Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.777
Peer-review started: December 17, 2023
First decision: January 4, 2024
Revised: January 9, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 27, 2024
Processing time: 90 Days and 11.6 Hours
Colorectal cancer is the third most common cancer and the second highest cause of cancer-related mortality worldwide. About 5%-10% of patients are diagnosed with locally advanced rectal cancer (LARC) on presentation. For LARC invading into other structures (i.e. T4b), multivisceral resection (MVR) and/or pelvic ex
To assess the feasibility and safety of minimally invasive MVR (miMVR), and compare post-operative outcomes between robotic and laparoscopic MVR.
This is a single-center retrospective cohort study from 1st January 2015 to 31st March 2023. Inclusion criteria were patients diagnosed with cT4b rectal cancer and underwent MVR, or stage 4 disease with resectable systemic metastases. Pa
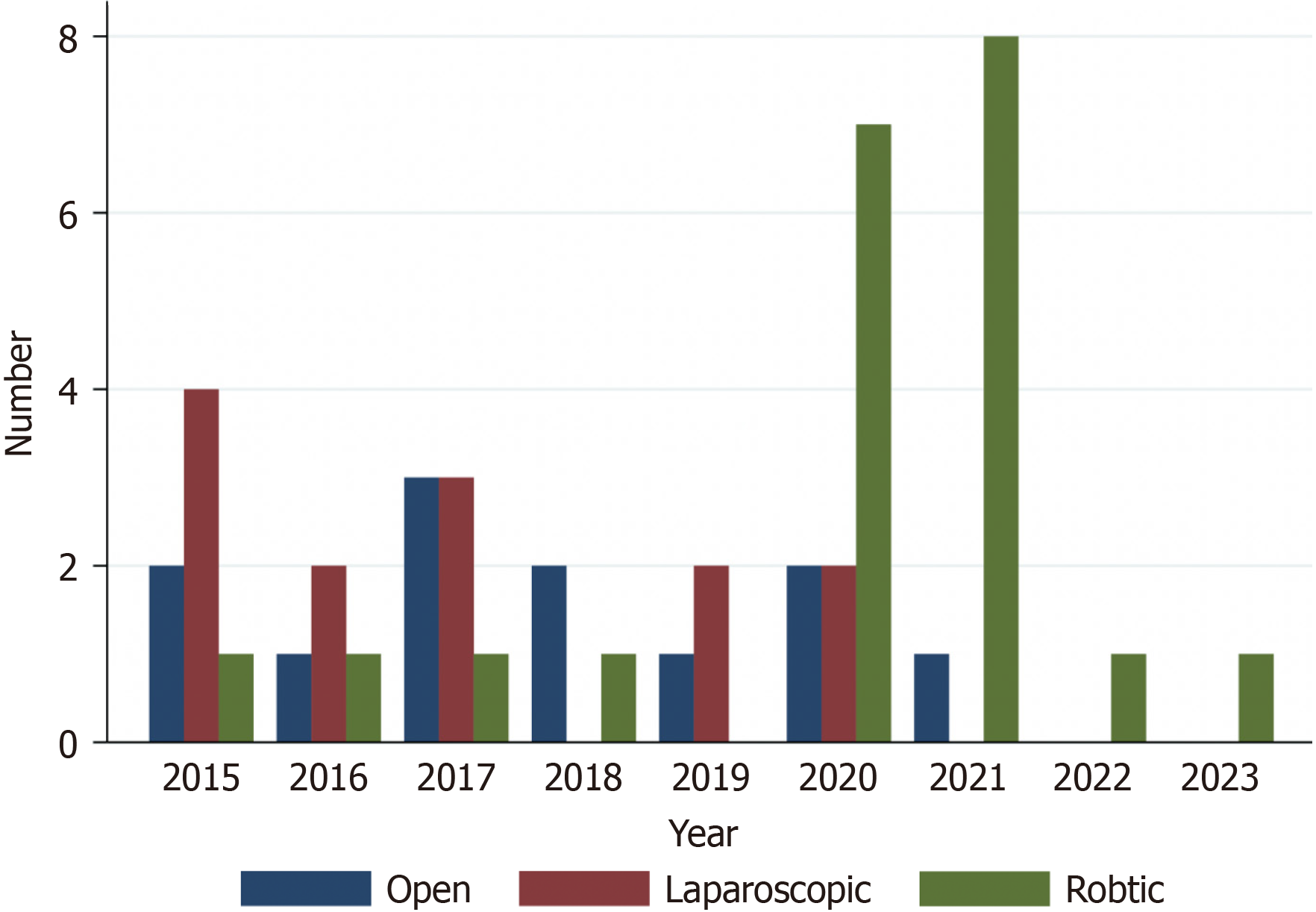
A total of 46 patients were included in this study [open MVR (oMVR): 12 (26.1%), miMVR: 36 (73.9%)]. Patients’ American Society of Anesthesiologists score, body mass index and co-morbidities were comparable between oMVR and miMVR. There is an increasing trend towards robotic MVR from 2015 to 2023. MiMVR was associated with lower estimated blood loss (EBL) (median 450 vs 1200 mL, P = 0.008), major morbidity (14.7% vs 50.0%, P = 0.014), post-operative intra-abdominal collections (11.8% vs 50.0%, P = 0.006), post-operative ileus (32.4% vs 66.7%, P = 0.04) and surgical site infection (11.8% vs 50.0%, P = 0.006) compared with oMVR. Length of stay was also shorter for miMVR compared with oMVR (median 10 vs 30 d, P = 0.001). Oncological outcomes–R0 resection, recurrence, OS and RFS were comparable between miMVR and oMVR. There was no 30-d mortality. More patients underwent robotic compared with laparoscopic MVR for complex cases (robotic 57.1% vs laparoscopic 7.7%, P = 0.004). The operating time was longer for robotic compared with laparoscopic MVR [robotic: 602 (400-900) min, laparoscopic: Median 455 (275-675) min, P < 0.001]. Incidence of R0 resection was similar (laparoscopic: 84.6% vs robotic: 76.2%, P = 0.555). Overall complication rates, major morbidity rates and 30-d readmission rates were similar between la
MiMVR had lower post-operative complications compared to oMVR. Robotic MVR was also safe, with acceptable post-operative complication rates. Prospective studies should be conducted to compare short-term and long-term outcomes between robotic vs laparoscopic MVR.
Core Tip: Multivisceral resection (MVR) remains the only potential curative surgical treatment for locally advanced rectal cancer but bears high morbidity. Literature on minimally invasive MVR (miMVR) is scarce. Our results showed that miMVR had lower major morbidity and shorter length of stay compared to open MVR with comparable R0 resection and long-term survival. Robotic MVR was used for more complex cases but had similar post-operative complications compared to laparoscopic MVR. Use of robotic MVR is feasible and safe even in lower volume institutions for locally advanced rectal cancer.
- Citation: Chan KS, Liu B, Tan MNA, How KY, Wong KY. Feasibility and safety of minimally invasive multivisceral resection for T4b rectal cancer: A 9-year review. World J Gastrointest Surg 2024; 16(3): 777-789
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/777.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.777
Colorectal cancer is the third most common cancer and the second highest cause of cancer-related mortality worldwide[1]. About 5%-10% of patients are diagnosed with locally advanced rectal cancer (LARC) on presentation[1-3]. Neo
Nevertheless, MVR and/or PE is a major and complex surgery with post-operative morbidity ranging from 20%-80%[8], and 30-d mortality of 0.5%-2.0%[9-11]. Minimally invasive surgery (MIS) which has been well-established as standard of care both benign and malignant intra-abdominal pathologies[12-15], may prove its utility on short-term post-operative outcomes. A retrospective study by Kazi et al[16] on 158 patients who underwent PE for LARC showed reduced intra-operative blood loss (900 vs 1600 mL, P < 0.001) and incidence of wound infections (8.2% vs 17.5%, P = 0.02) for minimally invasive PE compared with open PE, with similar 3-year overall survival (OS)[16]. However, existing randomized controlled trials on MIS rectal surgeries exclude T4 rectal tumours requiring extended resections with MVR[17-19]. In addition, while meta-analyses have compared MIS vs open PE for pelvic malignancies, only a minority of included pa
This is a single-center retrospective review of a prospectively maintained database from 1st January 2015 to 31st March 2023. Inclusion criteria were patients diagnosed with cT4b rectal cancer and underwent MVR, or stage 4 disease with resectable systemic metastases; patients with stage 4 disease were included in our study in view of the small sample size in our data set, and also because the presence of systemic metastases will not affect our primary aim (i.e. assessing fea
Patient demographics, presence of neoadjuvant treatment, intra-operative characteristics, histopathological findings and post-operative outcomes, and oncological outcomes were studied. In view of the heterogenous cohort of patients with varying extents of MVR, we arbitrarily classified patients who underwent laparoscopic or robotic MVR into simple or complex; complex cases were defined as cases which were more technically challenging in view of extent of resection, and/or difficulty with reconstruction (i.e. total PE, bladder-sparing prostatectomy and/or pelvic lymph node dissection (PLND), need for flap creation, or sacrectomy)[22]. Other cases were defined as simple (i.e. posterior PE, vaginectomy or seminal vesiculectomy).
Post-operative outcomes include the presence of intra-abdominal collection, anastomotic leak, ileus, wound infection, pneumonia, overall complication (defined as presence of any of the above complications), major morbidity, 30-d readmission and 30-d mortality. Major morbidity was defined as Clavien-Dindo ≥ Grade 3A complications[23]. Thirty day readmission was defined as re-admission within 30 d from date of discharge, while 30-d mortality was defined as death within 30 d from the date of surgery. Oncological outcomes include presence of local and systemic recurrence, time to local and systemic recurrence, OS and recurrence-free survival (RFS). OS and RFS were defined as proportion of pa
Enhanced Recovery After Surgery (ERAS) was introduced in our colorectal surgery unit from 2016. Pre-operative workup for newly diagnosed rectal cancer includes basic biochemistry investigations including full blood count and carcinoembryonic antigen, radiological imaging with computed tomography of the thorax, abdomen and pelvis and magnetic resonance imaging of the rectum, and colonoscopy with histological diagnosis. All patients diagnosed with rectal cancer were discussed in a multidisciplinary tumour board consisting of colorectal surgeons, medical oncologists and radiation oncologists both pre-operatively and post-operatively, except for patients who had emergency surgery (only post-ope
All patients planned for elective surgical resection from 2016 were enrolled into the standard ERAS pathway with standard peri-operative management[27]. Mechanical bowel preparation with polyethylene glycol solution and an
Categorical values were described as percentages and analysed by the chi-square test. Continuous variables were ex
A total of 46 patients were included in this study [oMVR: 12 (26.1%), miMVR: 34 (73.9%)]. Patients’ American Society of Anesthesiologists (ASA) score, body mass index and co-morbidities were comparable between oMVR and miMVR. How
| Total (n = 46) | Minimally invasive (n = 34) | Open (n = 12) | P valuea | |
| Age, yr | 68.0 (44.0-85.0) | 68.0 (44.0-85.0) | 67.5 (45.0-85.0) | 0.573 |
| Sex, male (%) | 21 (45.7) | 15 (44.1) | 6 (50) | 0.725 |
| BMI, kg/m2 | 22.5 (12.6-37.9) | 22.0 (12.6-32.1) | 23.3 (16.4-37.9) | 0.708 |
| ASA score, median | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.966 |
| 1 | 13 (28.3) | 10 (29.4) | 3 (25.0) | |
| 2 | 27 (58.7) | 19 (55.9) | 8 (66.7) | |
| 3 | 6 (13.0) | 5 (14.7) | 1 (8.3) | |
| Co-morbidities | ||||
| Hypertension | 23 (50.0) | 17 (50.0) | 6 (50.0) | 1.000 |
| Hyperlipidemia | 18 (39.1) | 14 (41.2) | 4 (33.3) | 0.632 |
| Diabetes mellitus | 11 (23.9) | 9 (26.5) | 2 (16.7) | 0.494 |
| Ischemic heart disease | 3 (6.5) | 3 (8.8) | 0 (0) | 0.287 |
| Previous abdominal surgery | 7 (15.2) | 5 (14.7) | 2 (16.7) | 0.871 |
| Neoadjuvant chemotherapy | 31 (67.4) | 25 (73.5) | 6 (50.0) | 0.135 |
| Neoadjuvant radiotherapy | 36 (78.3) | 29 (85.3) | 7 (58.3) | 0.052 |
| Previous local recurrence | 3 (6.5) | 2 (5.9) | 1 (8.3) | 0.768 |
The extent of MVR is summarised in Table 2. Of the patients who underwent oMVR, two cases had bilateral PLND, two cases had sacrectomy and three cases had vertical rectus abdominis myocutaneous (VRAM) flap reconstruction. None of the patients had resectable distant metastases. miMVR is associated with lower EBL (median 450 vs 1200 mL, P = 0.008), major morbidity (14.7% vs 50.0%, P = 0.014), post-operative intra-abdominal collections (11.8% vs 50.0%, P = 0.006), post-operative ileus (32.4% vs 66.7%, P = 0.04) and surgical site infection (SSI) (11.8% vs 50.0%, P = 0.006) compared with oMVR. Length of stay (LOS) was also shorter for miMVR compared with oMVR (median 10 vs 30 d, P = 0.001). Onco
| Total (n = 46) | Minimally invasive (n = 34) | Open (n = 12) | P value | |
| Surgical access | N/A | |||
| Laparoscopic | 13 (28.3) | 13 (38.2) | - | |
| Robotic | 21 (45.7) | 21 (61.8) | - | |
| Urgency of surgery | 0.015a | |||
| Elective | 44 (95.7) | 34 (100) | 10 (83.3) | |
| Emergency | 2 (4.3) | 0 (0) | 2 (16.7) | |
| Type of surgery | 0.147 | |||
| ULAR with DI | 30 (65.2) | 25 (73.5) | 5 (41.7) | |
| APR | 11 (23.9) | 6 (17.6) | 5 (41.7) | |
| Hartmann’s procedure | 3 (6.5) | 2 (5.9) | 1 (8.3) | |
| LAR | 1 (2.2) | 0 (0) | 1 (8.3) | |
| taTME | 1 (2.2) | 1 (2.9) | 0 (0) | |
| Extent of resection | - | |||
| En-bloc seminal vesicles | 5 (10.9) | 5 (14.7) | 0 (0) | |
| Posterior vaginectomy | 4 (8.7) | 4 (11.8) | 0 (0) | |
| Salpingo-oopherectomy | 1 (2.2) | 1 (2.9) | 0 (0) | |
| Bladder sparing prostatectomy | 5 (10.9) | 4 (11.8) | 1 (8.3) | |
| Posterior exanteration | 22 (47.8) | 16 (47.1) | 6 (50.0) | |
| Total pelvic exanteration | 9 (19.6) | 4 (11.8) | 5 (41.7) | |
| Small bowel resection | 1 (2.2) | 1 (2.9) | 0 (0) | |
| Sacrectomy | 3 (6.5) | 0 (0) | 3 (25.0) | |
| Initial stoma creation | 35 (76.1) | 27 (79.4) | 7 (58.3) | 0.153 |
| Eventual reversal | 18 (51.4) | 15 (55.6) | 3 (42.9) | |
| Intra-operative ureteric injury | 2 (4.3) | 2 (5.9) | 0 (0) | 0.390 |
| Operating time, min | 562 (225-900) | 566.5 (275-900) | 502.5 (225-751) | 0.150 |
| Estimated blood loss, mL | 500 (0-4000) | 450 (0-2400) | 1200 (200-4000) | 0.008a |
| Pathological TNM stage | 0.836 | |||
| Complete pathological response | 1 (2.2) | 1 (2.9) | 0 (0) | |
| 1 | 1 (2.2) | 1 (2.9) | 0 (0) | |
| 2 | 20 (43.5) | 15 (44.1) | 5 (41.7) | |
| 3 | 17 (37.0) | 11 (32.4) | 6 (50.0) | |
| 4 | 7 (15.2) | 6 (17.6) | 1 (8.3) | |
| R0 resection | 37 (80.4) | 27 (79.4) | 10 (83.3) | 0.768 |
| Total (n = 46) | Minimally invasive (n = 34) | Open (n = 12) | P value | |
| Short-term complications | ||||
| Anastomotic leak | 7 (15.2) | 4 (11.8) | 3 (25.0) | 0.272 |
| Intra-abdominal collection | 10 (21.7) | 4 (11.8) | 6 (50.0) | 0.006a |
| Ileus | 19 (41.3) | 11 (32.4) | 8 (66.7) | 0.038a |
| SSI | 10 (21.7) | 4 (11.8) | 6 (50.0) | 0.006a |
| Pneumonia | 2 (5.9) | 2 (5.9) | 0 (0) | 0.390 |
| Overall complications | 30 (65.2) | 20 (58.8) | 10 (83.3) | 0.125 |
| Major morbidity | 11 (23.9) | 5 (14.7) | 6 (50.0) | 0.014a |
| Length of stay, d | 12.0 (3.0-62.0) | 10.0 (3.0-42.0) | 30.0 (6.0-62.0) | < 0.001a |
| 30-d readmission | 11 (23.9) | 8 (23.5) | 3 (25.0) | 0.918 |
| 30-d mortality | 0 (0) | 0 (0) | 0 (0) | N/A |
| Adjuvant chemotherapy | 31 (67.4) | 24 (70.6) | 7 (58.3) | 0.436 |
| Follow-up, months | 32.2 (1.1-100.8) | 29.2 (1.1-100.8) | 36.2 (2.2-73.9) | 0.582 |
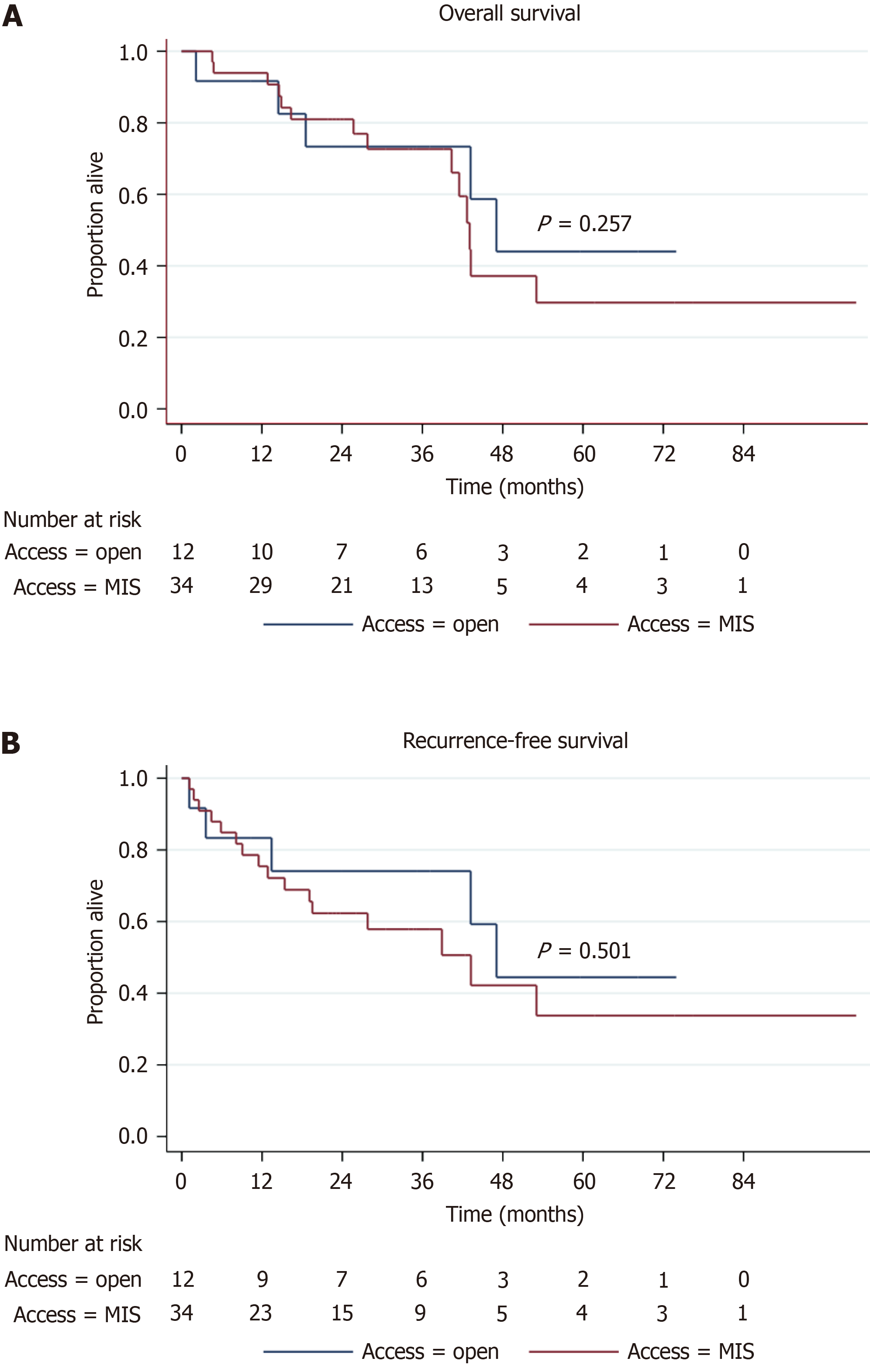
| Overall survival | ||||
| Median, months | 43.2 (41.5-N/A) | 43.1 (40.4-N/A) | 47.1 (14.5-N/A) | 0.257 |
| 1-year | 93.2 (80.3-97.8) | 93.9 (77.6-98.4) | 91.3 (52.4-98.7) | 0.774 |
| 3-year | 71.5 (54.1-83.3) | 71.1 (49.8-84.6) | 72.1 (35.9-90.1) | 0.473 |
| 5-year | 36.5 (18.2-55.0) | 32.1 (11.9-54.6) | 45.9 (13.6-73.8) | 0.446 |
| Recurrence-free survival | ||||
| Median, months | 43.2 (19.5-N/A) | 43.2 (15.4-N/A) | 47.1 (3.6-N/A) | 0.501 |
| 1-year | 77.3 (61.9-87.1) | 75.4 (56.8-86.9) | 82.6 (46.5-95.3) | 0.800 |
| 3-year | 60.8 (43.8-74.1) | 56.1 (36.0-72.2) | 72.9 (37.1-90.4) | 0.879 |
| 5-year | 37.1 (19.0-55.3) | 33.7 (13.1-55.8) | 17.2 (13.8-74.2) | 0.371 |
Additional comparison was also made between laparoscopic and robotic MVR. More patients who had complex surgeries underwent robotic compared to laparoscopic MVR (robotic 57.1% vs laparoscopic 7.7%, P = 0.004). The ope
| Robotic (n = 21) | Laparoscopic (n = 13) | P value | |
| Intra-operative ureteric injury | 1 (4.8) | 1 (7.7) | 0.724 |
| Open conversion | 1 (4.8) | 3 (23.1) | 0.107 |
| Surgical complexity | 0.004a | ||
| Simple | 9 (42.9) | 12 (92.3) | |
| Complex | 12 (57.1) | 1 (7.7) | |
| Short-term complications | |||
| Anastomotic leak | 1 (4.8) | 3 (23.1) | 0.107 |
| Intra-abdominal collection | 1 (4.8) | 3 (23.1) | 0.107 |
| Ileus | 6 (28.6) | 5 (38.5) | 0.549 |
| SSI | 1 (4.8) | 3 (23.1) | 0.107 |
| Pneumonia | 1 (4.8) | 1 (7.7) | 0.724 |
| Overall complications | 12 (57.1) | 8 (61.5) | 0.800 |
| Major morbidity | 3 (14.3) | 2 (15.4) | 0.930 |
| Length of stay, d | 11.0 (4.0-28.0) | 0.807 | |
| 30-d readmission | 5 (23.8) | 3 (23.1) | 0.961 |
| 30-d mortality | 0 (0) | 0 (0) | N/A |
| Adjuvant chemotherapy | 15 (71.4) | 9 (69.2) | 0.891 |
| Follow-up, months | 24.2 (4.8-100.8) | 41.5 (1.1-76.5) | 0.158 |
| Overall survival | |||
| Median, months | 43.2 (43.1-N/A) | 40.4 (12.9-53.0) | 0.149 |
| 1-year | 95.0 (69.5-99.3) | 92.0 (55.3-98.8) | 0.083 |
| 3-year | 83.1 (56.1-94.3) | 58.6 (27.2-80.2) | 0.008a |
| 5-year | 49.9 (13.1-78.8) | 21.0 (3.7-47.9) | 0.175 |
| Recurrence-free survival | |||
| Median, months | 43.2 (19.5-N/A) | 12.9 (2.6-53.0) | 0.096 |
| 1-year | 85.0 (60.4-94.9) | 60.0 (29.0-81.0) | 0.607 |
| 3-year | 72.9 (46.2-87.8) | 34.3 (10.8-59.8) | 0.002a |
| 5-year | 40.5 (9.3-70.9) | 22.9 (4.3-50.1) | 0.070 |
MVR is a complex surgery with high short-term morbidity and mortality[8-11]. MIS has been shown to improve short-term outcomes in various surgeries for gastrointestinal malignancies[12-14]. Our study demonstrated the safety of mi
One of the reasons why MVR is associated with high post-operative morbidity is due to the extent of resection; this results in longer operating time and is more technically challenging for en-bloc resection due to the need for more extensive dissection and higher risk of injury to other structures. Despite the low numbers of 46 cases over a period of 9 years, our institution demonstrated acceptable post-operative outcomes with no 30-d mortality and overall complication rate of 65.2%. This is similar to that reported in existing literature, with complication rates of 20%-80%[8], and 30-d mortality of 0.5%-2.0%[9-11].
Since the advent of robotic surgery, there has been an increasing adoption of its use in various institutions over the past decade[30]. Our unit adopted robotic surgery in 2011 and the first use of the robotic platform for MVR was in 2015. As our surgeons gained more experience and proficiency in robotic surgery, we have achieved nearly 100% utilization of robotic surgery in elective MVR rectal surgery over the last three years within our unit. Robotic surgery allows for a 3-dimensional view with depth perception and high-resolution imaging, free manipulation of robotic endowrists with a wider degree of movement, and ergonomic advantages (e.g. elimination of tremors, surgeon’s comfort)[31]. This allows for easier manoeuvring within the narrow pelvis, and also makes intracorporeal suturing and reconstruction (e.g. ureterectomy with Boari flap reconstruction for patients with ureteric involvement) much easier[32]. Another benefit of robotic surgery-which may be frequently overlooked–is the provision of an ergonomic environment for the operating surgeon which reduces physical strain and fatigue, especially in the context of complex surgeries requiring long operating hours[33,34]. This is evident in our study, where there were significantly more patients who underwent robotic MVR for com
Another benefit of the robotic platform includes the inbuilt integrated fluorescence capability with indocyanine green (ICG) (Firefly on the Da Vinci© robotic system), which can be activated at the surgeon’s console without the need to change to an ICG-enabled imaging system unlike laparoscopic surgery. In low rectal surgery, use of ICG allows for easy identification of ureters to avoid ureteric injury, as well as guide lymph node dissection[36]. While our series had 2 cases of intra-operative ureteric injury (1 laparoscopic, 1 robotic), both cases occurred prior to the routine pre-operative ureteric stenting and intra-operative ICG. No further cases of intra-operative ureteric injury were noted thereafter.
Nevertheless, despite the advantages of the robotic system described above, our study did not show a statistically significant difference in post-operative complications between laparoscopic and robotic MVR, which may be due to the initial learning curve of robotic MVR[37]. The surgeons’ experience in our robotic group had varying experience levels, with some having completed 10 to 20 robotic cases before undertaking MVR rectal surgery. However, for complex MVR procedures, an experienced robotic surgeon was always present. This underscores the notion that acquiring competence in robotic surgery can be achieved more swiftly compared to laparoscopic surgery. Nevertheless, an absolute reduction of 18.3% in anastomotic leak is clinically significant. Lack of statistical significance may be because our study was un
One criticism regarding the use of robotic MVR would be the longer operating time without any additional benefits compared to laparoscopic MVR. Robotic surgery has traditionally been shown to be associated with longer operating time, possibly because of initial learning curves and familiarity with the use of a robotic system[39], but this has been largely mitigated by the ease of use of the latest Da Vinci Xi© system. Although our study showed that robotic MVR had a longer median operating time of 602 min, compared to 455 min in laparoscopic MVR, it was not surprising given that most of these cases were more complex surgeries. These surgeries would also have been technically more challenging if performed laparoscopically.
With the theoretical benefits of robotic surgery over laparoscopic surgery, it is postulated that long-term survival will be higher, and recurrence will be lower in the robotic group. This was supported by our study which showed superior 3-year OS and 3-year RFS in robotic MVR compared with laparoscopic MVR. However, the Robotic vs Laparoscopic Resection for Rectal Cancer (ROLARR) trial in 2017 failed to show any statistical significance between robotic vs laparoscopic rectal resection in rectal adenocarcinoma [local recurrence hazards ratio (HR) 1.137, 95%CI: 0.554, 2.335, P = 0.756][18]. However, the primary aim of the ROLARR trial was to compare the risk of conversion to open laparotomy, rather than assessing long-term survival outcomes. Sample size calculation was performed based on the primary aim, and may have been underpowered to detect statistical significance in long-term survival between robotic and laparoscopic surgery. Use of learning effects model also showed that increasing level of robotic experience was associated with better treatment effects when comparing robotic vs laparoscopic surgery; benefits of robotic surgery may not be reaped when surgeons are still in the initial learning curve[40].
Conflicting results on long-term survival were shown by Kim et al[41], who performed a 1:1 propensity score matching (PSM) (n = 224 patients per arm) for patients who underwent robotic vs laparoscopic TME for rectal cancer[41]. While 5-year OS (robotic 90.5% vs laparoscopic 78.0%) and 5-year cancer-specific survival (CSS) (robotic 90.5% vs laparoscopic 79.5%) were statistically insignificant between robotic vs laparoscopic TME, this may be due to the reduced sample size following PSM (prior to PSM, 5-year OS and 5-year CSS was superior in the robotic group). Multivariate analysis also showed that robotic TME was a significant prognostic factor for OS (HR 0.333, P = 0.004) and CSS (HR 0.367, P = 0.0161). We postulate that robotic surgery may improve long-term survival with more precise dissection and adequacy of re
Comparing miMVR as a whole vs oMVR, our study demonstrated better short-term outcomes. This is not surprising; benefits of MIS have been shown to be superior compared with open surgery in both benign and malignant conditions, such as omental patch repair for perforated peptic ulcer, oesphagectomy, colorectal resection and liver resection[12-15]. The concept behind the benefits of MIS remains the same regardless of the type of surgery. The incision from MIS is smaller and less traumatic. As a result, this leads to lesser pain, LOS, incidence of SSI and intra-abdominal collection, as shown by our results. It is noteworthy that the incidence of post-operative ileus and SSI were high in the oMVR group, at 66.7% and 50.0% respectively. One plausible reason for this finding may be due to the extent of MVR, with 2 cases of bilateral PLND, 2 cases of sacrectomy, and 3 cases with VRAM flap. Ileus is expected following sacrectomy due to the denervation of the distal gastrointestinal tract during neural transection[42]. Use of VRAM flap also increases operating time, which consequently increases risk of post-operative ileus[43].
There are a few limitations to this study. Firstly, this is a retrospective cohort study with inherent selection bias. This is primarily a single-arm study with an aim of looking at the feasibility and safety profile of minimally miMVR; while we compared minimally invasive vs oMVR, sample size for the open group is small and the study is possibly underpowered to detect significant differences between the group[44]. Long-term oncological outcomes such as OS and DFS may also not be conclusive or representative of other cohorts, especially for the oMVR group, with a small sample size of 12 only. However, the main aim of this study was to assess safety profile of miMVR and also robotic MVR in our institution-a low volume centre-for cT4b rectal cancer; hence the results presented here are promising. Lastly, quality of life outcome mea
Our study showed the feasibility and safety of miMVR even in a low-volume institution for cT4b rectal cancer, with acceptable short-term morbidity, 30-d mortality and long-term survival. Additionally, miMVR was associated with shorter LOS and lower incidence of ileus and SSI. More prospective studies are required to evaluate the long-term oncological outcomes of miMVR; further studies should also compare robotic vs laparoscopic approach in MVR.
About 5%-10% of patients are diagnosed with locally advanced rectal cancer (LARC) on presentation. Multivisceral resection (MVR) and/or pelvic exenteration (PE) remains the only potential curative surgical treatment for LARC invading into other structures (i.e. cT4b tumours). However, MVR and/or PE is a major surgery with significant post-operative morbidity. There is currently no randomized controlled trial assessing T4 rectal tumours requiring extended resections with MVR.
Minimally invasive surgery (MIS) for other intra-abdominal pathologies has been shown to improve post-operative outcomes. However, evidence on the use of MIS for MVR and PE is not well established. Evidence on the use of robotic MVR and/or PE is even more scarce and needs to be reported.
Our primary aim is to assess the feasibility and safety of minimally invasive MVR (miMVR) in terms of the margin status and post-operative complications, and compare the outcomes between robotic and laparoscopic MVR. Our secondary aims are to assess the long-term survival of patients who underwent miVR, as well as compare between miVR compared to open MVR (oMVR).
This is a single-center retrospective review of a prospectively maintained database from 1st January 2015 to 31st March 2023. Inclusion criteria were patients diagnosed with cT4b rectal cancer and underwent MVR, or stage 4 disease with resectable systemic metastases. Comparison in outcomes were made between miMVR and oMVR. Categorical values were described as percentages and analysed by the chi-square test. Continuous variables were expressed as median (range) and analysed by Mann-Whitney U test. Cumulative overall survival and RFS were analysed using Kaplan-Meier estimates with life table analysis. Subgroup analysis was performed with the above statistical methods to compare between robotic and laparoscopic MVR.
Forty-six patients were included in this study [oMVR: 12 (26.1%), miMVR: 34 (73.9%)]. Patients’ American Society of Anesthesiologists score, body mass index and co-morbidities were comparable between oMVR and miMVR. The in
miMVR is safe and feasible even in a low-volume institution for cT4b rectal cancer with acceptable R0 resection, short-term morbidity, 30-d mortality and long-term survival.
Robotic MVR should be considered even in low volume institutions in view of the advantages conferred by robotic surgery in the presence of a proctor.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68488] [Article Influence: 13697.6] [Reference Citation Analysis (201)] |
| 2. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1835] [Reference Citation Analysis (5)] |
| 3. | Kokelaar RF, Evans MD, Davies M, Harris DA, Beynon J. Locally advanced rectal cancer: management challenges. Onco Targets Ther. 2016;9:6265-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 1106] [Article Influence: 221.2] [Reference Citation Analysis (1)] |
| 5. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3157] [Article Influence: 126.3] [Reference Citation Analysis (9)] |
| 6. | Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644-5650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 591] [Article Influence: 28.1] [Reference Citation Analysis (8)] |
| 7. | Yang TX, Morris DL, Chua TC. Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum. 2013;56:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 8. | Koh CE, Solomon MJ, Brown KG, Austin K, Byrne CM, Lee P, Young JM. The Evolution of Pelvic Exenteration Practice at a Single Center: Lessons Learned from over 500 Cases. Dis Colon Rectum. 2017;60:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | PelvEx Collaborative. Factors affecting outcomes following pelvic exenteration for locally recurrent rectal cancer. Br J Surg. 2018;105:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Peacock O, Waters PS, Kong JC, Warrier SK, Wakeman C, Eglinton T, Heriot AG, Frizelle FA, McCormick JJ. Complications After Extended Radical Resections for Locally Advanced and Recurrent Pelvic Malignancies: A 25-Year Experience. Ann Surg Oncol. 2020;27:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Venchiarutti RL, Solomon MJ, Koh CE, Young JM, Steffens D. Pushing the boundaries of pelvic exenteration by maintaining survival at the cost of morbidity. Br J Surg. 2019;106:1393-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Mederos MA, de Virgilio MJ, Shenoy R, Ye L, Toste PA, Mak SS, Booth MS, Begashaw MM, Wilson M, Gunnar W, Shekelle PG, Maggard-Gibbons M, Girgis MD. Comparison of Clinical Outcomes of Robot-Assisted, Video-Assisted, and Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e2129228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 14. | Zhuang CL, Huang DD, Chen FF, Zhou CJ, Zheng BS, Chen BC, Shen X, Yu Z. Laparoscopic versus open colorectal surgery within enhanced recovery after surgery programs: a systematic review and meta-analysis of randomized controlled trials. Surg Endosc. 2015;29:2091-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Chan KS, Ng STC, Tan CHB, Gerard G, Oo AM. A systematic review and meta-analysis comparing postoperative outcomes of laparoscopic versus open omental patch repair of perforated peptic ulcer. J Trauma Acute Care Surg. 2023;94:e1-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Kazi M, Kumar NAN, Rohila J, Sukumar V, Engineer R, Ankathi S, Desouza A, Saklani A. Minimally invasive versus open pelvic exenterations for rectal cancer: a comparative analysis of perioperative and 3-year oncological outcomes. BJS Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E; COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 959] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 18. | Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA. 2017;318:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1009] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 19. | Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 662] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 20. | Ryan OK, Doogan KL, Ryan ÉJ, Donnelly M, Reynolds IS, Creavin B, Davey MG, Kelly ME, Kennelly R, Hanly A, Martin ST, Winter DC. Comparing minimally invasive surgical and open approaches to pelvic exenteration for locally advanced or recurrent pelvic malignancies - Systematic review and meta-analysis. Eur J Surg Oncol. 2023;49:1362-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 7427] [Article Influence: 618.9] [Reference Citation Analysis (1)] |
| 22. | Heah NH, Wong KY. Feasibility of robotic assisted bladder sparing pelvic exenteration for locally advanced rectal cancer: A single institution case series. World J Gastrointest Surg. 2020;12:190-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9191] [Article Influence: 540.6] [Reference Citation Analysis (1)] |
| 24. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) - Rectal Cancer. 2023. Access Date: Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. |
| 25. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4550] [Article Influence: 206.8] [Reference Citation Analysis (7)] |
| 26. | Schrag D, Shi Q, Weiser MR, Gollub MJ, Saltz LB, Musher BL, Goldberg J, Al Baghdadi T, Goodman KA, McWilliams RR, Farma JM, George TJ, Kennecke HF, Shergill A, Montemurro M, Nelson GD, Colgrove B, Gordon V, Venook AP, O'Reilly EM, Meyerhardt JA, Dueck AC, Basch E, Chang GJ, Mamon HJ. Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med. 2023;389:322-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 297] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 27. | Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations: 2018. World J Surg. 2019;43:659-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1371] [Article Influence: 195.9] [Reference Citation Analysis (0)] |
| 28. | Mohan R, Huey CWT, Junnarkar S, Low JK, Shelat VG. Prehabilitation in elderly patients scheduled for liver resection and protocol for Recovery Of Surgery in Elderly. Hepatoma Res. 2020;6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Wong KY, Tan AM. Short term outcomes of minimally invasive selective lateral pelvic lymph node dissection for low rectal cancer. World J Gastrointest Surg. 2020;12:178-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Sheetz KH, Claflin J, Dimick JB. Trends in the Adoption of Robotic Surgery for Common Surgical Procedures. JAMA Netw Open. 2020;3:e1918911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 573] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 31. | Goh BK, Teo RY. Current Status of Laparoscopic and Robotic Pancreatic Surgery and Its Adoption in Singapore. Ann Acad Med Singap. 2020;49:377-383. [PubMed] |
| 32. | Sun JY, Granieri MA, Zhao LC. Robotics and urologic reconstructive surgery. Transl Androl Urol. 2018;7:545-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Wee IJY, Kuo LJ, Ngu JC. A systematic review of the true benefit of robotic surgery: Ergonomics. Int J Med Robot. 2020;16:e2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 34. | Shugaba A, Lambert JE, Bampouras TM, Nuttall HE, Gaffney CJ, Subar DA. Should All Minimal Access Surgery Be Robot-Assisted? A Systematic Review into the Musculoskeletal and Cognitive Demands of Laparoscopic and Robot-Assisted Laparoscopic Surgery. J Gastrointest Surg. 2022;26:1520-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, Zhang W, Zhao R, Zhang C, Cheng L, Zhang X, Liang F, He G, Wei Y, Xu J; REAL Study Group. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 299] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 36. | Ryu S, Hara K, Kitagawa T, Okamoto A, Marukuchi R, Ito R, Nakabayashi Y. Fluorescence vessel and ureter navigation during laparoscopic lateral lymph node dissection. Langenbecks Arch Surg. 2022;407:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 37. | Shaw DD, Wright M, Taylor L, Bertelson NL, Shashidharan M, Menon P, Menon V, Wood S, Ternent CA. Robotic Colorectal Surgery Learning Curve and Case Complexity. J Laparoendosc Adv Surg Tech A. 2018;28:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Flynn J, Larach JT, Kong JCH, Waters PS, Warrier SK, Heriot A. The learning curve in robotic colorectal surgery compared with laparoscopic colorectal surgery: a systematic review. Colorectal Dis. 2021;23:2806-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Wang X, Cao G, Mao W, Lao W, He C. Robot-assisted versus laparoscopic surgery for rectal cancer: A systematic review and meta-analysis. J Cancer Res Ther. 2020;16:979-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 40. | Tang B, Li T, Gao G, Shi J. Learning Curve of Robotic-Assisted Total Mesorectal Excision for Rectal Cancer. Front Oncol. 2022;12:931426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Kim J, Baek SJ, Kang DW, Roh YE, Lee JW, Kwak HD, Kwak JM, Kim SH. Robotic Resection is a Good Prognostic Factor in Rectal Cancer Compared with Laparoscopic Resection: Long-term Survival Analysis Using Propensity Score Matching. Dis Colon Rectum. 2017;60:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Gallia GL, Bagley GA, Witham GA, Wolinsky JP, Gokaslan ZL. Total Sacrectomy. In: Wolfla CE, Resnick DK. Neurosurgical Operative Atlas: Spine and Peripheral Nerves. New York: Thieme, 2007: 266-272. [DOI] [Full Text] |
| 43. | Funder JA, Tolstrup R, Jepsen BN, Iversen LH. Postoperative paralytic ileus remains a problem following surgery for advanced pelvic cancers. J Surg Res. 2017;218:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod. 2014;19:27-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 510] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 45. | Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann Surg. 2018;267:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Baratti D, Italy; Tian BL, China S-Editor: Li L L-Editor: A P-Editor: Xu ZH