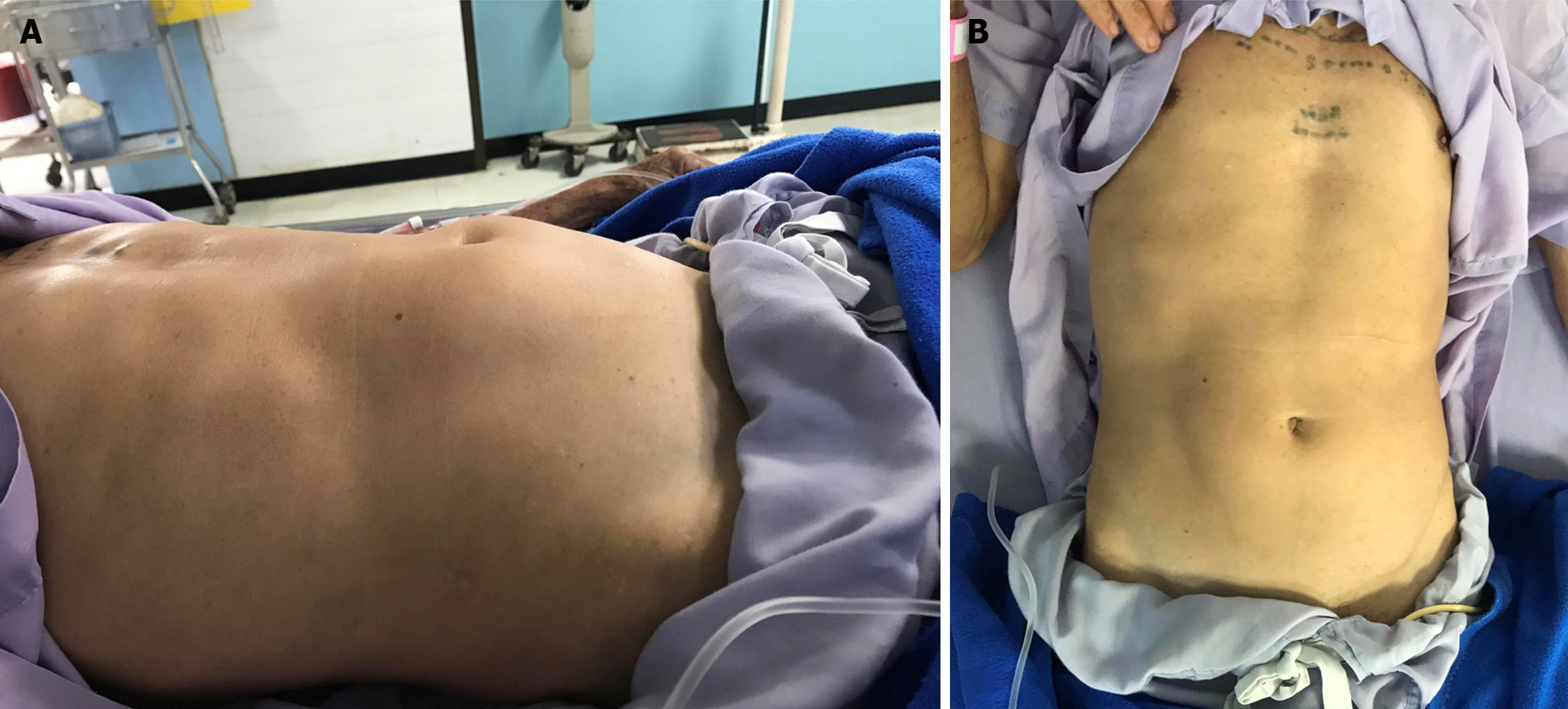
Case 2: During the outpatient evaluation, a physical examination revealed a 10 cm × 10 cm abdominal mass protruding in the mid-abdominal region (Figure 1). The patient had mild tenderness at mass without rebound tenderness or guarding.
Case 3: Upon examination in the ward, a distended abdomen with moderate tenderness in the lower left abdomen (more pronounced than the right) was noted.
Case 1: Laboratory analysis revealed a slight elevation in leukocyte count [white blood counts (WBC) 11000/μL] and C-reactive protein (CRP) was 175 mg/dL while other parameters remained within normal ranges.
Case 2: Laboratory analysis revealed no leukocytosis [WBC 8100/cumm, polymorphonuclear neutrophil (PMN) 72%] and normal electrolyte levels (initial setting).
Case 3: Laboratory analysis revealed minimal leukocytosis (WBC 11300/cumm, PMN 81%) and normal electrolyte levels.
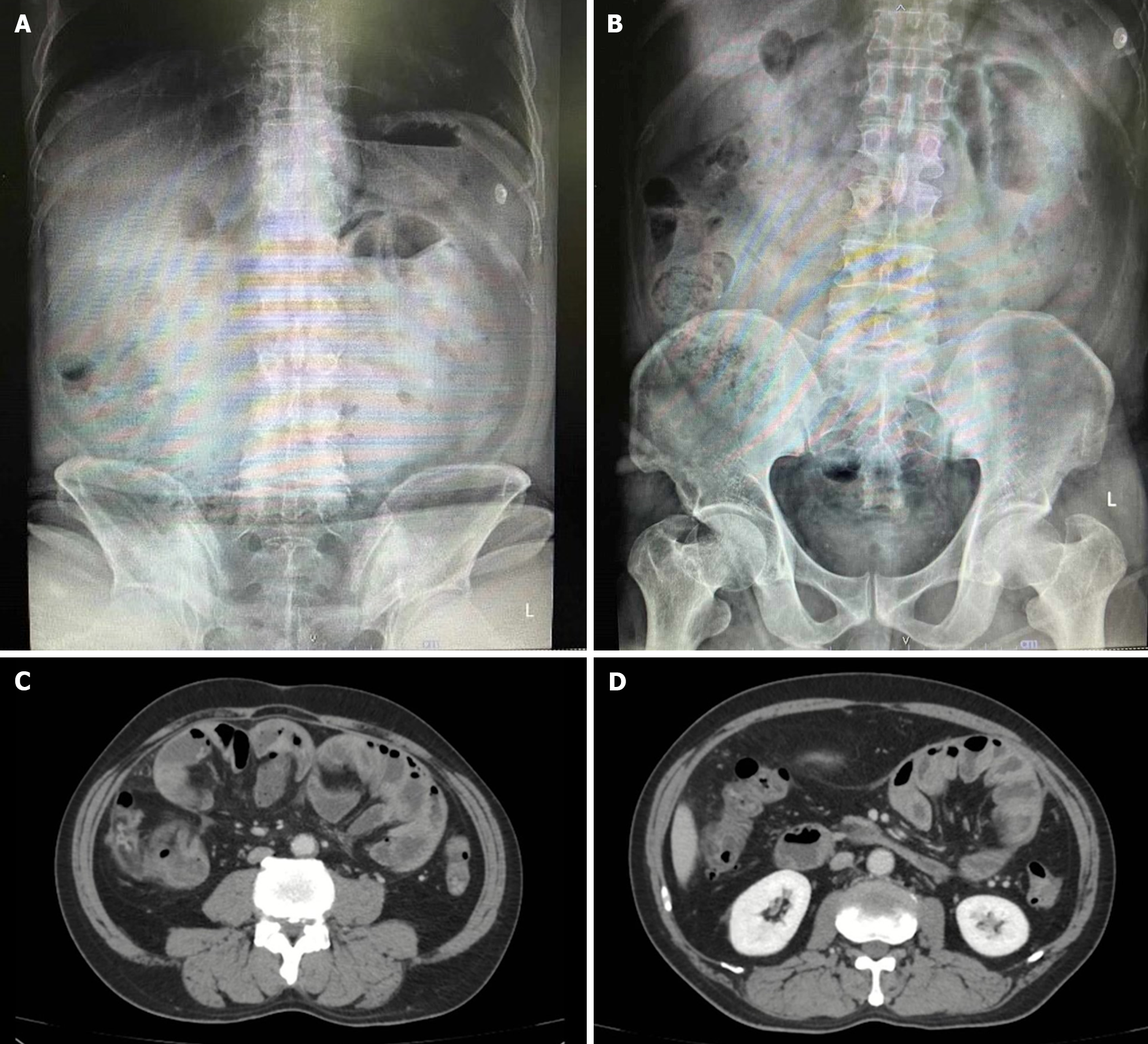
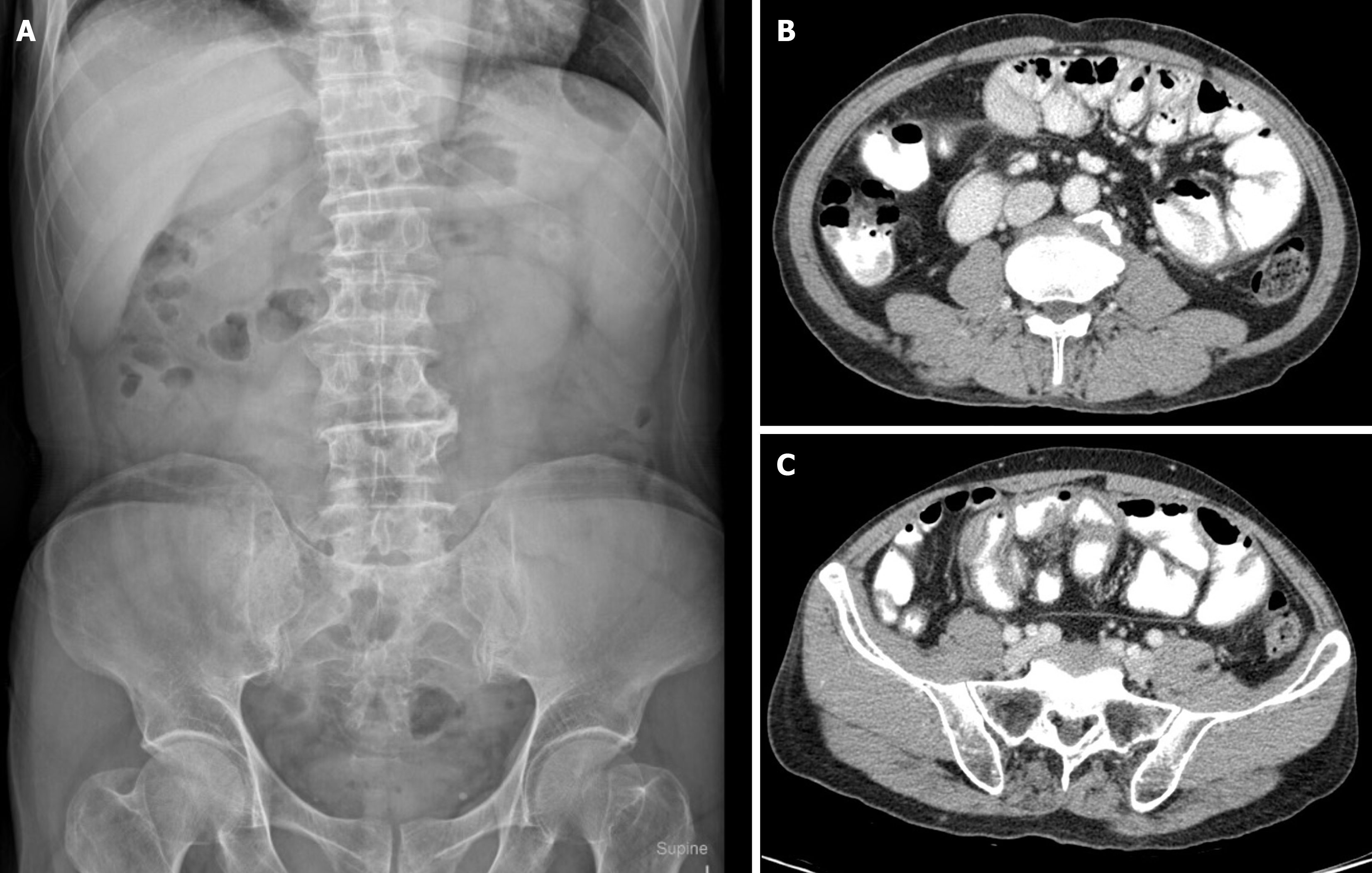
Case 1: Plain abdominal X-rays yielded unremarkable results (Figure 2A and B), whereas abdominal CT scans displayed an encapsulated thickening of the peritoneum with enhanced features, coupled with ascites between bowel loops. No signs of bowel dilation or obstruction were evident (Figure 2C and D).
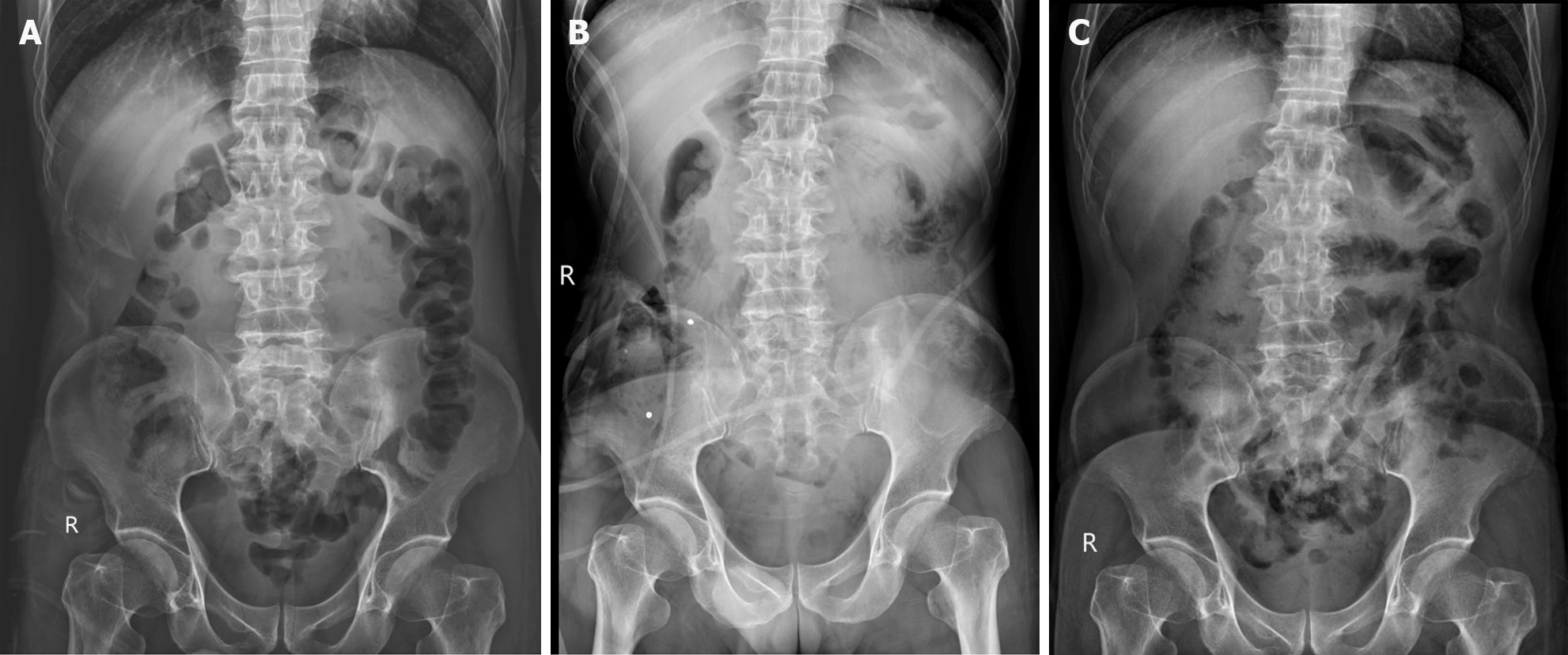
Case 2: Abdominal X-rays indicated normal gas distribution in the colon and rectum, they revealed a gasless small bowel (Figure 3A). The intern referred the patient for an abdominal ultrasound examination due to the presence of a mid-abdominal mass. The ultrasound revealed multiple bowel loops, raising suspicion of a mass near the cecum, warranting a differential diagnosis that included the possibility of colon cancer. The primary surgeon elected an abdominal CT scan, revealing diffuse thickening of the small bowel wall without any blockage suggestive of encapsulating peritoneal sclerosis (Figure 4A).
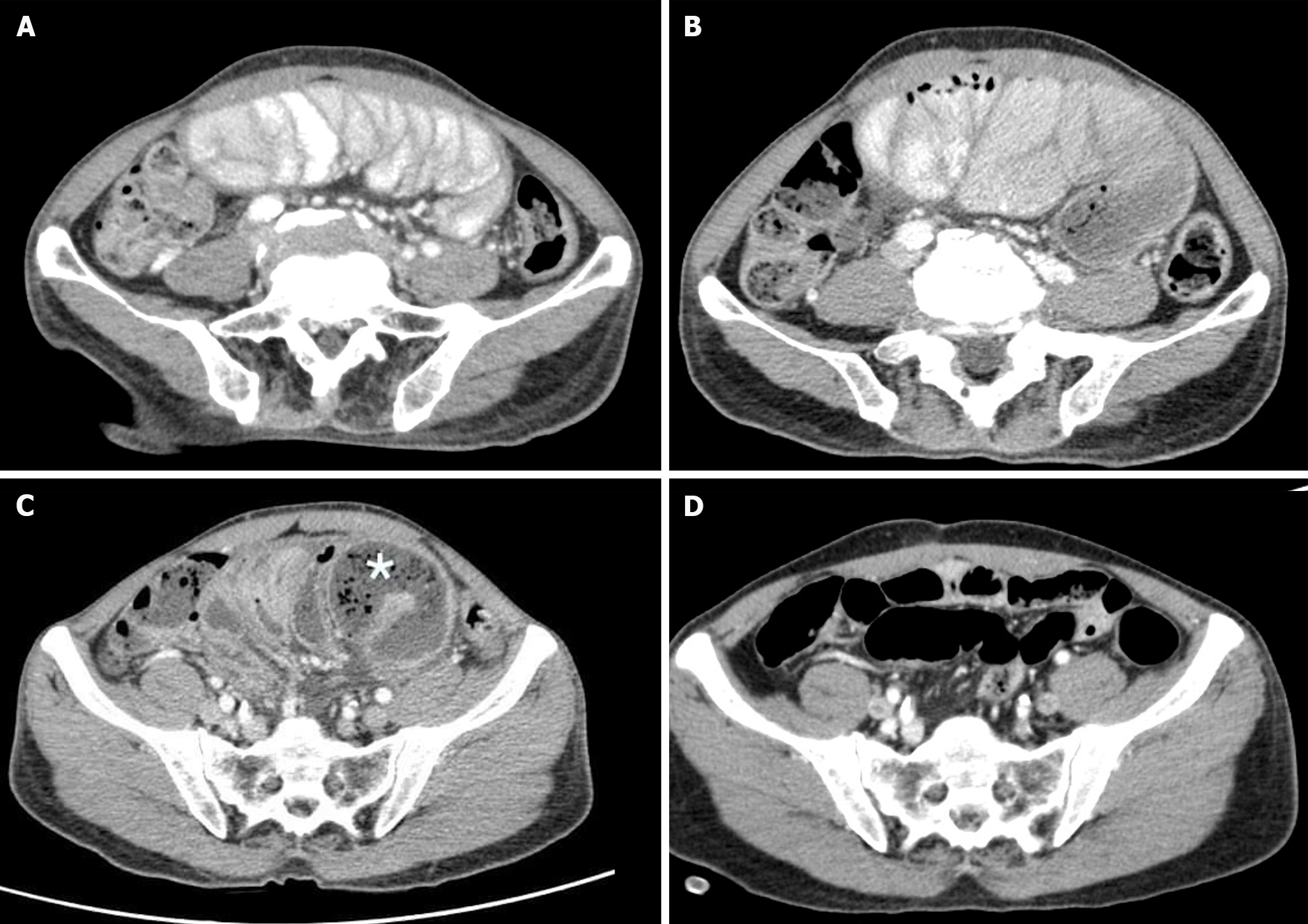
Case 3: The abdominal plain film demonstrated a gasless small bowel (Figure 5A) with slight air retention in the colon and rectum. An abdominal CT scan, initially suspected to indicate sigmoid diverticulitis, was reported by the radiologist as suggestive of enteritis. However, a closer review of the CT scan raised suspicion of encapsulating peritoneal sclerosis with complications, as it exhibited stacked small bowel loops within a thick, membrane-like sac (Figure 5B and C).
All of the patients received a diagnosis of “acute ACS” with associated complications.
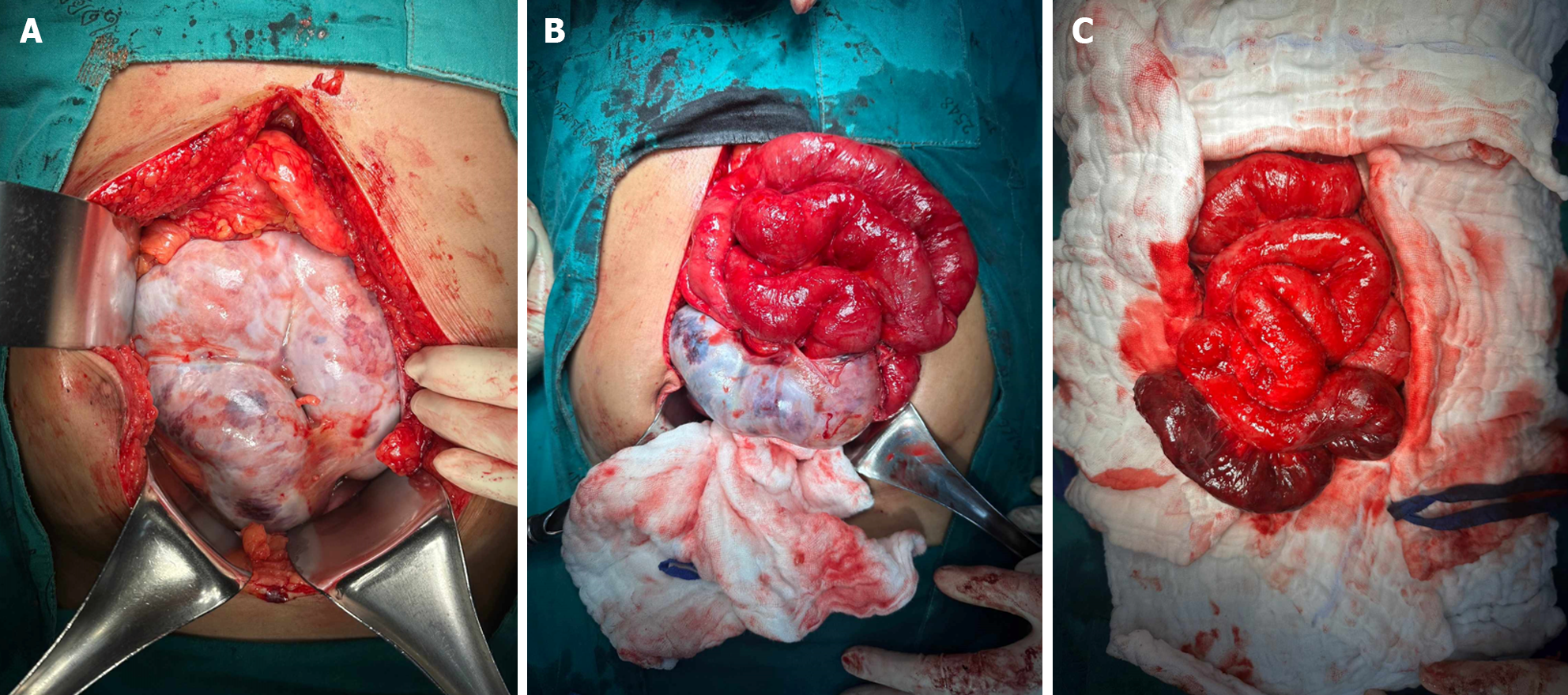
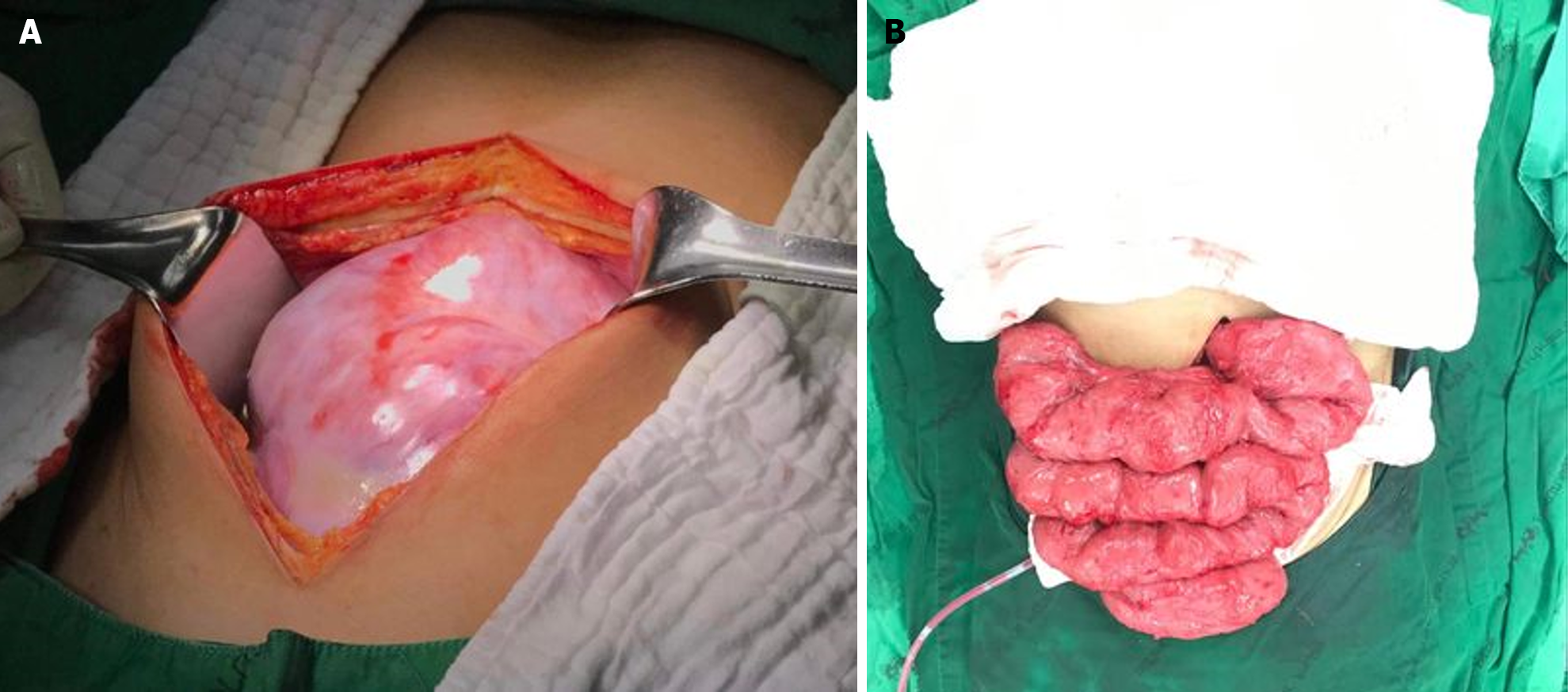
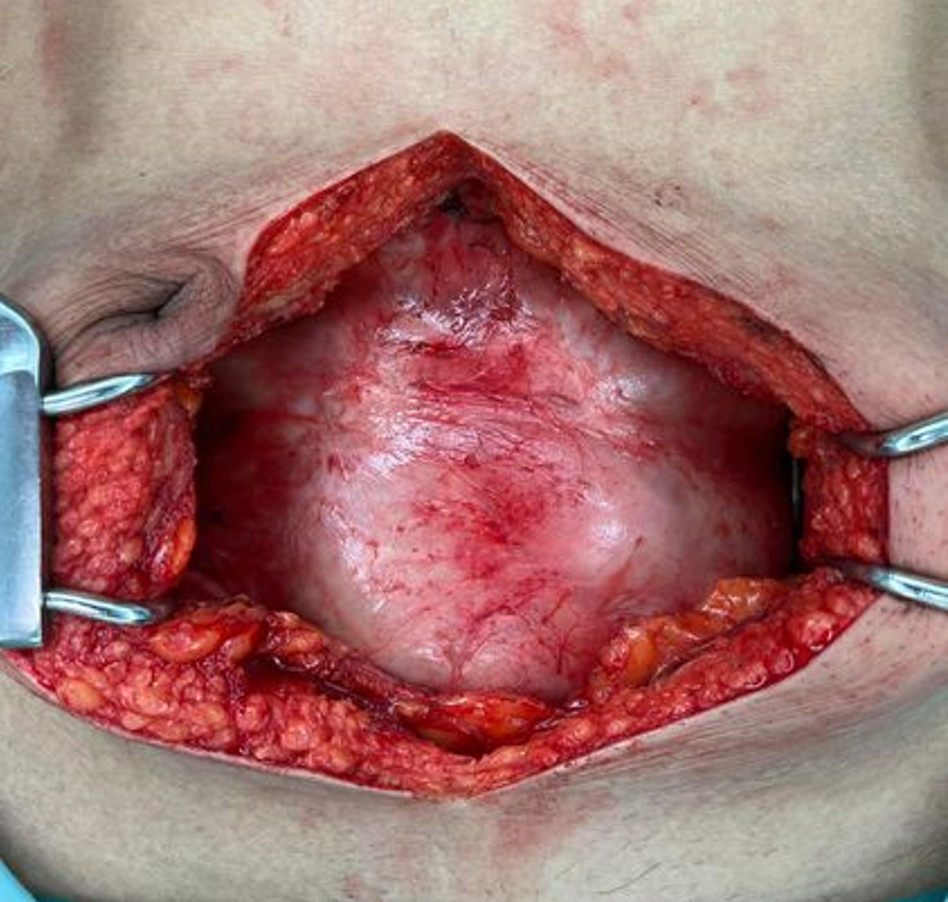
Our initial approach prioritized conservative treatment, considering the absence of peritonitis during the patient's initial presentation at the ER. However, within the subsequent six hours during follow-up, the patient exhibited a fever along with increased abdominal pain. Physical examination revealed significant tenderness around the mass and guarding specifically in the right lower quadrant area. Subsequently, due to the emergence of peritonitis symptoms, an exploratory laparotomy was considered necessary. During the laparotomy, extensive dense fibrotic tissue enveloping the third part of the duodenum up to the upper rectum was observed (Figure 6A), presenting a cocoon-like appearance with multiple loops. Subsequent resection of this fibrotic tissue revealed approximately 20 cm of mid-ileal ischemia attributed to the wrapping of fibrotic tissue around the mesentery (Figure 6B and C). Surgical intervention involved the resection of the affected segment of the small bowel followed by a hand-sewn end-to-end anastomosis by polydioxanone 4-0. Moreover, we decided to perform an appendectomy during the same setting to avoid entering a hostile abdomen in the future, especially with atypical presentation.
After CT scan was done, a subsequent referral to a gastroenterologist raised suspicion of encapsulating peritoneal sclerosis. Despite negative results for sputum acid-fast bacilli (AFB) and Gene X-pert for tuberculosis (TB), esophagogastroduodenoscopy and colonoscopy were performed, uncovering gastritis, erosive esophagitis, and an unremarkable colonoscopy. Histopathological analysis from random biopsies exhibited significant chronic gastritis with moderate activity and persistent inflammation of the terminal ileum. Regrettably, five days after the colonoscopy procedure, the patient developed fresh-onset abdominal pain, prompting an ER visit. Physical examination corroborated the previously documented abdominal mass, devoid of fever, alongside moderate tenderness and voluntary guarding. Subsequent abdominal X-rays exhibited decreasing air in the colon (Figure 3B). Laboratory findings indicated minimal leukocytosis (WBC 9800 /cu.mm, PMN 69%) and normal electrolyte levels. The attending surgeon elected to perform a repeat CT scan, revealing small bowel loops arranged within a substantial, membrane-like sac and a newly developed small bowel feces sign (Figure 4B and C), arousing suspicion of encapsulating peritoneal sclerosis accompanied by complications. Upon exploratory laparotomy, an extensive fibrous sheath enveloping the entirety of the small bowel and colon was revealed (Figure 7A). The fibrous sheath encapsulating the small bowel underwent meticulous dissection through sharp dissection with excision of the sheath. Successful adhesiolysis between the small bowel loops was achieved. While observing small bowel congestion, normal peristalsis was preserved (Figure 7B). A minimal quantity of reddish ascites was collected for laboratory analysis.
Due to bowel obstruction and peritonitis, surgical management was performed. During the surgery, an extensive fibrous sheath covered the entire small bowel and colon, reminiscent of the previous case, albeit with minimal soft adhesions between the parietal peritoneum and the fibrous sheath (Figure 8). Remarkably, the small bowel remained viable. A meager amount of yellowish ascitic fluid was collected for laboratory examination.
The patient was discharged on the seventh-day post-surgery, exhibiting no postoperative complications. After a two-week follow-up, the blood chemistry was within a normal range (CRP 0.2 mg/dL). The culture results were negative for viruses, bacteria, and mycobacteria. The small bowel pathology disclosed acute transmural hemorrhagic infarction, devoid of thrombus in the vascular margin. Analysis of the resected dense, cocoon-like tissue solely indicated fibrosis and fibrous formations, with no signs of malignancy. The whole cocoon contained only fibro-collagenous tissue. Further staining of the specimen for IgG4/IgG revealed a plasma cell ratio of 0%.
After surgery, the patient abstained from food until the resolution of bowel ileus and was discharged on the seventh postoperative day, progressing toward an uncomplicated recovery. Pathological analysis of the fibrous membrane revealed benign fibrotic tissue with mild, persistent inflammation, lacking granulomas, or malignancy. Assessments of ascitic fluid exhibited negative findings for both gram and AFB stains. Cultures for bacteria, fungi, and TB demonstrated uniformly negative results. Follow-up evaluations conducted two weeks after surgery indicated the patient's complete resolution of symptoms and regained body weight. Subsequent abdominal X-rays demonstrated normal air distribution in the small bowel and colon (Figure 3C).
Nevertheless, a year later, the patient required readmission to the trauma unit due to multiple injuries sustained in a fall. Despite the injuries, the patient remained asymptomatic, maintaining a stable body weight of 50 kg. During this hospital stay, the patient encountered hematemesis, leading to a comprehensive whole abdomen CT angiogram performed by a trauma surgeon, revealing normal alignment of the small bowel (Figure 4D).
Postoperative management mirrored that of the previous case, and the patient was discharged on the fifth postoperative day, experiencing an uncomplicated recovery. Pathological scrutiny of the fibrous membrane indicated benign fibrous tissue without granulomas or malignancy. Ascitic fluid analyses showed negative results for gram stain, Wright’s stain, mycobacterial AFB stain, and AFB stain. Bacterial culture yielded negative results. Two weeks postoperatively, the patient remained asymptomatic during the follow-up.
Peritoneal encapsulation, an unusual anatomical condition initially termed peritonitis chronica fibrosa incapsulata in 1907 due to an additional peritoneal membrane, gained recognition as an 'abdominal cocoon' by Foo et al[2] in 1978[1,2]. Despite extensive research, the precise etiology of SEP remains enigmatic. SEP is classified into primary or secondary types based on etiology and pathogenesis[7].
Primary SEP, also known as idiopathic SEP or ACS, denotes instances where alternative causative factors are excluded or when the phenomenon defies explanation within the patient's medical context. Literature analysis reveals a higher prevalence of ACS in specific regions such as China, India, and Turkey, exhibiting a twofold higher incidence in males compared to females[8]. In contrast, secondary SEP is associated with clearly identified etiological causes like peritoneal dialysis, TB infection, sarcoidosis, abdominal trauma, surgery, autoimmune diseases, or beta-blockers used[9,10].
In delineating the complexities of SEP, an intriguing observation surfaces: a seemingly elevated prevalence in Asia, aligning with the reported distribution in previous scholarly works. This regional correlation hints at a distinctive trend within the epidemiological landscape, suggesting a potentially heightened incidence or recognition of SEP cases in Asian populations. This noteworthy observation corresponds to previously documented reports, underscoring a particular focus on Asia in the prevalence and incidence of SEP cases[4,7,10,11].
The rarity of ACS often leads to underdiagnosis, with most patients presenting recurrently with abdominal discomfort, tenderness, nausea/vomiting, intestinal distention, and abdominal masses[7]. Manifestations vary across acute, subacute, and chronic settings, frequently accompanied by weight loss and malnutrition. Notably, Li et al's study highlighted that 75% of chronic ACS patients exhibited a body mass index of < 18.5 kg/m2[4]. The challenging diagnostic landscape results in severe presentations like intestinal obstruction or bowel ischemia, an uncommon occurrence without concomitant bowel obstruction, typically attributed to fibrotic band entanglement within the small bowel mesentery.
Initial diagnosis utilizing a plain X-ray abdomen aids in identifying characteristic signs such as air-fluid levels and dilated bowel loops associated with bowel obstruction cases[12]. Reports suggest ultrasound's potential in diagnosing ACS by revealing dilated small bowel loops, trilaminar bowel wall appearance, membrane formation, and bowel tethering to the posterior abdominal wall. However, its operator-dependent nature limits sensitivity and specificity as shown in the second case report[13]. CT scans emerge as the most reliable diagnostic tool for ACS, revealing thickened peritoneum (> 2 mm), peritoneal or mural calcification, and indications of bowel obstruction[14,15]. Due to the rare etiology of this disease, it is imperative that both surgeons and radiologists possess awareness and understanding of this condition.
The absence of standardized ACS treatment guidelines prompts surgical interventions primarily in cases complicated by intestinal obstruction. Surgical objectives encompass the complete release of fibrotic bands along the intestine and mesenteric root. Bowel resection is reserved for nonviable segments, avoiding unnecessary resections to prevent complications like short bowel syndrome. In select cases, appendectomy during the same procedure may preclude future diagnostic ambiguities, given the potential for atypical presentations in ACS patients[16,17]. Mild cases may warrant conservative treatment; ongoing trials have shown promising outcomes with steroids, colchicine, tamoxifen, and azathioprine[11].
Presently, histopathological analysis is infrequently deemed necessary due to the diagnostic confidence achieved through a combination of CT imaging patterns and clinical manifestations in identifying SEP. Histologically, examination of the peritoneum reveals an overgrowth of fibro-connective tissue, accompanied by inflammatory infiltrates and dilated lymphatics. Notably absent are indications of foreign body granulomas, giant cells, or birefringent material. The term 'sclerosing' delineates the progressive development of dense collagenous layers, 'encapsulating' refers to the newly formed fibrous tissue sheath enveloping and constricting the small bowel, thereby restricting its motility. Moreover, 'peritonitis' signifies an ongoing inflammatory state, evidenced by a mononuclear inflammatory infiltrate within the evolving fibrosing tissue[6,18,19].
This comprehensive overview of SEP and ACS highlights the multifaceted nature of these conditions, emphasizing the need for further research to establish standardized diagnostic protocols and effective treatment guidelines to improve patient outcomes.
In conclusion, the intricate nature of SEP, particularly in its manifestation as ACS, underscores the challenges in understanding its origins and diagnosing its diverse presentations. While primary and secondary classifications offer insights into potential causative factors, the rarity of ACS often leads to underdiagnosis, accentuating the need for heightened clinical suspicion. Diagnostic modalities like plain X-ray, ultrasound, and CT scans provide crucial tools, yet their limitations necessitate further refinement for accurate and timely identification. Surgical interventions, guided by tailored approaches, remain pivotal in managing complications, advocating for meticulous band release while preserving bowel integrity. Amidst ongoing trials exploring conservative treatments, a standardized approach is paramount, urging continued research to unravel the complexities of SEP and ACS, thereby paving the way for enhanced diagnostic stra
| 1. | Singhal M, Krishna S, Lal A, Narayanasamy S, Bal A, Yadav TD, Kochhar R, Sinha SK, Khandelwal N, Sheikh AM. Encapsulating Peritoneal Sclerosis: The Abdominal Cocoon. Radiographics. 2019;39:62-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (3)] |
| 2. | Foo KT, Ng KC, Rauff A, Foong WC, Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978;65:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 207] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Awe JA. Abdominal cocoon syndrome (idiopathic sclerosing encapsulating peritonitis): how easy is its diagnosis preoperatively? A case report. Case Rep Surg. 2013;2013:604061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Li N, Zhu W, Li Y, Gong J, Gu L, Li M, Cao L, Li J. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014;38:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Chorti A, Panidis S, Konstantinidis D, Cheva A, Papavramidis T, Michalopoulos A, Paramythiotis D. Abdominal cocoon syndrome: Rare cause of intestinal obstruction-Case report and systematic review of literature. Medicine (Baltimore). 2022;101:e29837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25 Suppl 4:S19-S29. [PubMed] |
| 7. | Machado NO. Sclerosing Encapsulating Peritonitis: Review. Sultan Qaboos Univ Med J. 2016;16:e142-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015;21:675-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 9. | Xia J, Xie W, Chen L, Liu D. Abdominal cocoon with early postoperative small bowel obstruction: A case report and review of literature in China. Medicine (Baltimore). 2018;97:e11102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Aziz W, Malik Y, Haseeb S, Mirza RT, Aamer S. Abdominal Cocoon Syndrome: A Laparoscopic Approach. Cureus. 2021;13:e16787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kappauf C, Rahaman J, Popowich D. Abdominal cocoon: an unexpected cause of ascites in a healthy patient. J Surg Case Rep. 2019;2019:rjz310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Karona P, Blevrakis E, Kastanaki P, Tzouganakis A, Kastanakis M. Abdominal Cocoon Syndrome: An Extremely Rare Cause of Small Bowel Obstruction. Cureus. 2021;13:e14351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012;18:1999-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (5)] |
| 14. | Dave A, McMahon J, Zahid A. Congenital peritoneal encapsulation: A review and novel classification system. World J Gastroenterol. 2019;25:2294-2307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 15. | Gupta S, Shirahatti RG, Anand J. CT findings of an abdominal cocoon. AJR Am J Roentgenol. 2004;183:1658-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (3)] |
| 16. | Solak A, Solak İ. Abdominal cocoon syndrome: preoperative diagnostic criteria, good clinical outcome with medical treatment and review of the literature. Turk J Gastroenterol. 2012;23:776-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wei B, Wei HB, Guo WP, Zheng ZH, Huang Y, Hu BG, Huang JL. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009;198:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 18. | Clatworthy MR, Williams P, Watson CJ, Jamieson NV. The calcified abdominal cocoon. Lancet. 2008;371:1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Okada K, Onishi Y, Oinuma T, Nagura Y, Soma M, Saito S, Kanmatsuse K, Takahashi S. Sclerosing encapsulating peritonitis: regional changes of peritoneum. Nephron. 2002;92:481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Esch M, Germany S-Editor: Qu XL L-Editor: A P-Editor: Cai YX